Abstract
Native to the northeast USA, highbush blueberry is a crop domesticated for close to 100 years and that has been selected mainly for high yields and bigger fruit. We hypothesized that, due to domestication and associated agronomic selection (i.e., cultivation practices), cultivated blueberries differ from their wild ancestors in fruit volatile emissions, affecting the response of a frugivorous pest. To test this hypothesis, we compared the attraction of adult spotted-wing drosophila (Drosophila suzukii) to wild and cultivated blueberry fruit volatiles in choice assays. We also conducted headspace volatile chemical analysis and electroantennographic detection (EAD) analysis to identify and quantify any antennally active compounds. For this, fruit from wild and cultivated blueberries, growing in proximity, was sampled from six farms located in the Pinelands National Reserve (New Jersey, USA)—a blueberry-producing region with a forest understory consisting largely of wild blueberries. On a per gram basis, we found that wild blueberries are more attractive to D. suzukii flies and have higher volatile emission rates than cultivated blueberries. Nine EAD-active compounds from wild blueberries (isobutyl acetate, ethyl butyrate, ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, hexanal, isoamyl acetate, 3-hydroxybutanone (acetoin), 6-methyl-5-hepten-2-one, and 1-hexanol) were attractive individually and as a blend to D. suzukii flies. However, a 4-component blend composed of isoamyl acetate, acetoin, 6-methyl-5-hepten-2-one, and 1-hexanol was more attractive to D. suzukii than the 9-component blend. Altogether, our results show that the domestication/cultivation of blueberries is associated with lower rates of fruit volatile emissions, which has resulted in decreased attraction of a frugivorous pest, D. suzukii.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Key Message
-
Humans can affect insect-plant interactions through crop domestication/cultivation practices.
-
In highbush blueberries, Drosophila suzukii was more attracted to volatiles from wild than cultivated fruit.
-
Nine volatiles from wild fruit elicited strong antennal and behavioral responses in D. suzukii.
-
A 4-component blend from wild blueberries was attractive to D. suzukii.
-
Our study shows the impact of domestication/cultivation on a frugivorous pest attraction to fruit volatiles.
Introduction
Humans have modified plant phenotypes by altering the plant’s genetic makeup through domestication and by changing its environment through cultivation. Domestication is a process in which plants are morphologically and physiologically modified by humans (Darwin 1868; Hancock 2005; Fuller et al. 2010). Crop domestication began over 10,000 years ago (Diamond 2002; Purugganan and Fuller 2009; Meyer et al. 2012; Fuller et al. 2014), resulting in the selection of various traits, including increased yield of the harvested organ (e.g., seeds, fruits, and roots), reduced seed dispersal and dormancy, and altered secondary metabolites (Bai and Lindhout 2007; Meyer et al. 2012; Fuller et al. 2014; Li et al. 2018). In addition, agronomic selection (i.e., cultivation practices) associated with the domestication of crops could play an important role in the expression of plant traits. For example, soil quality improvements through crop fertilization and water use can affect the susceptibility of cultivated plants to insect pests via changes in nutrient content (Altieri and Nicholls 2003).
Furthermore, several studies have shown that cultivated crops are less resistant to herbivores than their wild counterparts (Rodriguez-Saona et al. 2011, 2018; Chen et al. 2015a, 2018; Whitehead et al. 2017). However, little is known about the effects of domestication/cultivation on fruit volatiles and their interactions with frugivorous pests. Native to the northeast USA, highbush blueberry (Vaccinium corymbosum L., Ericaceae) is an ideal cropping system in which to study these effects because it has a relatively short domestication/cultivation history—it was first domesticated in New Jersey (USA) about 100 years ago (Eck and Childers 1966). This crop was mainly selected for increased yield and fruit size—cultivated fruits are bigger and plants produce a higher quantity of and more uniform fruit than their wild ancestors (Moore 1965; Ehlenfeldt 2009). In New Jersey, highbush blueberries are commonly cultivated near wild Vaccinium plants in a region known as the Pinelands National Reserve (McCormick 1979). In a previous study, Hernandez-Cumplido et al. (2018) showed that domestication reduced the resistance of highbush blueberry leaves against a non-native folivore but had fewer effects on the performance of two native folivores; however, the effects of blueberry domestication on frugivorous insects remain unknown.
Native to Southeast Asia, spotted-wing drosophila, Drosophila suzukii Matsumura (Diptera: Drosophilidae), has become an important pest of soft, thin-skinned fruits, including cultivated blueberries (Burrack et al. 2013), in many countries throughout North and South America and Europe (Hauser 2011; Calabria et al. 2012; Cini et al. 2012; Asplen et al. 2015; Arnó et al. 2016). In the USA, this pest was first detected in 2008 and quickly spread to other states; by 2011, it was found in most Northeastern states (Hauser 2011; Asplen et al. 2015). In laboratory studies, Rodriguez-Saona et al. (2018) demonstrated that domestication/cultivation practices made blueberries more susceptible to D. suzukii—oviposition preference and immature performance were higher on cultivated than wild blueberry fruit. They also found that several physico-chemical traits differed between cultivated and wild blueberry fruit. For example, cultivated fruits are bigger and have lower concentrations of defensive compounds (e.g., anthocyanin and phenolic content) than wild fruits, which may explain their greater susceptibility to D. suzukii (Rodriguez-Saona et al. 2018). However, under field conditions, D. suzukii was found to exploit both cultivated and wild blueberries for oviposition and development, indicating that wild Vaccinium hosts likely serve as a source of D. suzukii to adjacent blueberry fields (Urbaneja-Bernat et al. 2020).
