Abstract
A new fluorescent sensor, 4-allylamine-N-(N-salicylidene)-1,8-naphthalimide (1), anchoring a naphthalimide moiety as fluorophore and a Schiff base group as receptor, was synthesized and characterized. The photophysical properties of sensor 1 were conducted in organic solvents of different polarities. Our study revealed that, depending on the solvent polarity, the fluorescence quantum yields varied from 0.59 to 0.89. The fluorescent activity of the sensor was monitored and the sensor was consequently applied for the detection of Cu2+ with high selectivity over various metal ions by fluorescence quenching in Tris-HCl (pH = 7.2) buffer/DMF (1:1, v/v) solution. From the binding stoichiometry, it was indicated that a 1:1 complex was formed between Cu2+ and the sensor 1. The fluorescence intensity was linear with Cu2+ in the concentration range 0.5–5 μM. Moreso, the detection limit was calculated to be 0.32 μM, which is sufficiently low for good sensitivity of Cu2+ ion. The binding mode was due to the intramolecular charge transfer (ICT) and the coordination of Cu2+ with C = N and hydroxyl oxygen groups of the sensor 1. The sensor proved effective for Cu2+ monitoring in real water samples with recovery rates of 95–112.6 % obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the recent few decades, much attention has been paid to the science of the design and synthesis of probes for the monitoring of physiologically- and environmentally-relevant metal ions [1–16]. There is no doubt that many metal ions play great and varied roles, however, the ecotoxicological impacts of such metal ions on environmental and biological systems have constituted major challenges for a long time [17]. Among other metal ions, Cu2+ ion is an eminent metal ion owing to the indispensable roles it plays by acting as a catalyst and structural cofactor for many metalloenzymes, mitochondrial respiration, iron absorption, free radical scavenging and elastin cross-linking [18–20]. Though regulated amounts of Cu2+ ion in human bodies are essential for normal healthy living, however, excess concentrations of Cu2+ ion are both toxic and detrimental and can lead to oxidative stress that can in turn be implicated in a number of neuronal cytoplasm neurodegenerative diseases like Wilson’s disease, dyslexia, hypoglycemia, Alzheimer’s diseaese, Menkes diesease, Parkinson’s disease, amyotrophic lateral sclerosis, infant liver damage, and gastrointestinal disease [18, 21–27]. Due to the obvious threatful implications of excess amounts of Cu2+ ion, the search for methods for the detection of Cu2+ ion has been directed towards the synthesis of chemosensors.
Unlike other conventional analytical techniques, the fluorescence technique is a highly efficient analytical method and has received much research attention due to its relative advantages of high selectivity and sensitivity, intrinsic specificity, real time monitoring and fast response time [28–30]. Owing to their strong absorption and emission in the visible region, high photostability, large Stokes shift and high fluorescence quantum yields, much work is currently in progress in investigating the potent uses of 1,8-naphthalimide derivatives, which are a special class of environmentally sensitive chromophores [31–39]. This explains the reason why 1,8-naphthalimide derivatives enjoy a wide range of applications as colourants in the polymer industry [40, 41], laser dyes [42, 43], chemosensors [44, 45] and fluorescence probes for biomedical purposes, including fluorescence cell makers [46] and anti-cancer agents [47].
As such, recently much emphasis has been placed on the applications of Schiff bases in diverse fields including biological medicine, catalytic synthesis, analytical chemistry, anticorrosion, light-induced discoloration, thereby making them one of the most important categories of compounds in biology and chemistry [48].
Herein, we report a Schiff base based fluorescent chemosensor for Cu2+ ion, which induces a fluorescence quenching effect upon chelation with the metal ion. The chemosensor displays high and singular selectivity towards Cu2+ ion and could thereby distinguish Cu2+ when in coexistence with other metal ions under investigation.
