Abstract
Water-soluble Mn2+-doped ZnS quantum dots (QDs) were prepared using mercaptoacetic acid as the stabilizer. The optical properties and structure features were characterized by X-Ray, absorption spectrum, IR spectrum and fluorescence spectrum. In pH 7.8 Tris-HCl buffer, the QDs emitted strong fluorescence peaked at 590 nm with excitation wavelength at 300 nm. The presence of sulfide anion resulted in the quenching of fluorescence and the intensity decrease was proportional to the S2− concentration. The linear range was from 2.5 × 10−6 to 3.8 × 10−5 mol L−1 with detection limit as 1.5 × 10−7 mol L−1. Most anions such as F−, Cl−, Br−, I−, CH3CO2 −, ClO4 −, CO3 2−, NO2 −, NO3 −, S2O3 2−, SO3 2− and SO4 2− did not interfere with the determination. Thus a highly selective assay was proposed and applied to the determination of S2− in discharged water with the recovery of ca. 103%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The risk of sulfide anion toxicity is associated with exposure in a number of occupational settings [1–3]. Continuous and high concentration exposure of sulfide can cause various physiological and biochemical problems. When sulfide anion is protonated, it becomes even more toxic. Thus the detection of sulfide anion has become very important from industrial, environmental, and biological point of view [4]. Many methods have been developed so far including titration [5], spectrophotometry [6–10], inductively coupled plasma atomic emission spectroscopy (ICP-AES) [11], hydride generation atomic fluorescence spectrometry (HGAFS) [12], electrochemical methods [13–16], ion chromatography [17, 18], chemiluminescence (CL) methods [4, 19–21] and fluorimetry [22–26]. Among them, fluorimetry has received considerable attention because of high sensitivity and easy detection. Recently semiconductor nanocrystals or quantum dots (QDs) have been widely used because they offer many advantages over conventional organic fluorophores due to their generally high luminescence quantum yield, good photochemical stability, broad excitation band and narrow emission band, size-dependent emission wavelength and large effective Stokes shift [27]. They are most frequently used to detect cations [28–35]. Recently, they have been applied to the detection of anions as well. Lakowicz et al. [36] reported the detection of I− by surface modified CdS. Alfredo et al. [37, 38] reported the selective determination of CN− in methanol by tert-butyl-N-(2-mercaptoethyl)-carbamate modified CdSe QDs. Liu et al. [39] developed a new method to detect NO2 − by CdSe QDs. Wu et al. [40] realized the highly selective detection of HSe− by water soluble CdS QDs with slight interference from Cu2+ and S2−. Multrooney et al. [41] detected F− by CdSe/ZnS QDs. Usually the fluorescence emission of doping ions has higher photostability than the defect-related luminescence of semiconductive nanomaterials, because the defects are greatly affected by synthesis conditions and environments. Because of its wide band gap (3.7 eV), zinc sulfide (ZnS) is particularly suitable for use as a host material for a large variety of dopants. Mn2+-doped ZnS had strong orange emission at ca. 600 nm and high quantum yield [42–46].The luminescence lifetime of Mn2+-doped ZnS nanocrystals was ca. 1 ms. Such a long lifetime made the luminescence from the nanocrystal readily distinguishable from the background luminescence [47]. Therefore Mn2+-doped ZnS nanocrystals have been extensively used as fluorescent sensor in various applications. Tu et al. [48] synthesized the amine-capped Mn2+-doped ZnS nanocrystals for the ultra sensitive detection of 2,4,6-trinitrotoluene(TNT) in liquid and gas explosive substance. Yan et al. [49] applied the phosphoresence of ZnS:Mn2+ to the assay of enoxacin in biological fluids. Wang et al. [50] proposed an assay for pentachlorophenol based on SiO2 modified ZnS:Mn2+. Swadeshmukul et al. [51] synthesized water soluble mercaptoacetic CdS/ZnS:Mn2+ QDs which were used in biological imaging. However until now the detection of sulfide anion by QDs has never been reported yet. Herein, we synthesized mercaptoacetic coated ZnS:Mn2+ QDs which showed excellent water solubility and optical properties. At the same time, the QDs highly selectively responded to sulfide anion in pH 7.8 Tris-HCl buffer.