Because D. suzukii attacks ripening fruit (Lee et al. 2011), fruit volatiles likely serve as important cues to females during oviposition site location (Revadi et al. 2015; Abraham et al. 2015; Cloonan et al. 2018). In Y-tube olfactometer assays, D. suzukii females were attracted to odors from fresh blackberry, blueberry, cherry, raspberry, and strawberry (Revadi et al. 2015). In coupled gas chromatography–electroantennographic detection (GC-EAD) experiments, D. suzukii antennae detected odors from various fruits, including blueberries (Abraham et al. 2015). This latter study identified an 11-component blend from the headspace of raspberry fruit extracts that were attractive to D. suzukii (Abraham et al. 2015). Still, it is unknown whether D. suzukii responds differently to volatiles emitted from cultivated and wild blueberry fruits.
To fill these knowledge gaps, we tested the hypothesis that D. suzukii flies are differentially attracted to fruit volatiles from cultivated and wild blueberries. For this, we conducted a series of laboratory studies to (1) determine the behavioral response of D. suzukii to volatiles from wild and cultivated blueberry fruit; (2) identify the antennally active compounds in the most attractive fruit tested (wild); (3) investigate the behavioral response of D. suzukii females to each of the identified EAD-active compounds from wild blueberry fruit; and (4) evaluate the behavioral response of D. suzukii females (mated and virgin) and males to two synthetic blends (a 9-component [full] and a 4-component [partial] blend) formulated based on the EAD-active compounds.
Materials and methods
Plant material
Ripe (blue) fruits were collected from six commercial highbush blueberry farms located in the Pinelands National Reserve in southern New Jersey. In each farm, a blueberry field (cv. ‘Duke’ or ‘Bluecrop’) was used as the source of cultivated plants. The early-season ‘Duke’ and the mid-season ‘Bluecrop’ are two of the most common highbush blueberry cultivars grown in New Jersey and in the USA (Gallardo et al. 2018). Three of the farms had ‘Duke’ blueberries, and the other three had ‘Bluecrop’ blueberries. The cultivated field in each of these farms was paired with adjacent forest land dominated by Pitch pine, Pinus rigida Mill., where wild blueberry plants are typically found in the understory (McCormick 1979). Urbaneja-Bernat et al. (2020) found that fruit from both cultivated and wild blueberries ripen at the same time in this region. At each site, we randomly selected five bushes (total of 25–30 cultivated and 25–30 wild plants across all sites); cultivated bushes were located inside the fields within 30 m from the field edge, and wild bushes were located inside the forest, within 30 m from the forest edge facing the blueberry field. Three fruit clusters per cultivated highbush and wild blueberry were bagged at fruit set (i.e., green-pink fruit, early June) to prevent pesticide exposure and natural insect infestation, considering that fruits are susceptible to D. suzukii once they start to color (Lee et al. 2011). Fruits were harvested during maturation (i.e., blue fruit) on June 27 and July 7, 2018, placed in polyethylene Ziplock clear bags, transported to the laboratory, and used for choice assays and headspace volatile collections on the same day of collection. At all sites, we collected sufficient fruit (unknown exact amount) such that, later in the laboratory, we could select fruits used in the experiments—only healthy (undamaged) fruits were used in all experiments.
Insects
Drosophila suzukii flies were obtained from a laboratory colony initiated in 2013 from wild specimens captured in Atlantic County (New Jersey) and maintained at the Rutgers P.E. Marucci Center in Chatsworth, New Jersey. The colony was reared on a standard Drosophila artificial diet (Dalton et al. 2011; Jaramillo et al. 2015) in 50-mL polystyrene vials (Fisher Scientific, Nazareth, PA, USA) filled with ~ 15 mL of diet and plugged with BuzzPlugs (Fisher Scientific). Insects were kept in an incubator (Percival Scientific, Perry, IA, USA) set at 25 °C with 55% relative humidity (RH) and a 16:8 (light: dark [L:D]) h cycle. Drosophila suzukii males and mated and virgin females were used in experiments; flies were 3–7 days old and were starved for ~ 8 h before the start of experiments to enhance their response to fruit volatiles.
Chemicals
We purchased all chemicals from Sigma-Aldrich (St. Louis, MO, USA), including isobutyl acetate (≥ 97% purity, CAS 110-19-0); ethyl butyrate (≥ 98%, CAS 105-54-4); ethyl 2-methylbutyrate (≥ 98%, CAS 7452-79-1); ethyl 3-methylbutyrate (≥ 99%, CAS 108-64-5); hexanal (≥ 98%, CAS 66-25-1); isoamyl acetate (≥ 95%, CAS 123-92-2); 3-hydroxybutanone (acetoin) (≥ 96%, CAS 513-86-0); 6-methyl-5-hepten-2-one (≥ 98%, CAS 110-93-0); 1-hexanol (98%, CAS 111-27-3); as well as solvents, hexane, and methylene chloride, which were high-performance liquid chromatography grade.