Experimental
Materials and General Methods
Unless otherwise stipulated, all chemicals and solvents used for the synthesis were analytical reagent grade and used without further purification. Stock solutions of various metal ions (1 mM) were prepared using the nitrate salt of each metal in double-distilled water. Britton–Robinson (B–R) buffer was prepared with 40 mM acetic acid, boric acid and phosphoric acid. Dilute hydrochloric acid or sodium hydroxide was used for adjusting pH values. Tris-HCl buffer (pH = 7.2) were prepared using bidistilled water.
C, H and N contents were determined using a Carlo Erba 1106 elemental analyzer. The Infrared Radiation (IR) spectra were recorded in the 4000–400 cm−1 region with a Nicolet FT-VERTEX 70 spectrometer using KBr pellets. Absorption spectra were scanned using a Lab-Tech UV Bluestar spectrophotometer. Proton nuclear magenetic resonance (1H-NMR) spectra and carbon-13 nuclear magnetic resonance (13C-NMR) spectra were obtained with a Mercury plus 400 MHz NMR spectrometer with trimethyl silane (TMS) as internal standard and DMSO-d 6 as the solvent. Mass spectra (MS) were recorded on a Mass Spectrometer micrOTOF. The corrected excitation and fluorescence spectra were taken on a F97 Pro fluorescence spectrophotometer. A 1 × 1 cm2 quartz cuvette was used for the spectroscopic analysis. Relative fluorescence quantum yields (Ф F) were measured using N-butyl-4-n-butylamino-naphthalimide (Ф F = 0.81 in ethanol) [49] as standards. Thin Layer Chromatography (TLC) was performed on silica gel, Fluka F60 254, 20 × 20, 0.2 mm. The melting points were determined by means of a Kofler melting point microscope. The synthetic route to 1 is shown in Scheme 1.
Synthesis of Sensor 1
A suspension of 4-bromo-1,8-naphthalic anhydride (2.77 g,10 mmol) and 80 % hydrazine hydrate (1.25 g, 20 mmol) in 30 mL of ethanol was refluxed with stirring for 4 h [50]. The progress of the reaction was monitored by thin layer chromatography (TLC) using dichloromethane as the eluent. The mixture was cooled and the precipitated solids were filtered, recrystallized from ethanol and dried to afford N-amido-4-bromine-1,8-naphthalimide (2) as yellow-brown crystals in 2.49 g (85.6 %) yield. 1HNMR (CDCl3): δ (ppm) = 8.68 (d, 1H, J = 7.2 Hz), 8.60 (d, 1H, J = 8.4 Hz); 8.44 (d, 1H, J = 7.6 Hz); 8.06 (d, 1H, J = 8.06 Hz), 7.87 (t, 1H, J = 7.6 Hz), 5.30 (s, 2H).
To a solution of 1.47 g (5 mmol) of N-amido-4-bromine-1,8-naphthalimide (2) in 20 mL 2-methoxyethanol, 1.425 g (15 mmol) of allylamine was added. The resulting mixture was refluxed and stirred for 64 h and then poured into 100 mL of water. The precipitate was collected by filtration, washed with water and dried to give 1.25 g of (92.68 %) of N-amido-4-allylamine-1,8-naphthalimide (3). mp:180–182 °C.
Compound 3 (1 g, 3.75 mmol) was dissolved in absolute ethanol (40 mL). An excess of salicylic aldehyde (0.68 g, 5.57 mmol) was added and the mixture was refluxed for 6 h. After the mixture was cooled to room temperature, the precipitate that resulted was filtered, washed with water (3 × 10 mL) and dried to give orange crude product. The crude product was purified by column chromatography on flash silica gel using dichloromethane-acetone (v:v = 5:1, Rf = 0.78) as eluent to give 0.68 g (48.9 %) of the final product (1). mp:205–206 °C. Anal. calcd. C 71.15; H 4.61; N 11.31 %; found: C 71.08; H 4.58; N 11.29 %. IR (KBr; v/cm−1):1701, 1669, 1653, 1582, 1542, 1343, 763. UV-Visible (in DMF, nm): 274, 327, 441. M-S([C22H17N3O3] + 1) m/z = 372.1697. 1H NMR (DMSO-d 6 , 400 MHz): δ (ppm) = 8.978(s, 1H), 8.745 (d, 1H, J = 8.4 Hz); 8.50 (d, 1H, J = 7.6 Hz); 8.30 (d, 1H, J = 8.4 Hz); 7.727–7.785 (m, 2H); 7.473–7.511 (m, 1H); 7.011–7.057(m, 2H); 6.768 (d, 1H, J = 8.8 Hz); 5.961–6.003 (m, 1H); 5.196–5.304 (m, 2H); 4.092 (s, 2H). 13C NMR (DMSO-d 6 , 400 MHz): δ (ppm) = 169.632, 160.746, 160.175, 158.704, 150.931, 134.700, 134.472, 134.091, 131.264, 130.844, 128.932, 124.596, 122.005, 120.229, 119.719, 117.762, 116.762, 116.335, 107.542, 104.714, 44.933.