Experimental
Apparatus
All fluorescence measurements were carried out on a F-4600 spectrofluorimeter (Hitachi, Japan) equipped with a xenon lamp source and a 1.0 cm quartz cell, both slits of emission and excitation were 5 nm and the scan speed was 1,200 nm min−1. Absorption spectra were recorded on a Shimadzu-2501 UV-Vis spectrophotometer (Shimadzu Japan) using a 1.0 cm quartz cell. X-ray spectroscopy was collected on XRD DI SYSTEM (Bede England). Infrared spectra were obtained as KBr pellets on a Nicolet 5700 FTIR spectrometer. Size distribution of ZnS:Mn2+ was performed on Hydrosol Nano-particle size analyzer and Zeta Potential Analyzer (PSA NANO2590, Malvern Companies, UK). The multi-elemental analysis of ZnS:Mn2+ QDs was performed on ICP-AES OPTIMA 5300DV (Perkin-Elmer,U.S.A). All pH measurements were made with a pHS-3 digital pH-meter (Shanghai REX Instrument Corp., Shanghai, China) with a combined glass-calomel electrode.
Reagents
All chemicals were of analytical grade and were used without further purification. All solutions were prepared using doubly distilled water. Zn(CH3CO2)2•2H2O and Mn(CH3CO2)2• 4H2O were the products of the Shanghai Qingxi Technology Co., Ltd., NaS•9H2O was purchased from Shanghai Chemical Technology Development Co., Ltd., Tris (Sigma Chemical Co.) buffer had a pH of 7.8. The sodium salts of the tested anions and the chloride of the tested cations were Sigma-Aldrich Corp. products.
Preparation of mn-doped ZnS QDs [52]
To the three-necked flask, 5.0 mL of 0.1 mol L−1 Zn(CH3CO2)2, 20 mL of 0.1 mol L−1 mercaptoacetic acid and 1.5 mL of 0.01 mol L−1 Mn(CH3CO2)2 were added and diluted to 50 mL with doubly deionized water. The pH of the mixed solution was adjusted to be 10.5 using 2.0 mol L−1 NaOH and nitrogen gas was passed for 30 min at room temperature in order to remove oxygen. Then, 5.0 ml of 0.1 mol L−1 Na2S was quickly injected into the solution under vigorous stirring under nitrogen atmosphere for 15 min. At last, the solution was aged at 50 °C in air for 2 h. The aged solution was precipitated with anhydrous ethanol, the precipitate was centrifuged and washed with ethanol, then dried in vacuum. The ZnS:Mn2+ nanocrystals were obtained.
Procedure of measurement
A certain amount of ZnS:Mn2+ powder was dispersed in deionized water and the solution of 100 μg mL−1 was obtained. Different concentrations of sulfide anion were added into 0.6 mL QDs solution and diluted to 2.0 ml with Tris-HCl aqueous solution of pH 7.8. The fluorescence spectra were obtained with excitation wavelength at 300 nm. The scan speed was 1,200 nm min−1 and the band-slits of both excitation and emission were set as 5.0 nm.
Results and discussion
IR spectra of mercaptoacetic acid and ZnS:Mn2+ QDs capped TGA
The IR spectra of mercaptoacetic acid (TGA) and QDs capped with TGA were shown in Fig. 1. It was clear that the absorption band at 2,567 cm−1 which was ascribed to sulfhydryl group disappeared in the spectrum of QDs. The asymmetric and symmetric stretch vibrations of the carboxyl group of TGA were at 1,710 cm−1 and 1,384.4 cm−1 respectively. After binding to QDs, they shifted to 1,587.1 cm−1 and 1,387.8 cm−1 respectively. It could be concluded that mercaptoacetic acid has bound to the surface of QDs. As a result, the solubility of QDs in water was improved.
Content of doped manganese
The amount of Mn doped will affect the fluorescent intensity of QDs. The experiment results displayed that the optimal molar ratio between Mn2+ and Zn2+ was 0.03 in synthesis of Mn-Doped ZnS QDs. The actual concentration ratio of Mn2+ and Zn2+ was 0.028 which was obtained from inductively coupled plasma analyzer (ICP).