Behavioral response of D. suzukii to cultivated and wild blueberry volatiles
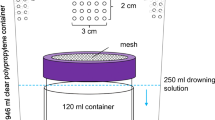
A dual-choice behavioral bioassay (Feng et al. 2018) was conducted to compare the response of D. suzukii to wild and cultivated blueberry fruit volatiles. On the first sampling date (27 June), fruit was collected from all six farms, but, on the second sampling date (7 July), we only sampled fruit from five farms due to availability (‘Duke’ had been harvested in one farm). The experimental arena (replicate) consisted of a transparent polypropylene, cylindrical plastic cup (946 mL, 114 mm diameter, 127 mm height; Paper Mart, CA, USA), with a 8-cm-diameter circular hole cut in the lid and covered with a nylon mesh to provide ventilation (Fig. 1a). Two 50-mL polystyrene vials (95 mm × 28.5 mm, same as those used in insect rearing) were placed vertically on opposite sides of the cup, and one was labeled “W” (for wild fruit) and the other “C” (for cultivated fruit). We used ~ 10 g per each fruit genotype (~ 5 and ~ 25 cultivated and wild blueberry fruits, respectively) to account for differences in fruit size. The top of each trap vial was sealed with Parafilm®, leaving only a 4-mm-diameter hole in the center to allow flies to enter. The vials were wrapped in aluminum foil to prevent any visual cues from affecting fly behavior. A container (20 mL; VWR, PA, USA) filled with deionized water and plugged with a cotton ball was placed in the middle of the plastic cup (Fig. 1a). Twenty D. suzukii (10 males and 10 females) adults were released in the center of each cup, and their positions were recorded after 24 h. The experiment was replicated 44 times (N = 4 replicates/farm × 5 or 6 farms [depending on sampling date] × 2 sampling dates × 20 flies/replicate = 880 flies tested) and was carried out under a fume hood at 25 °C, 60% RH, 16:8 (L:D) h, and ~ 1,700 lx.

(adapted from Feng et al. 2018). b Percent (mean ± 1 SE) response of adult Drosophila suzukii (mixed sexes) to wild and cultivated highbush blueberry (Vaccinium corymbosum) fruit volatiles in choice tests after 24 h. Each bar is the mean of 44 replicates, and 20 D. suzukii flies were released per replicate (N = 880 flies)
a Arena used for the dual-choice experiments with intact fruit
Sampling of fruit volatiles
Headspace volatile samples were taken from healthy (undamaged) wild and cultivated blueberry fruit (~ 30 g) collected on 07 July from the three ‘Bluecrop’ farms. Volatile sampling was done in 236-mL clear glass jars (Sigma-Aldrich). The lid of the jars had two perforations; one served as an air inlet and the other as an air outlet. We inserted a Pasteur pipette filled with activated charcoal in the inlet to filter the air. A HayeSep Q (30 mg) adsorbent trap (Volatile Assay Systems, Rensselaer, NY) was inserted into the outlet such that the volatiles were pulled at ~ 2 L/min using a 12-V pump (Sensidyne, Clearwater, FL, USA) for 3 h (11:00–14:00 h). Trapped volatiles were eluted with 150 mL methylene chloride with the help of a gentle stream of N2. Headspace volatiles from an empty 236-mL glass jar were collected concurrently to produce blank samples. Samples were stored in a freezer at − 10 °C until GC-EAD, GC-mass spectrometry (GC–MS), and GC-flame ionization detection (GC-FID) analyses were conducted. We collected four fruit volatile samples (replicates) for each of the blueberry genotypes (wild and cultivated).
GC-EAD and GC–MS analyses
First, we conducted GC-EAD analysis to screen for antennal responses of D. suzukii flies to volatile compounds from blueberry fruit. The coupled GC-EAD system used was as previously described (Zhang et al. 1999; Abraham et al. 2015). Both the headspace volatile samples and a synthetic blend composed of the EAD-active compounds from wild blueberry fruit (the most attractive fruit genotype; see Results section) were tested for D. suzukii female antennal activity. The GC-EAD analysis was conducted on a Hewlett-Packard (HP) 6890 GC equipped with a DB-WAXetr capillary column (60-m length, 0.25-mm internal diameter, 0.25-μm film thickness; J&W Scientific Inc., Folsom, CA, USA). The GC-EAD was run in the splitless mode, with hydrogen as the carrier gas (3 mL/min). The oven temperature was programmed at 40 °C held isothermally for 2 min and then at 15 °C/min to 260 °C and held for 10 min. The capillary column effluent and nitrogen makeup gas (10 mL/min) were split (~ 1:1) by a fixed outlet splitter (SGE Inc., Austin, TX, USA) to the FID and EAD. The head of a D. suzukii fly was excised from the body, and both antennae were positioned between two gold wire electrodes, which were immersed in saline-filled (0.9% NaCl) wells (1.25 mm in diameter; ~ 3 mm apart) in a small, acrylic plastic holder (8 cm in length by 0.8 cm in width by 0.6 cm in breadth). The output recording electrodes were connected to a high-impedance 1:100 amplifier with automatic baseline drift compensation. The airstream flowing over the antennae (~ 500 mL/min) was humidified by bubbling through distilled water before entering the EAD interface. The antennal preparation was cooled to ~ 5 °C inside a condenser (at 100% humidity) by circulating near 0 °C water from a bench-top refrigeration unit (RTE-100; NESLAB instruments Inc., Portsmouth, NH, USA) through the insulation layer of the modified condenser containing the acrylic plastic holder mounted on top of the GC. The flame ionization and electrophysiological output signals were recorded using the HP Chem Station software.
Next, we identified the EAD-active compounds using GC–MS. GC electron impact (EI) MS was conducted on a HP 6890 GC coupled to a HP 5973 mass selective detector and equipped with a DB-WAXetr column identical to the one described above. The GC–MS was run in the splitless mode, with helium as the carrier gas (2 mL/min) (Zhang et al. 2004). A 70-eV electron beam was used for sample ionization. The oven temperature program started at 40 °C held isothermally for 2 min and then at 10 °C/min to 250 °C and held for 25 min. The chemical identification of EAD-active compounds from blueberry headspace volatiles was based on comparison of their mass spectra with the NIST 11 (Gaithersburg, MD, USA) and Wiley 7 N (John Wiley, NY, USA) mass spectral libraries, and identities were confirmed by mass spectra and GC retention times of authentic standards on both polar and non-polar GC capillary columns (Zhang et al. 2004, 2005).