Results and Discussion
Molecular Design of Sensor 1 and Synthesis
The design methods of many fluorescent sensors are based on two fundamental principles of photoinduced electron transfer (PET) and internal charge transfer (ICT) [51, 52]. The PET system based on the “fluorophore-spacer-receptor” array stands out as the most widely used method for the design of fluorescent sensors and switches [53, 54]. In the ICT system, there is a direct attachment of the receptor to the electron-donating/withdrawing unit that is conjugated to the fluorophore [51, 52]. During excitation of the system, the fluorophore undergoes donor-acceptor intramolecular charge transfer.
In this current investigation, sensor 1 was designed as a fluorescent probe for the detection of Cu2+ ion based on the ICT mechanism. The sensor is based on the “fluorophore-receptor’ paradigm in such a way that the fluorophore is directly connected to a receptor sensor with the sensor void of a spacer.
The synthesis of sensor 1 was accomplished via three steps as depicted in Scheme 1. It was fully characterized by elemental analysis, 1H NMR, 13C NMR, UV-vis and IR spectroscopy and mass spectrometry. The spectral and elemental analysis data are all in good agreement with their chemical structures.
Photophysical Characteristics of Sensor 1
As widely known, the photophysical properties of substituted 1,8-naphthalimides are largely dependent on the polarization of their chromophoric systems. Absorption of light in these molecules generates a charge transfer interaction between the substituents at C-4 position and the imide carbonyl groups. We thereby carried out the photophysical characteristics of 1 in acetone, acetonitrile, DMF, tetrahydrofuran and dichloromethane solvents. The absorption (λA) and fluorescence (λF) maxima, the extinction coefficient (ε), the Stokes shift (υ A - υ F), and quantum fluorescence yield (Ф F) of 1 are presented in Table 1.
As seen from the data in Table 1, compound 1 shows absorption band with maximum range of about 427–441 nm, which is typical for 1,8-naphthalimides substituted in C-4 position with alkylamines. The molar extinction coefficient (ε) falls within the range 10,670–19,390 M−1 cm−1, which is higher than 10,000 M−1 cm−1, indicating that the absorption spectra band at about 430 nm is attributed to S0 → S1 transition. The respective fluorescence maxima are in the region 502–526 nm.
The photophysical properties of 1 under study is largely influenced by the polarity of the organic solvents, most especially is the quantum fluorescence yield and Stokes shift. The Stokes shift (υ A - υ F) reflects the difference in the properties and structure of the fluorophore between the ground state S0, and the first exited state S1. We obtained the value of the Stokes shift (cm−1) using the equation (1) [55]:
The Stokes shift values for 1 under investigation are in the region of 3498–3938 cm−1. It is obvious that the value of the Stokes shift depends on the solvent media, with polar solvents yielding larger Stokes shift values than non-polar solvents owing to their more favored hydrogen bond formation or dipole-dipole interactions (Table 1). This observation is in good agreement with investigations on other 1,8-naphthalimide derivatives [56, 57]. The fluorescent quantum yield, Ф F is another interesting photophysical parameter, which is used to characterize the ability of 1 to emit absorbed light energy. We obtained the value of the fluorescence quantum yield using N-butyl-4-n-butylamino-naphthalimide (Ф F = 0.81 in ethanol) according to equation (2) [49]:
where Ф F is the emission quantum yield of the sample, Ф ref is the emission quantum yield of the standard, Aref and Asample represent the absorbance of the standard and sample at the excited wavelength, respectively, while Sref and Ssample are the integrated emission band areas of the standard and sample, respectively, and nref and nsample are the solvent refractive index of the standard and sample, respectively. As expected, the fluorescence quantum yield of 1 in polar solvents is lower than that in non-polar solvents. This is explained on the basis of the photoinduced electron transfer process which is favored in polar solvents with the consequent result of low fluorescence emission [58].