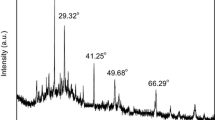
Characterization of the Mn-doped ZnS QDs by XRD
For different drying condition under 60 °C, 120 °C and 210 °C, the XRD spectra of QDs showed no significant discrepancy. Figure 2 presented the XRD patterns of ZnS:Mn2+ QDs which were obtained under 210 °C for drying. These diffraction features appealing at 28.5°, 47.5°, and 56.3° corresponded to the (111), (220), and (311) planes of cubic sphalerite ZnS, which was very consistent with the values in the standard card (PDF-card 5-566). It implied that the Mn-doped ZnS possessed the cubic sphalerite ZnS crystal model. According to Debye-Scherrer formula,
where D is the averaged crystallite size, λ is the wavelength of X-ray, usually using line Kα of Cu with wavelength of 0.15,406 nm, θ is a glancing angle between X-rays and a crystal face, k is a constant as 0.89, andβis the full width at half maximum of the diffraction line. From the equation (1), D of Mn-doped ZnS was estimated as 3.8 nm.
Spectroscopic properties of ZnS:Mn2+ QDs in aqueous solution
The fluorescence spectrum and absorption spectrum of ZnS:Mn2+ QDs in pH 7.8 aqueous solution were presented in Fig. 3. The absorption peak was 294 nm (4.22 eV) which showed the band gap absorption of ZnS:Mn2+ nano-crystalline particles. Compared with the phase material of ZnS whose absorption peak was at 340.6 nm (3.64 eV), the absorption peak blue shifted 46 nm (0.58 eV). The result indicated that ZnS:Mn2+possessed quantum confinement effect. According to Brus effective mass approximation [53], the average size of ZnS:Mn2+ was estimated as 4.1 nm which was consistent with the result of XRD. Two emission bands peaked at 400 nm and 590 nm, respectively were observed when excitation wavelength set at 300 nm. The weak emission at 400 nm originated from the defect-related emission of the ZnS [54]. The strong emission peak at 590 nm is attributed to the 4T1−6A1 transition of Mn2+ impurity which indicated Mn2+ entered into the ZnS lattice to form ZnS: Mn2 + QDs. However, the green fluorescence at 480 nm of ZnS phase wasn’t observed which was attributed to energy transfer from ZnS QDs to Mn2+ [48].
Size distribution of ZnS:Mn2+ hydrosol
In order to explore the emission mechanism, the size of QDs in aqueous solution was measured. As described in the section of procedure of measurement, the nanocrystal hydrosol was prepared and the size distribution curve was presented in Fig. 4. It was clear that the size of nanocrytal concentrated in the range of 33–50 nm. The narrow scope suggested the nanocrystal hydrosol was relatively uniform in size distribution. Placed the nanocrystal hydrosol in the refrigerator at 4 °C for 3 days, no observable precipitation was found and the size distribution curve kept constant. Such a transparent solution implied that Mn-doped ZnS nanocrystal has good water solubility and stability which was promising for the application. Obviously, the size of the hydrated molecule was significantly larger than that of solid owing to the hydration of nanocrystal hydrosol. But the emission profile and peak position in solid state and in hydrosol solution were the same which implied that the hydration action did not change the luminescent properties of QDs.
Spectral changes of ZnS: Mn2+ QDs in the presence of anions
Figure 5 showed the fluorescent spectrum changes of QDs upon addition of S2−. In the pH 7.8 Tris-HCl buffer, the addition of S2− resulted in the fluorescence quenching. The fluorescence quenching was best described by the equation:1 − F/F0 = −0.018 + 0.016[Q], where F0 and F were the fluorescent intensity of QDs in the absence and presence of S2−, [Q] was the concentration of the quencher (S2−). The linear relationship of the intensity ratio vs S2− concentration was presented in the insert of Fig. 5. The linear range of the calibration curve was from 2.5 × 10−6 to 3.8 × 10−5 mol L−1 with the detection limit as 1.5 × 10−7 mol L−1.
Fluorescent spectral changes of QDs in pH 7.8 Tris-HCl buffer upon addition of S2−. The excitation wavelength was 300 nm. The inset was the fitted liner relationship between intensity ratio and concentration of S2−.The concentration of S2− was 0, 0.25, 0.75, 1.75, 2.75, 3.75, 4.75, 5.75, 6.75, 7.75, 8.75, 9.75, 11.75, and 13.75 × 10−5 mol L−1, respectively. Arrow indicants increasing of S2− concentration
In the above quenching experiment, the excitation wavelength was set at 300 nm. However, the absorbance of ca. 300 nm increased upon addition of S2−. It was concluded that the fluorescence quenching of QDs upon addition of S2− was ascribed to be the binding action between QDs and S2− instead of the change of absorbance.