GC-FID analysis
We used GC-FID to compare the emission rates of all and EAD-active volatiles between wild and cultivated blueberry fruit. The headspace volatile samples were run on a HP 6890 GC equipped with an HP-1 column (10-m length, 0.53-mm internal diameter, 2.65-μm film thickness; Agilent, Santa Clara, CA, USA). The GC-FID was run in the splitless mode, with helium as the carrier gas (5 mL/min). The oven temperature was programmed at 40 °C held isothermally for 1 min, then at 14 °C/min to 180 °C and held for 2 min, then at 40 °C/min to 200 °C and held for 2 min and then to 220 °C and held isothermally for 5 min. Prior to GC-FID analysis, 400 ng of n-octane was added to the samples as an internal standard. The relative amounts of the compounds in the samples were quantified based on a comparison of peak areas with that of the internal standard. The peaks of EAD-active compounds were identified by comparison of retention times with those of the synthetic standards.
Behavioral assays with individual EAD-active volatiles
A dual-choice bioassay was used to test the behavioral response of D. suzukii mated females to the nine EAD-active compounds identified (see Results below): isobutyl acetate, ethyl butyrate, ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, hexanal, isoamyl acetate, acetoin, 6-methyl-5-hepten-2-one, and 1-hexanol. We used a similar experimental setup used for assays with intact fruit described above (Fig. 4a). The two vials inside the arena (cup) were labeled either as “chemical compound” or as “control.” The chemical compound vials contained 20 µl of a pure individual compound loaded onto a small cotton ball inside a small polyethylene tube (26 mm × 8 mm × 1.5 mm thickness; Just Plastic Ltd., Norwich, UK). Control vials contained cotton only. The tubes (open lid) were placed vertically within the vials, and the top of the vials was sealed with Parafilm, leaving a 4-mm-diameter hole in the center to allow flies to enter. Ten mated D. suzukii females (3–7 days old, starved for ~ 8 h) were released in the center of each arena, and their positions were recorded after 24 h. The experiment was replicated 10 times for each of the nine EAD-active compounds (N = 9 compounds × 10 flies/replicate × 10 replicates = 900 flies tested) and was conducted under a fume hood at 25 °C, 60% RH, 16:8 (L:D) h, and ~ 1,700 lx.
Behavioral assays with volatile blends
Dual-choice bioassays were used to assess the behavioral response of D. suzukii flies to a synthetic blend of the 9-component EAD-active blend (full blend) composed of isobutyl acetate, ethyl butyrate, ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, hexanal, isoamyl acetate, acetoin, 6-methyl-5-hepten-2-one, and 1-hexanol at a natural ratio of 10:1:1:10:1:1:4:1:1 (by volume). Based on the behavioral results of the individual EAD-active compounds (see Results section), we selected the four most attractive to D. suzukii to also create a 4-component EAD-active (partial) blend. The 4-component blend consisted of isoamyl acetate, acetoin, 6-methyl-5-hepten-2-one, and 1-hexanol at a 1:1:1:1 ratio. These four compounds were chosen because D. suzukii antenna was highly sensitive to them, i.e., all elicited a strong EAD response at relatively low amounts. We used the same experimental setup described above to test these two blends in three choice combinations: (1) 9-component (full) blend versus blank control, (2) 4-component (partial) blend versus blank control, and (3) 9-component (full) blend versus 4-component (partial) blend. Ten D. suzukii flies were released in the center of each arena and their position recorded after 24 h. For these experiments, we used males and mated and virgin females (3–7 days old, starved for ~ 8 h), and each choice test combination was replicated 12 times (N = 3 choice tests × 3 sex status × 10 flies/replicate × 12 replicates = 1,080 flies tested). Assays were carried out under a fume hood at 25 °C, 60% RH, 16:8 (L:D) h, and ~ 1,700 lx.
Statistical analyses
All choice data were analyzed using Student's t tests. We used generalized linear models assuming a Poisson distribution and log link function to determine differences among the nine EAD-active compounds to select the most attractive compounds used to make the 4-component blend.
Analysis of variance (ANOVA) was used to test for differences between wild and cultivated blueberries on the total amount of fruit volatiles and amounts of individual EAD-active volatiles. Prior to the ANOVA, volatile data were checked for normality and equal variances using Anderson–Darling and Levene’s tests, respectively. If needed, volatile data were ln (x or x + 0.1)-transformed to meet ANOVA assumptions. In addition, we subjected the data from the GC-FID to principal component analysis (PCA) to visualize, using the score plot, differences in the volatile blends emitted from wild and cultivated blueberry fruit. Only volatile compounds present in at least 75% of the samples (i.e., 3 of 4 replicates) were included in the data analyses.
Choice data analyses were performed using SPSS Statistics version 23.0 (SPSS 2015). Volatile data were analyzed using Minitab version 17 (Minitab 2013).
Results
Behavioral response of D. suzukii to cultivated and wild blueberry volatiles
In dual-choice tests, D. suzukii was more attracted to fruit volatiles from wild than cultivated blueberries (Fig. 1b). This response was the same regardless of fly sex (t = 1.33, df = 174; P = 0.184), blueberry cultivar (t = 0.46, df = 42; P = 0.65), and date of the experiment (t = 1.19, df = 86; P = 0.234).