Influence of pH on the Photophysical Properties of Sensor 1
The pH sensing properties of sensor 1 arise from ICT process. After protonation of sensor’s imine (C = N) nitrogen, the ICT interaction would be affected and the fluorescence of the system quenched [59]. Therefore, the emission of sensor 1 would experience an “on-state” at neutral media and “switched off” in both acidic and alkaline media. This principle provides the foundation for the effect of pH on the photophysical properties of sensor 1.
Generally, the sensing behavior of fluorescent sensors is influenced by the protonation or deprotonation of the fluorophore, which in turn affects their sensitivities in the detection of an analyte [60]. The light-harvesting system under this present study was designed as a molecular fluorescent probe for determination of pH changes over a wider pH scale. This is why we chose to investigate the photophysical behavior of 1 in Britton-Robison buffer/dimethyl formamide (1:1) solution at varying pH values. To acquire a great depth of insight, we utilized a wide pH range of 1.81–11.82, adjusted with 0.1 M HCl and/or 0.1 M NaOH, starting from the acidic region. As depicted in Fig. 1 , in the 1.81–11.82 pH region, the fluorescence intensity is pH-dependent. The result reveals that the fluorescence intensity of 1 suffered a dramatic decrease when the pH value goes down from 3.78 to 1.81 or goes up from 11.82 to 7.96. However, the fluorescence intensity of 1 did not undergo any significant changes in the range of pH from 3.78 to 7.96, implying that 1 was actually pH-independent between pH 3.78 to 7.96. In the light of this, we conducted the following experiments in solution at pH 7.2.
Recognition Experiment of Sensor 1 in the Presence of Various Metal Ions
A very crucial feature of any chemosensor is its ability for singular selectivity towards a specific analyte in the presence of competing species. We obtained insights into the fluorescent intensity of 1 as a ligand in the presence of various transition metal cations (Na+, K+, Ca2+, Mg2+, Al3+, Pb2+, Fe3+, Ni2+, Zn2+, Cu2+, Hg2+, Ag+, Co2+, Cr3+, Mn2+ or Cd2+, respectively) in Tris-HCl (pH = 7.2) buffer/DMF(1:1,v/v) solution. The fluorescence emission spectra were recorded at room temperature with an excitation at 444 nm. In the presence of metal cations in the solution, 1 acts as a ligand during the interaction between the components which is signaled by the changes in the fluorescence intensity. As seen from Fig. 2a, we found that only Cu2+ caused a significant fluorescence quenching at 539 nm among the various transition metal ions tested under the same conditions. These results clearly indicate that 1 could be used as a Cu2+ selective fluorescent sensor.