The effect of various anions on the fluorescence spectrum of ZnS:Mn2+ QDs was also investigated and presented in Fig. 6. Upon the presence of 2.5 × 10−4 mol L−1 for each anion such as Cl−, Br−, CH3CO2 −, ClO4 −, CO3 2−, F−, I−, NO2 −, NO3 −, S2O3 2−, SO3 2− and SO4 2−, no obvious fluorescent intensity change was observed. However with the addition of 2.5 × 10−5 mol L−1 S2−, the fluorescence intensity decreased at ca. 30%.
a Intensity change of QDs (30 μg mL−1) in the presence and absence of anions. The concentration of sulfide anion was 2.5 × 10−5 mol L−1 while the concentrations of other anions were 2.5 × 10−4 mol L−1. b The intensity change of QDs and 2.5 × 10−5 mol L−1 sulfide anion mixture upon addition of different anions (2.5 × 10−4 mol L−1)
Analytical application
Optimal experimental condition
The effect of pH on the luminescence of ZnS:Mn2+ was investigated and presented in Fig. 7a. When pH value was lower than 9.0 and higher than 7.0, the intensity of QDs was strong and kept constant and the system was stable at room temperature for at least 3 days. In addition, the effect of pH value on the intensity of QDs-S2− was also studied (shown in Fig. 7b). The intensity stabilized in the pH range of 6.7 to 10.4. In the strong acid the interaction between QDs and S2− was weak because sulfur existed in the form of HS− instead of S2−. At the same time, the response was also weak in strong base medium because the existence of too much OH− groups on the surface of QDs hindered the interaction between S2− and QDs. In order to make it feasible, Tris-HCl buffer of pH 7.8 was selected.
At the same time, the effect of concentration of ZnS:Mn2+ on the sensitivity was also studied. The fluorescent intensity increased with the increase of concentration of ZnS:Mn2+. However, the degree of fluorescent intensity upon addition of S2− was decreased with the concentration of ZnS:Mn2+. Take both into consideration, the concentration of ZnS:Mn2+ was 30 μg mL−1.
Thus, the optimal experiment condition was under room temperature in Tris-HCl buffer of pH 7.8 and the concentration of QDs was 30 μg mL−1.
Interference of foreign substances
Under optimal experimental condition, in the mixture solution of 5.0 × 10−6 mol L−1 S2− and 30 μg mL−1 QDs, the presence of following amounts of foreign substances compared with the concentration of S2− resulted in less than ±5% error: 1,000-fold NO3 −, Na+, K+, Li+ and NH4 +, 600-fold SO3 2− ,500-fold CO3 2−, ClO4 − and F−, 400-fold NO2 − and SO4 2−, 300-fold Br−, 100-fold I−, CH3CO2 −, 20-fold Mg2+, Ca2+, Ba2+. The presence of heavy metal ions such as Cu2+, Hg2+ and Ag+ also quenched the fluorescence of QDs, because the insoluble sulfides formation between metal ions and S2− led to ZnS precipitation transfer which resulted in the change of photophysical properties or the surface state of thiol-capped QDs [32]. However, those ions of high concentration couldn’t coexist with S2−, their existence didn’t interfere the determination.
Sample analysis
To investigate the possibility of practical application, the determination of sulfide anion was performed on a lake water sample. The recovery was about 103% shown in Table 1.
Proposed mechanism
ZnS dopped Mn2+ quantum dots emit two emission bands peaked at 400 nm and 590 nm. The emission at 400 nm originated from the defect-related emission of the ZnS [54] and the emission of 590 nm is attributed to the 4T1−6A1 transition of Mn2+ impurity [48]. When S2− was adsorbed on the surface of the QDs, S2− vacancy of the surface of QDs decreased. As the result, surface fluorescence of ZnS was quenched effectively. At the same time, the adsorption of S2− increased dangling bonds originating from the lone pairs on surface S2− which resulted in more no-radiation pathways of luminescent center, consequently the fluorescence of Mn2+ was quenched as well [55].