GC-EAD and GC–MS analyses
Based on GC-EAD analysis, nine volatile compounds from wild blueberry fruit consistently elicited strong responses in D. suzukii antennae (Fig. 2a). These EAD-active compounds were identified by GC–MS as isobutyl acetate, ethyl butyrate, ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, hexanal, isoamyl acetate, acetoin, 6-methyl-5-hepten-2-one, and 1-hexanol. A synthetic blend of these EAD-active compounds at a natural ratio also elicited similar antennal responses (Fig. 2b).
Simultaneous responses of electroantennographic detection (EAD) of an adult Drosophila suzukii (female) antenna and gas chromatographic (GC) flame ionization detection (FID) to (a) wild blueberry headspace volatile extract and (b) synthetic chemical blend on a DB-WAXetr capillary column. Identified compounds from wild blueberry are: 1 = isobutyl acetate; 2 = ethyl butyrate; 3 = ethyl 2-methylbutyrate; 4 = ethyl 3-methylbutyrate; 5 = hexanal; 6 = isoamyl acetate; 7 = acetoin; 8 = 6-methyl-5-hepten-2-one; 9 = 1-hexanol with 10:1:1:10:1:1:4:1:1 ratios
GC-FID analysis
Volatile emission rates from wild blueberries were 2.3 times higher than from cultivated blueberries (mean emission rate [ng/g/h] ± SE: cultivated = 3.29 ± 0.59; wild = 7.53 ± 1.42; F = 6.77, df = 6, P = 0.041). Out of the 46 fruit volatiles consistently detected by GC-FID analysis, 22 (48%) were differentially emitted from wild and cultivated blueberry fruit (Table S1). Out of these 22 volatiles, 20 (91%) were emitted at higher emission rates from wild than cultivated blueberry fruits (Table S1). The PCA shows differences in volatile blend composition between wild and cultivated blueberry fruit, with the PC1 and PC2 explaining ~ 77% of the total variance and the first PC component clearly separating the two blends (Fig. 3a). The PCA also shows a larger variation in the volatile blends of wild blueberries than in the blends of cultivated blueberries, which may reflect the greater genetic diversity of the wild blueberry genotypes.
The principal component analysis (PCA) score plot of the first two PCs of the wild and cultivated highbush blueberry (Vaccinium corymbosum) fruit for the complete volatile blends (a) and for the 9 EAD-active compounds (b). The ellipses enclose the volatile data points for wild and cultivated blueberry fruits
The EAD-active compounds constituted ~ 40% of the total fruit volatile emissions. Although the emission rates of the EAD-active volatiles were also 2.3 times higher in wild blueberries than in cultivated blueberries, this difference was not significant (mean emission rate [ng/g/h] ± SE: cultivated = 1.34 ± 0.49; wild = 3.13 ± 1.52; F = 1.97, df = 6, P = 0.21). However, the emission rates of some individual EAD-active volatiles differed between wild and cultivated blueberries. Two of the nine EAD-active compounds (the ester ethyl 3-methylbutyrate and the ketone 6-methyl-5-hepten-2-one) were emitted at significantly higher rates from wild blueberry fruit, whereas the emission rate of the ester ethyl butyrate was higher from cultivated blueberries (Table 1). The esters isobutyl acetate and ethyl 2-methylbutyrate were also emitted at a greater rate from wild and cultivated fruit, respectively, but these differences were only marginally significant (0.05 < P < 0.1; Table 1). The PCA shows differences in the blends composed of the 9 EAD-active compounds between wild and cultivated blueberry fruit, with the PC1 and PC2 explaining ~ 75% of the total variance and the first PC component clearly separating the two blends (Fig. 3b).
Behavioral assays with individual EAD-active volatiles
When the nine EAD-active compounds were tested individually against the blank controls in dual-choice assays, all of them attracted D. suzukii mated females (Fig. 4b). However, four of them (the ester isoamyl acetate, the ketones acetoin and 6-methyl-5-hepten-2-one, and the C-6 alcohol 1-hexanol) showed 2-times greater fly attraction than the other five EAD-active compounds (F = 63.96; df = 8; P < 0.011; Fig. 4b) and were thus used to prepare a 4-component (partial) blend. Relatively small amounts of these four compounds also elicited high antennal responses in D. suzukii as compared with the other EAD-active compounds (Fig. 2).

(adapted from Feng et al. 2018). (b) Percent (mean ± 1 SE) response of adult Drosophila suzukii (mated females) to each of the nine EAD-active compounds from wild blueberry fruit against distilled water (control) after 24 h. Each bar is the mean of 10 replicates, and 10 female flies were released per replicate (N = 100 flies)
(a) Arena used for dual-choice experiments with EAD-active volatiles
Behavioral assays with volatile blends
In dual-choice tests, when the 9-component (full) and the 4-component (partial) blends were tested individually against a blank control, D. suzukii females (virgin and mated) (Fig. 5a, b) and males (Fig. 5c) were similarly attracted to both blends. However, when the full and partial blends were tested against each other, D. suzukii females (virgin and mated [Fig. 5a, b]) and males (Fig. 5c) were significantly more attracted to the 4-component blend (partial) than the 9-component blend (full).
Percent (mean ± 1 SE) response of adult Drosophila suzukii virgin (a) and mated (b) females and males (c) to the following three choice tests: 9-component (full) blend versus blank control, 4-component (partial) blend versus blank control, and 9-component (full) blend versus 4-component (partial) blend, after 24 h. The choice arena was the same as in Fig. 4a. Each bar is the mean of 12 replicates, and 10 flies of each sex and mating status were tested per replicate (N = 120 flies). White bars = blank control; gray bars = 4-component blend; dark bars = 9-component blend.