a Fluorescence spectra of sensor 1 (5 × 10−6 M) in Tris-HCl (pH = 7.2) buffer/DMF(1:1,v/v) solution towards Na+, K+, Ca2+, Mg2+, Al3+, Pb2+, Fe3+, Ni2+, Zn2+, Cu2+, Hg2+, Ag+, Co2+, Cr3+, Mn2+ or Cd2+ (2 eq) with an excitation at 444 nm; b Competitive experiments in sensor 1 + Cu2+ system with interfering metal ions at 539 nm; c Fluorescence titration spectra of sensor 1 with Cu2+ (0-2 eq) in Tris-HCl (pH = 7.2) buffer/DMF (1:1, v/v) solution; d Job’s plot at 539 nm
Selectivity Study of Sensor 1 for Cu2+ Ion over Competitive Metal Ions
To evaluate the affinity of 1 to Cu2+, a competitive binding experiment between Cu2+ and other metals to 1 was carried out by using a mixed solution containing Cu2+ (10 μM) and each of the other metal cations (Na+, K+, Ca2+, Mg2+, Al3+, Pb2+, Fe3+, Ni2+, Zn2+, Hg2+, Ag+, Co2+, Cr3+, Mn2+ or Cd2+) at a concentration of 10 μM in Tris-HCl (pH = 7.2) buffer/DMF(1:1,v/v) solution. From the inset Fig. 2b, we can see that there was no obvious discrimination of Cu2+ rendered by 1 even in the presence of competitive metal ions, which implies that compound 1-Cu2+ system was hardly affected by these coexistent ions.
Fluorescence Titration of Sensor 1 in the Presence of Increasing Concentration of Cu2+ Ion
In order to gain insight into the binding interaction between 1 with Cu2+, we performed fluorescence titration experiments of 1 with Cu (NO3)2 in Tris-HCl (pH = 7.2) buffer/DMF(1:1, v/v) solution. With an increasing concentration of Cu2+, the intensity of the maximum emission at 539 nm suffered a gradual decrease (Fig. 2c). The fluorescence intensity almost reached a minimum when the amount of added Cu2+ ion was about 5 × 10−6 M. As more Cu2+ was titrated, the fluorescence intensity showed negligible changes. The nonlinear curve fitting of the fluorescence titration gives a 1:1 stoichiometric ratio between compound 1 and Cu2+. Moreover, Job’s plot [61], which exhibits a maximum at 0.5 M fraction of Cu2+, indicates that only a 1:1 complex is formed (Fig. 2d).
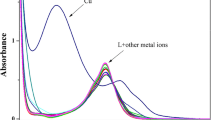
UV-Vis Titration of Sensor 1 in the Presence of Increasing Concentration of Cu2+ Ion
Furthermore, we performed the UV-vis titration experiment of 1 with Cu2+ in Tris-HCl (pH = 7.2) buffer/DMF (1:1, v/v) solution. Figure 3 reveals the UV-vis absorption spectra of 1 (5 × 10−6 M) in the presence of various concentrations of Cu2+ ion (0–1 × 10−5 M, 0-2 eq) while the inset shows the plot of changes in 326 and 373 nm maxima as a function of increasing concentrations of Cu2+. The absorbance of 1 at 326 nm gradually decreases with an increasing concentration of Cu2+ ion, showing a clear evidence of C = N coordination to Cu2+ ion. Owing to the coordination of 1 with Cu2+ ion, the absorbance at 373 nm increases with an increasing concentration of Cu2+ ion. When the amount of Cu2+ ion added was about 5 × 10−6 M, the absorbance of 1 at 326 nm and 373 nm reached a minimum and maximum, respectively. As more Cu2+ was titrated, the absorbance showed negligible changes, implying a 1:1 stoichiometry complex was formed. More interesting to note is that two isobestic points appeared at 304 nm and 353 nm. Moreover, the absorption at 274 nm and 448 nm could be assigned to π–π* transitions of benzene and naphthalene ring, respectively.
Determination of Association Constant and Detection Limit
Based on the fluorescence titration of compound 1 with Cu2+, the association constant has been calculated to be 1.088 × 106 M−1 (error limits ≤10 %) by a Benesi-Hildebrand equation [62–64] (Fig. 4).
Herein, F is the fluorescence intensity at 539 nm at any given Cu2+ concentration, F0 is the fluorescence intensity at 539 nm in the absence of Cu2+, and Fm is the minimum fluorescence intensity at 539 nm in the presence of Cu2+ in solution. The association constant Ka was evaluated graphically by the plot of log [(F-F0)/(Fm-F)] versus log[Cu2+].