Since the surface of QDs was coated with negatively charged mercaptoacetic ions, it was very difficult for other anions to interact with the surface of the QDs. However S2− could fit well the sulfide defect of the surface and S2− could bind strongly with Zn2+, thus S2− could easily interact with the QDs [40]. Therefore, QDs showed highly selective response to sulfide anion than other anions.
Conclusions
Water-soluble Mn2+-doped ZnS quantum dots (QDs) were prepared which showed selective response to sulfide anion over other anions such as F−, Cl−, Br−, I−, CH3CO2 −, ClO4 −, CO3 2−, NO2 −, NO3 −, S2O3 2−, SO3 2− and SO4 2−. The high selectivity was assumed that S2− could fit well the sulfide defect of the QDs surface and S2− could bind strongly with Zn2+.
References
Puacz W, Szahun W (1995) Catalytic determination of sulfide in blood. Analyst 120(3):939–941
Hendrickson RG, Chang A, Hamilton RJ (2004) Co-worker fatalities from hydrogen sulfide. Am J Ind Med 45(4):346–350
Lawrence NS, Davis J, Compton RG (2000) Analytical strategies for the detection of sulfide: a review. Talanta 52(5):771–784
Huang RF, Zheng XW, Qu YJ (2007) Highly selective electrogenerated chemiluminescence (ECL) for sulfide ion determination at multi-wall carbon nanotubes-modified graphite electrode. Anal Chim Acta 582(2):267–274
Balasubramanian S, Pugalenthi V (2000) A comparative study of the determination of sulphide in tannery waste water by ion selective electrode (ISE) and iodimetry. Water Res 34(17):4201–4206
Ferrer L, de Armas G, Miró M, Estela JM, Cerdà V (2004) A multisyringe flow injection method for the automated determination of sulfide in waters using a miniaturised optical fiber spectrophotometer. Talanta 64(5):1119–1126
Silva MSP, Galhardo CX, Masini JC (2003) Application of sequential injection-monosegmented flow analysis (SI-MSFA) to spectrophotometric determination of sulfide in simulated waters samples. Talanta 60(1):45–52
Silva MSP, da Silva IS, Abate G, Masini JC (2001) Spectrophotometric determination of acid volatile sulfide in river sediments by sequential injection analysis exploiting the methylene blue reaction. Talanta 53(4):843–850
Pouly F, Touraud E, Buisson JF, Thomas O (1999) An alternative method for the measurement of mineral sulphide in waste water. Talanta 50(4):737–742
Kuban V, Dasgupta PK, Marx JN (1992) Nitroprusside and methylene blue methods for silicone membrane differentiated flow injection determination of sulfide in water and waste water. Anal Chem 64(1):36–43
Colon M, Todoli JL, Hidalgo M, Iglesias M (2008) Development of novel and sensitive methods for the determination of sulfide in aqueous samples by hydrogen sulfide generation-inductively coupled plasma-atomic emission spectroscopy. Anal Chim Acta 609(2):160–168
Jin Y, Wu H, Tian Y, Chen LH, Cheng JJ, Bi SP (2007) Indirect determination of sulfide at ultratrace levels in natural waters by flow injection on-line sorption in a knotted reactor coupled with hydride generation atomic fluorescence spectrometry. Anal Chem 79(18):7176–7181
Lawrence NS, Deo RP, Wang J (2004) Electrochemical determination of hydrogen sulfide at carbon nanotube modified electrodes. Anal Chim Acta 517(1–2):131–137
Spilker B, Randhahn J, Grabow H, Beikirch H, Jeroschewski P (2008) New electrochemical sensor for the detection of hydrogen sulfide and other redox active species. J Electroanal Chem 612(1):121–130
Tsai DM, Kumar AS, Zen JM (2006) A highly stable and sensitive chemically modified screen-printed electrode for sulfide analysis. Anal Chim Acta 556(1):145–150
GarcõÂa-Calzada M, MarbaÂn G, Fuertes AB (1999) Potentiometric determination of sulphur in solid samples with a sulphide selective electrode. Anal Chim Acta 380(1):39–45