Discussion
In highbush blueberries, V. corymbosum, anthropogenic selection for large fruit and high yields could have unintentionally resulted in changes in fruit chemistry. Four key findings on the effects of domestication/cultivation on blueberry-insect pest interactions can be drawn from our study: (1) D. suzukii flies are more attracted to wild than cultivated blueberry fruit volatiles; (2) volatile emission rates were higher in wild than in cultivated blueberries; (3) D. suzukii antenna responds to nine volatiles present in the wild blueberry fruit blend; and (4) a blend composed of four EAD-active volatiles from wild blueberries attracts D. suzukii females (mated and virgin) and males under laboratory conditions.
Crop domestication/cultivation has the potential to alter plant–insect interactions (Benrey et al. 1998; Chen et al. 2015a, b, 2018). In previous choice and no-choice experiments, we showed that D. suzukii prefers to oviposit on and larvae perform better in fruits of cultivated blueberries when compared with wild blueberries (Rodriguez-Saona et al. 2018). These positive effects of crop domestication/cultivation on the fly’s oviposition preference and performance were correlated with fruit size and defenses—cultivated blueberries have bigger fruit with lower concentrations of phenolics and anthocyanins. In the present followed-up study, we showed a greater attraction of both male and female D. suzukii flies toward volatiles from wild than cultivated blueberry fruit, likely explained by differences in the composition of the volatile blends (i.e., quantities and ratios of individual compounds) between wild and cultivated blueberries. These findings are counterintuitive: why are flies more attracted to fruits that are less preferred as oviposition sites and of lower quality for the survival of their offspring? This could be explained by the role secondary plant metabolites play in nature. In nature, wild plants experience a dilemma—should plants increase fruit apparency to attract seed dispersers (Feeny 1973) or increase defenses in fruits to deter antagonists, such as frugivorous insects (Cipollini and Stiles 1993; Cipollini 2000; Rodríguez et al. 2013)? It is likely that wild blueberries produce fruit volatiles to increase their apparency to seed dispersers (Johnson et al. 1985), and frugivorous insects, such as D. suzukii, eavesdrop on these volatiles to locate their host. Our findings indicate that humans, through the process of domestication/cultivation of blueberries, have visually made fruits more apparent to animals by increasing fruit size, but, by doing so, the emission rates of fruit volatiles have been reduced. Evidently, domestication/cultivation of blueberries has reduced levels of different classes of secondary metabolites, including volatiles, phenolics, and anthocyanins, in fruits making cultivated fruits less attractive, but more susceptible, to D. suzukii. Under field conditions, however, cultivated and wild blueberries were similarly infested by D. suzukii throughout the harvest period (Urbaneja-Bernat et al. 2020). It is likely that in nature, various factors determine D. suzukii attraction and oviposition preference, such as host plant and fruit apparency, availability of resources, and background odors. Thus, even though wild blueberries emit higher volatile emission rates under laboratory conditions, this does not necessarily mean that they will be more attractive to D. suzukii than cultivated blueberries under natural (field) conditions.
Although a recent meta-analysis showed that cultivated crops generally emit higher levels of herbivore-induced volatiles than their wild relatives (Rowen and Kaplan 2016), studies have shown that domestication can reduce volatile emissions in various crops, including cranberries (Rodriguez-Saona et al. 2011), tomato (Goff and Klee 2006), maize (Köllner et al. 2008; Tamiru et al. 2011), and strawberry (Ulrich et al. 2007). However, little is known about the effects of crop domestication/cultivation on fruit volatiles and their effects on the interactions between fruits and frugivorous insect pests, such as D. suzukii. Similar to other Drosophila spp. (Lebreton et al. 2012; Stensmyr et al. 2012; Faucher et al. 2013; Linz et al. 2013), D. suzukii likely uses fruit volatiles to locate hosts and oviposition sites (Abraham et al. 2015; Revadi et al. 2015; Cloonan et al. 2018; Liu et al. 2018). Unlike other Drosophila spp., however, D. suzukii is more responsive to odors from ripening than overripe fruit (Keesey et al. 2015). In the present study, we found that the emission rates of fruit volatiles were reduced by the domestication/cultivation of blueberries. Still, because of their larger size and thus larger emission surface (i.e., fruit skin), cultivated blueberries could compensate for the reduced volatile emission rates. In fact, on average, cultivated blueberries weigh 2 g and emit 3.3 ng of volatiles per g, while wild blueberries weigh 0.4 g and emit 7.5 ng of volatiles per g; thus, total amounts of volatiles are ~ 2-times higher in cultivated than wild fruit. According to this, if domestication/cultivation of blueberries had not caused a reduction in volatile emission rates, we would expect cultivated blueberries to emit 5-times more total amounts of volatiles than wild blueberries. Based on PCA, we also found that the composition of the volatile blend emitted from wild blueberries differs from that of cultivated blueberries. Baloga et al. (1995) also found differences among the volatile profiles of fruit extracts from highbush blueberries (V. corymbosum) and wild Vaccinium species. Other studies have provided a detailed profile of the volatiles emitted from Vaccinium spp. (Hirvi and Honkanen 1983; Horvat et al. 1983; ChunYu et al. 2009; Du 2014; Farneti et al. 2017); however, this was not the goal of our study. Instead, we sought to identify the specific volatiles within the blend of wild blueberry fruit that elicit antennal (EAD) and behavioral responses in D. suzukii to then create an EAD-active blend effective at attracting this pest.