Figure 5 also further confirms the good linearity between the emission at 539 nm and concentrations of Cu2+ in the range from 0.5 to 5 μM, indicating that sensor 1 can detect quantitatively relevant concentrations of Cu2+.
We calculated the limit of detection (LOD) based on the definition by IUPAC and the information obtained from the Stern-Volmer plot according to the equation below [65]:
where σ is the standard deviation of the blank solution and k is the slope between F0/F versus [Cu2+]. The value of LOD was obtained to be 0.32 μM, which is far lower than the WHO and U.S. EPA regulated limits of 31.5 μM and 20 μM, respectively [19, 66]. This result substantiates that the proposed sensor 1 could monitor Cu2+ ion in sufficiently low concentrations whether in environmental- or biological system.
Effects of Anions on the Sensing Properties of Sensor 1 towards Cu2+
In order to explore the effects of anionic counter-ions on the sensing behavior of 1 to Cu2+ ion, fluorescence responses of 1 to sulfate, chloride, bromide, oxalate and nitrate salts of copper were performed in Tris-HCl (pH = 7.2) buffer/DMF(1:1,v/v) solution. As can be seen from Fig.6, there were no noteworthy changes in the fluorescence responses of 1 to CuSO4, CuCl2, CuBr2, CuC2O4 and Cu (NO3)2.
Proposed Binding Mechanism
Based on the results of the absorption titration spectra, fluorescence titration spectra and Job’s plot, we hereby propose the binding mode of sensor 1 with Cu2+. As shown in Scheme 2, the mechanism for Cu2+ quenching is explained as due to intramolecular charge transfer (ICT) [1, 16, 52]. The chelation between sensor 1 and Cu2+ is facilitated by the additional coordination from: (i) the imine group, C = N group, which displays a high level of binding affinity with many transition and post-transition metal ions [67, 68] and (ii) the hydroxyl oxygen group. The sensor 1 was designed in such a way that these two functional groups are positioned in a way that facilitates their proximity to the fluorophore moiety. Upon coordination of Cu2+ with sensor 1, there is a chelation between the metal ion and N of the C = N group and O of the hydroxyl oxygen group, leading to the repression of the fluorescence emission intensity.
Application of Sensor 1 for Cu2+ Ion Analysis in Water Samples
To evaluate the potential applicability of sensor 1 in real water sample analysis, it was applied for the determination of Cu2+ in Yellow River water sample (Yellow River is a popular river in Northwest China that flows through Lanzhou City wherein our school, Lanzhou Jiaotong University is located) and tap water sample (sourced from our school). The pH of all the water samples were suitably adjusted by HEPES aqueous buffer solution (pH = 7.4) and analyzed with sensor 1. Summarized in Table 2 are the results obtained, which showed excellent recovery and mean S.D. values for all the samples. Obviously, sensor 1 could facilitate the detection Cu2+ in real water samples.
Conclusions
In summary, we have designed and synthesized a new fluorescent sensor for the detection of Cu2+ ion. The sensor, containing a 1,8-napthalimide moiety and a Schiff base unit, displayed a high selectivity and sensitive towards Cu2+ with little interference observed from other coexistent metal ions. According to the results of UV-vis titration, fluorescence titration and Job plot, we determined the binding ratio of sensor-Cu2+ complex to be 1:1. The proposed sensor exhibits a linear response towards Cu2+ in the concentration range covering from 0.5 to 5 μM with a detection limit of 0.32 μM. These results clearly demonstrate that our proposed sensor could be useful for analysis of Cu2+ in environmental samples and even for biological studies.