Giuriati C, Cavalli S, Gorni A, Badocco D, Pastore P (2004) Ion chromatographic determination of sulfide and cyanide in real matrices by using pulsed amperometric detection on a silver electrode. J Chromatogr A 1023(1):105–112
Divjak B, Goessler W (1999) Ion chromatographic separation of sulfur-containing inorganic anions with an ICP–MS as element-specific detector. J Chromatogr A 844(1–2):161–169
Maya F, Estela JM, Cerdá V (2007) Improving the chemiluminescence-based determination of sulphide in complex environmental samples by using a new, automated multi-syringe flow injection analysis system coupled to a gas diffusion unit. Anal Chim Acta 601(1):87–94
Safavi A, Karimi MA (2002) Flow injection chemiluminescence determination of sulfide by oxidation with N-bromosuccinimide and N-chlorosuccinimide. Talanta 57(3):491–500
Du JX, Li YH, Lu JR (2001) Investigation on the chemiluminescence reaction of luminol–H2O2–S2−/R–SH system. Anal Chim Acta 448(1–2):79–83
Choi MF, Hawkins P (1997) Development of sulphide-selective optode membranes based on fluorescence quenching. Anal Chim Acta 344(1–2):105–110
Axelrod HD, Cary JH, Bonelli JE, Lodge JP Jr (1969) Fluorescence determination of sub-parts per billion hydrogen sulfide in the atmosphere. Anal Chem 41(13):1856–1858
Spaziani MA, Tinani M, Carroll MK (1997) On-line determination of sulfide by the methylene blue method with diode-laser-based fluorescence detection. Analyst 122(12):1555–1557
Rodríguez-Fernández J, Costa JM, Pereiro R, Sanz-Medel A (1999) Simple detector for oral malodour based on spectrofluorimetric measurements of hydrogen sulphide in mouth air. Anal Chim Acta 398(1):23–31
Yang XF, Wang LP, Xu HM, Zhao ML (2009) A fluorescein-based fluorogenic and chromogenic chemodosimeter for the sensitive detection of sulfide anion in aqueous solution. Anal Chim Acta 631(1):91–95
Wang Q, Kun YC, Wang YW, Shin G, Ruengruglikit C, Huang QR (2006) Luminescent properties of water-soluble denatured bovine serum albumin-coated CdTe quantum dots. J Phys Chem B 100(34):16860–16866
Chen YF, Rosenzweig Z (2002) Luminescent CdS Quantum dots as selective ion probes. Anal Chem 74(19):5132–5138
Li J, Mei F, Li WY, He XW, Zhang YK (2008) Study on the fluorescence resonance energy transfer between CdTe QDs and butyl-rhodamine B in the presence of CTMAB and its application on the detection of Hg(II). Spectrochimica Acta Part A 70(4):811–817
Wu HM, Liang JG, Han HY (2008) A novel method for the determination of Pb2+ based on the quenching of the fluorescence of CdTe quantum dots. Microchim Acta 161(1–2):81–86
Ruedas-Rama MJ, Hall EAH (2008) Azamacrocycle activated quantum dots for zinc ion detection. Anal Chem 80(21):8260–8268
Liang JG, Ai XP, He ZK, Pang DW (2004) Functionalized CdSe quantum dots as selective silver ion chemodosimeter. Analyst 129(7):619–622
Li HB, Zhang Y, Wang XQ (2007) L-Carnitine capped quantum dots as luminescent probes for cadmium ions. Sensors and Actuators B 127(2):593–597
Kar S, Banerjee S, Santra S (2008) A simple strategy for quantum dot assisted selective detection of cadmium ions. Chem Commun 26:3037–3039
Ali EM, Zheng YG, Yu H, Ying JY (2007) Ultrasensitive Pb2+ detection by glutathione-capped quantum dots. Anal Chem 79(24):9452–9458
Lakowicz JR, Gryczynski I, Gryczynski Z, Murphy CJ (1999) Luminescence spectral properties of CdS nanoparticles. J Phys Chem B 103(36):7613–7620
Jin WJ, Costa-Fernández JM, Pereiro R, Sanz-Medel A (2004) Surface-modified CdSe quantum dots as luminescent probes for cyanide determination. Anal Chim Acta 522(1):1–8