Nine volatile compounds from wild blueberries elicited antennal and behavioral responses in D. suzukii females. These included five esters (isobutyl acetate, ethyl butyrate, ethyl 2-methylbutyrate, ethyl 3-methylbutyrate, and isoamyl acetate), a C-6 aldehyde (hexanal), a C-6 alcohol (1-hexanol), and two ketones (acetoin and 6-methyl-5-hepten-2-one). Esters constitute an important class of volatiles emitted from Vaccinium sp. fruits (Farneti et al. 2017); and ethyl butyrate, ethyl 2-methylbutyrate, and ethyl 3-methylbutyrate are commonly found in these blends (Lugemwa et al. 1989; Baloga et al. 1995; ChunYu et al. 2009; Du and Qian 2010; Du et al. 2011; Du 2014; Abraham et al. 2015; Farneti et al. 2017; Liu et al. 2019b). In a previous study, ethyl 2-methylbutyrate was shown to elicit strong antennal responses in another important frugivorous pest of blueberries, the blueberry maggot fly (Rhagoletis mendax Curran), which is native to North America (Lugemwa et al. 1989). Here, we showed that this ester not only elicits an antennal response in the invasive D. suzukii but also attracts this fly. Esters from other berry fruits have been shown to also elicit antennal responses and attract D. suzukii (Liu et al. 2018), indicating that this volatile group likely plays a critical role during host finding. The two esters isobutyl acetate and isoamyl acetate are often emitted from fermented blueberry fruits (Liu et al. 2019a, b) and from yeast (i.e., Hanseniaspora uvarum; Scheidler et al. 2015) associated with D. suzukii (Hamby et al. 2012). Thus, it is likely that these compounds are emitted by yeast during fruit fermentation. Interestingly, H. uvarum in diet improves D. suzukii adult fitness (i.e., survival and fecundity) (Bellutti et al. 2018; Spitaler et al. 2020). Isobutyl acetate and isoamyl acetate attract D. suzukii flies under laboratory conditions (Revadi et al. 2015; Cloonan et al. 2019), elicit stronger antennal responses in D. suzukii than in Drosophila melanogaster Meigen (Scheidler et al. 2015), and their rates of emissions were 4.4-times greater in wild blueberries than in cultivated blueberries. Agronomic practices, such as the use of fungicides, could have negatively affected yeast colonization (e.g., Agarbati et al. 2019) and, as a result, their volatile emissions in cultivated fruit. In future studies, we will investigate the role of yeasts associated with wild and cultivated blueberry fruits in volatile emissions and D. suzukii attraction.
Aldehydes and alcohols are also important components of the blueberry fruit blend, and hexanal and 1-hexanol are consistently identified from these blends (Hirvi and Honkanen 1983; Baloga et al. 1995; ChunYu et al. 2009; Du and Qian 2010; Du et al. 2011; Du 2014; Abraham et al. 2015; Farneti et al. 2017). In agreement with our findings, hexanal and 1-hexanol were shown to elicit antennal responses in D. suzukii in previous studies (Abraham et al. 2015; Revadi et al. 2015). 6-Methyl-5-hepten-2-one is a ketone commonly found in the fruit volatile blend of blueberries (Du et al. 2011; Farneti et al. 2017) that elicits strong antennal responses in D. suzukii (Abraham et al. 2015). On the other hand, acetoin is not a typical compound found in the volatile blend of ripening cultivated blueberries. This compound has been found in the volatile blends of bilberry, Vaccinium myrtillus L. (Hirvi and Honkanen 1983), raspberry fruit (Du and Qian 2010), and also from fermented fruit (Feng et al. 2018) and fermentation products (Cha et al. 2012). Acetoin at high concentrations repels D. melanogaster (Stensmyr et al. 2003); however, our experiments with blueberry fruit and volatile blends show no evidence of repellency effects of this or any of the other EAD-active volatiles on D. suzukii.
Although each of the nine identified EAD-active volatiles alone attracted D. suzukii, our aim here was to formulate an attractive blend based on blueberry fruit volatiles. According to our EAD results, we formulated and tested D. suzukii attraction to two fruit volatile blends: a full (9-component) and a partial (4-component) blend. The 4-component blend contained the ester isoamyl acetate, the two ketones acetoin and 6-methyl-5-hepten-2-one, and the alcohol 1-hexanol. We showed that the 4-component blend was more attractive to D. suzukii males and females (virgin and mated) than the 9-component blend. Two conclusions can be made from these findings. First, D. suzukii flies are more attracted to simpler volatile blends. A greater attraction of D. suzukii to less complex blends, containing only 4 or 5 key components, has been shown in previous studies (Cha et al. 2012, 2013; Feng et al. 2018). In fact, Cha et al. (2013) identified a 4-component (acetic acid, ethanol, acetoin, and methionol) blend from wine and vinegar that was attractive to D. suzukii. Recently, Feng et al. (2018) identified a quinary (acetoin + ethyl octanoate + ethyl acetate + acetic acid + phenethyl alcohol) blend from fermented apple juice that was attractive to D. suzukii. However, volatiles from ripening fruit could be more selective to D. suzukii, in particular mated females, than volatiles from fermented fruit (Cloonan et al. 2018). Future studies need to compare the attraction of D. suzukii and other drosophilids to our 4-component fruit blend versus these 4- and 5-component fermentation-based blends in the laboratory and field. Second, there were no differences between sexes or between virgin and mated females in their response to our 4-component fruit blend, which indicates that these volatiles likely provide general information about the location of hosts as opposed to cues on oviposition sites to mated females. Furthermore, the response of D. suzukii to volatiles depends on the context in which they are perceived (i.e., background volatiles) (Alkema et al. 2019). To date, several individual fruit volatiles and blends have been identified as attractive to D. suzukii flies (e.g., Abraham et al. 2015; Revadi et al. 2015; Briem et al. 2016; reviewed by Cloonan et al. 2018); however, their efficacy in attracting them in the field might depend on the competing background odors emitted by the crop itself. Also, volatiles that individually increase D. suzukii attraction could inhibit their response to otherwise attractive blends if presented in the “wrong” context (Cloonan et al. 2019). Thus, finding the “right” combination of fruit volatiles that attracts a specific sex, and in a particular mating status, of D. suzukii to multiple cropping systems can be challenging.