References
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Chem Rev 97:1515–1566
Gunnlaugsson T, Lee TC, Parkesh R (2003) Org Biomol Chem 1:3265–3267
Misra A, Shahid M, Srivastava P (2012) Sensors Actuators B 169:327–340
Bojinov VB, Panova IP, Simeonov DB, Georgiev NI (2010) J. Photochem. Photobiol A: Chem, 210:89–99
Li Q, Guo Y, Shao SJ (2012) Sensors Actuators B 171–172:872–877
Bargossi C, Fiorini MC, Montalti M, Prodi L, Zaccheroni N (2000) Coord Chem Rev 208:17–32
Aragay G, Pons J, Merkoci A (2011) Chem Rev 111(5):3433–3458
Quang DT, Kim JS (2010) Chem Rev 110:6280–6301
Nolan EM, Lippard SJ (2008) Chem Rev 108(9):3443–3480
Hewage HS, Anslyn EV, Am J (2009) Chem Soc 131(36):13099–13106
Kaur N, Kumar S (2011) Tetrahedron 67(48):9233–9264
Kuang GC, Allen JR, Baird MA, Nguyen BT, Zhang L, Morgan Jr TJ, Levenson CW, Davidson MW, Zhu L (2011) Inorg Chem 50(20):10493–10504
Dutta M, Das D (2012) Trac-trends Anal Chem 32:113–132
Kim HN, Ren WX, Kim JS, Yoon J (2012) Chem Soc Rev 41:3210–3244
Barba-Bon A, Costero AM, Gil S, Parra M, Soto J, Martínez-Máñez R, Sancenón F (2012) Chem Commun 48:3000–3002
Valeur B, Leray I (2000) Coord Chem Rev 205(1):3–40
Que EL, Domaille DW, Chang CJ (2008) Chem Rev 108(5):1517–1549
Cho J, Pradhan T, Lee YM, Kim JS, Kim S (2014) Dalton Trans 43:16178–16182
You GR, Park GJ, Lee JJ, Kim C (2015) Dalton Trans 44:9120–9129
Tapiero H, Townsend DM, Tew KD (2003) Biomed Pharmacother 57(9):386–398
Barnham KJ, Masters CL, Bush AI (2004) Nat Rev Drug Discov 3:205–214
Mare S, Penugonda S, Robinson SM, Dohgu S, Banks WA, Ercal N (2007) Peptides 28(7):1424–1432
Kim BE, Nevitt T, Thiele DJ (2008) Nat Chem Biol 4:176–185
Lee JC, Gray HB, Winkler JR (2008) J Am Chem Soc 130(22):6898–6899
Mokhir A, Kiel A, Herten D-P, Kraemer R (2005) Inorg Chem 44(16):5661–5666
Liu J, Lu Y, Am J (2007) Chem Soc 129(32):9838–9839
Løvstad RA (2004) Biometals 17(2):111–113
Chen X, Pradhan T, Wang F, Kim JS, Yoon J (2012) Chem Rev 112:1910–1956
Fabbrizzi L, Licchelli M, Pallavicini P, Sacchi D, Taglietti A (1996) Analyst 121:1763–1768
Kramer R (1998) Angew Chem Int Ed 37:772–773
Nakaya KI, Funabiki R, Muramatsu H, Shabata K, Matsui M (1999) Dyes Pigments 43:235–239
Kawai K, Kawabata K, Tojo S, Majima T (2002) Bioorg Med Chem Lett 12:2363–2366
Ramachandram B, Saroja G, Sankaran NB, Samanta A (2000) J Phys Chem B 104:11824–11832
Das SK, Sahu PK, Kar UP, Rahaman A, Sarkar M, Soni M (2013) J Phys Chem C 117:14338–14347
Huang X, Fang Y, Li X, Xie Y, Zhu W (2011) Dyes Pigments 90:297–303
Mohan V, Nijamudheen A, Das SK, Sahu PK, Kar UP, Rahaman A, Sarkar M (2012) Chem Phys Chem 13:3882–3892
Wang J, Yang L, Hou C, Cao H (2012) Org Biomol Chem 10:6271–6274
Gunnlaugsson T, Kruger P, Jensen P, Pfeffer F, Hussey G (2003) Tetrahedron Lett 44:8909–8913
Desislava S, Evgenia VT, Ivo G (2014) J Mol Struct 1071:88–94
McGehee MD, Heeger AJ (2000) Adv Mater 12:1655
Bojinov V, Grabchev I (2001) Dyes Pigments 51:57–61