Jin WJ, Fernandez-Arguelles MT, Cosat-fernandez JM, Pereiro R, Sanz-Medel A (2005) Photoactivated luminescent CdSe quantum dots as sensitive cyanide probes in aqueous solutions. Chem Commun 7:883–885
Liu X, Guo L, Cheng LX, Ju HX (2009) Determination of nitrite based on its quenching effect on anodic electrochemiluminescence of CdSe quantum dots. Talanta 78(3):691–694
Wu CL, Zhao YB (2007) CdS quantum dots as fluorescence probes for the sensitive and selective detection of highly reactive HSe− ions in aqueous solution. Anal Bioanal Chem 388(3):717–722
Mulrooney RC, Singh N, Kaur N, Callan JF (2009) An “off-on” sensor for fluoride using luminescent CdSe/ZnS quantum dots. Chem Commun (6):686–688
Norman TJ, Magana JD, Wilson T, Burns C, Zhang JZ (2003) Optical and Surface structural properties of Mn2+-dopped ZnSe nanoparticles. J Phys Chem B 107(26):6309–6317
Mahamuni S, Lad AD, Patole S (2008) Photoluminescence properties of manganese-dopped zinc selenide quantum dots. J Phys Chem C 112(7):2271–2277
Sapra S, Prakash A, Ghangrekar A, Perasamy N, Sarma DD (2005) Emission properties of manganese-dopped ZnS nanocrystals. J Phys Chem B 109(5):1663–1668
Quan ZW, Wang ZL, Yang PP, Lin J, Fang JY (2007) Synthesis and characterization of high-quality ZnS, ZnS:Mn2+and ZnS:Mn2+/ZnS (core/shell) luminescent nanocrystals. Inorg Chem 46(4):1354–1360
Wang XF, Xu JJ, Chen HY (2008) A new electrochemiluminescence emission of Mn2+-dopped ZnS nanocrystals in aqueous solution. J Phys Chem C 112(45):17581–17585
Dong CQ, Qian HF, Fang NH, Ren JC (2006) Study of fluorescence quenching and dialysis process of CdTe quantum dots, using ensemble techniques and fluorescence correlation spectroscopy. J Phys Chem B 110(23):11069–11075
Tu RY, Liu BH, Wang ZY, Gao DM, Wang F, Fang QL, Zhang ZP (2008) Amine-capped ZnS:Mn2+ nanocrystals for fluorescence detection of trace TNT explosive. Anal Chem 80(9):3458–3465
He Y, Wang HF, Yan XP (2008) Exploring Mn-doped ZnS quantum dots for the room-temperature phosphorescence detection of enoxacin in biological fluids. Anal Chem 80(10):3832–3837
Wang HF, He Y, Ji TR, Yan XP (2009) Surface molecular imprinting on Mn-Doped ZnS quantum dots for room-temperature phosphorescence optosensing of pentachlorophenol in water. Anal Chem 81(4):1615–1621
Santra S, Yang H, Holloway PH, Stanley JT, Mericle RA (2005) Synthesis of water-dispersible fluorescent, radio-opaque, and paramagnetic CdS:Mn/ZnS quantum dots: A multifunctional probe for bioimaging. J Am Chem Soc 127(6):1656–1657
Zhuang JQ, Zhang XD, Wang G, Li DM, Yang WS, Li TJ (2003) Synthesis of water-soluble ZnS:Mn2+ nanocrystals by using mercaptopropionic acid as stabilizer. J Mater Chem 13(7):1853–1857
Brus LE (1984) Elctron-electron and electron-hole interactions in small semiconductor crystallites: The size dependence of the lowest excited electronic state. J Chem Phys 80(9):4403–4409
Sooklal K, Cullum BS, Angel SM, Murphy CJ (1996) Photophysical properties of ZnS nanoclusters with spatially localized Mn2+. J Phys Chem 100(11):4551–4555
Chen L, Zhang JH, Luo YS, Lu SZ, Wang XJ (2004) Effect of Zn2+and Mn2+ introduction on the luminescent properties of colloidal ZnS:Mn2+ nanoparticles. Applied Phys Lett 84(1):112–114
Acknowledgements
The authors gratefully acknowledge the financial support of this study by Natural Science Foundation of China (No.20965006) and Jiangxi Province Education Ministry Foundation (No.GJJ09040).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, BH., Wu, FY., Wu, YM. et al. Fluorescent Method for the Determination of Sulfide Anion with ZnS:Mn Quantum Dots. J Fluoresc 20, 243–250 (2010). https://doi.org/10.1007/s10895-009-0545-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-009-0545-0