Tian H, He Y, Chang CP (2000) J Mater Chem 10:2049–2055
Yang S, Meng F, Tian H, Chen K (2002) Eur Polym J 38:911–919
Duke RM, Veale EB, Pfeffer FM, Kruger PE, Gunnlaugsson T (2010) Chem Soc Rev 39:3936–3953
Wang B, Anslyn EV (2011) Chemosensors: Principles, Strategies and Applications,. John Wiley & Sons, New York, pp. 229–252
Qian X, Xiao Y, Xu Y, Guo X, Qian J, Zhu W (2010) Chem Commun 46:6418–6436
M. F. Brana and A. Ramos, Curr. Med. Chem. Anti-cancer Agents., 1, 237–255 (2001)
Beezer A, Miles R, Shaw E, Willis P (1979) Experientia 35:795–796
Alexiou MS, Tychopoulos V, Ghorbanian S, Tyman JHP, Brown RG, Brittain PI (1990) J Chem Soc Perkin Trans 2:837–842
Chen ZJ, Wang LM, Zou G, Cao XM, Wu Y, Hu PJ (2013) Spectrochim Acta A 114:323–329
de Silva A, McCaughan B, McKinney B, Querol M (2003) Dalton Trans 10:1902–1913
Callan JF, de Silva AP, Magri DC (2005) Tetrahedron 61:8551–8588
T. Gunnlaugsson, C. McCoy, R. Morrow, C. Phelan and F. Stomeo, ARKIVOC VII, 216–28 (2003).
Ramachandram B (2005) J Fluoresc 15:71–83
Georgiev NI, Bojinov VB (2012) J Lumin 132:2235–2241
Sali S, Guittonneau S, Grabchev I (2006) Polym Adv Technol 17:180–185
Grabchev I, Chovelon JM (2008) Dyes Pigments 77:1–6
Poteau X, Brown AI, Brown RG, Holmes C, Matthew D (2000) Dyes Pigments 47:91–105
Qian J, Xu Y, Qian X, Wang J (2008) J Photochem Photobiol A: Chem 200:402–409
Han ZX, Zhang XB, Li Z, Gong YJ, Wu XY, Jin Z, He CM, Jian LX, Zhang J, Shen G-L, Yu R-Q (2010) Anal Chem 82(8):3108–3113
Job P (1928) Ann Chim 9:113–203
Benesi HA, Hildebrand JH, Am J (1949) Chem Soc 71:2703–2707
K. A. Connors, Binding Constants, John Wiley & Sons, New York, 339–343 (1987)
Valeur B (2000) Molecular Fluorescence. Wiley-VCH, Weinheim, pp. 339–346
Joshi BP, Park J, Lee WI, Lee KH (2009) Talanta 78:903–909
Jung HS, Kwon PS, Lee JW, Kim JII, Hong CS, Kim JW, Yan S, Lee JY, Lee JH, Joo T, Kim JS (2009) J Am Chem Soc 131(5):2008–2012
Bhardwaj VK, Pannu APS, Singh N, Hundal MS, Hundal G (2008) Tetrahedron 64:5384–5391
Pascu SI, Balazs G, Green JC, Green MLH, Vei IC, Warren JE, Windsor C (2010) Inorg Chim Acta 363:1157–1172
Acknowledgment
The authors appreciate the financial supports from the National Natural Science Foundation of China (Grant No. 21367017), Natural Science Foundation of Gansu Province (Grant No. 1212RJZA037) and Graduate Student Innovation Projects of Lanzhou Jiaotong University, which resulted in this article. Likewise, the first author thanks the Chinese Scholarship Council for awarding him a scholarship opportunity (CSC No. 2014BSZ528) to conduct his Master’s program in China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aderinto, S.O., Xu, Y., Peng, H. et al. A highly Selective Fluorescent Sensor for Monitoring Cu2+ Ion: Synthesis, Characterization and Photophysical Properties. J Fluoresc 27, 79–87 (2017). https://doi.org/10.1007/s10895-016-1936-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-016-1936-7