In conclusion, our study demonstrates that domestication/cultivation of blueberries, V. corymbosum, decreased fruit volatile emission rates, which in turn reduced attraction of the frugivorous pest D. suzukii to cultivated fruit. We identified nine compounds from wild blueberries that elicit EAD responses in D. suzukii and formulated a 4-component blend based on these compounds that attracts both males and females under laboratory conditions. Future studies are needed to determine whether this blend is effective in attracting D. suzukii under field conditions. Moreover, crop domestication/cultivation can also affect higher trophic interactions, such as the attraction of natural enemies through changes in volatiles induced by herbivore-damaged plants (Chen and Welter 2005; Huang et al. 2009; Wang et al. 2009; de Lange et al. 2016; Paudel et al. 2019). Parasitoids of D. suzukii are known to use fruit volatiles to locate their host (Biondi et al. 2017); whether domestication/cultivation of blueberries affects these tri-trophic interactions will be the subject of future investigation. Finally, fruit-based attractants identified in this study may help improve D. suzukii monitoring and management. Whether D. suzukii is attracted to our 4-component blend from blueberries in different crops or whether this attraction is context dependent (i.e., affected by background odors) needs investigation.
Author contributions
AZ performed GC-EAD and GC–MS analyses. PS-M and CR-S conducted volatile collections and GC-FID analysis. PU-B and KC performed behavioral assays. PU-B and CR-S performed statistical analyses and wrote the first draft of the manuscript. All authors contributed to the study conceptualization, commented on previous versions of the manuscript, and read and approved the final manuscript.
References
Abraham J, Zhang A, Angeli S et al (2015) Behavioral and antennal responses of Drosophila suzukii (Diptera: Drosophilidae) to volatiles from fruit extracts. Environ Entomol 44:356–367
Agarbati A, Canonico L, Ciani M, Comitini F (2019) The impact of fungicide treatments on yeast biota of Verdicchio and Montepulciano grape varieties. PLoS ONE 14:e0217385
Alkema JT, Dicke M, Wertheim B (2019) Context-dependence and the development of push-pull approaches for integrated management of Drosophila suzukii. Insects 10:454. https://doi.org/10.3390/insects10120454
Altieri MA, Nicholls CI (2003) Soil fertility management and insect pests: harmonizing soil and plant health in agroecosystems. Soil Tillage Res 72:203–211
Asplen MK, Anfora G, Biondi A et al (2015) Invasion biology of spotted wing Drosophila (Drosophila suzukii): A global perspective and future priorities. J Pest Sci 88:469–494
Bai Y, Lindhout P (2007) Domestication and breeding of tomatoes: what have we gained and what can we gain in the future? Ann Bot 100:1085–1094
Baloga DW, Vorsa N, Lawter L (1995) Dynamic headspace gas chromatography—mass spectrometry analysis of volatile flavor compounds from wild diploid blueberry species. In: Rouseff RL, Leahy MM (ed) Fruit Flavors. ACS Symposium Series vol. 596, pp 235–247
Bellutti N, Gallmetzer A, Innerebner G et al (2018) Dietary yeast affects preference and performance in Drosophila suzukii. J Pest Sci 91:651–660
Benrey B, Callejas A, Rios L et al (1998) The effects of domestication of Brassica and Phaseolus on the interaction between phytophagous insects and parasitoids. Biol Control 11:130–140
Biondi A, Wang X, Miller JC et al (2017) Innate olfactory responses of Asobara japonica toward fruits infested by the invasive spotted wing drosophila. J Insect Behav 30:495–506
Briem F, Eben A, Gross J et al (2016) An invader supported by a parasite: Mistletoe berries as a host for food and reproduction of spotted wing Drosophila in early spring. J Pest Sci 89:749–759
Burrack HJ, Fernandez GE, Spivey T, Kraus DA (2013) Variation in selection and utilization of host crops in the field and laboratory by Drosophila suzukii Matsumara (Diptera: Drosophilidae), an invasive frugivore. Pest Manag Sci 69:1173–1180
Calabria G, Máca J, Bächli G, Serra L, Pascual M (2012) First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. J Appl Entomol 136:139–147
Cha DH, Adams T, Rogg H, Landolt PJ (2012) Identification and field evaluation of fermentation volatiles from wine and vinegar that mediate attraction of spotted wing drosophila, Drosophila suzukii. J Chem Ecol 38:1419–1431
Cha DH, Hesler SP, Cowles RS et al (2013) Comparison of a synthetic chemical lure and standard fermented baits for trapping Drosophila suzukii (Diptera: Drosophilidae). Environ Entomol 42:1052–1060
Chen YH, Welter SC (2005) Crop domestication disrupts a native tritrophic interaction associated with the sunflower, Helianthus annuus (Asterales: Asteraceae). Ecol Entomol 30:673–683
Chen YH, Gols R, Benrey B (2015a) Crop domestication and its impact on naturally selected trophic interactions. Annu Rev Entomol 60:35–58
Chen YH, Gols R, Stratton CA et al (2015b) Complex tritrophic interactions in response to crop domestication: predictions from the wild. Entomol Exp Appl 157:40–59
Chen YH, Ruiz-Arocho J, von Wettberg EJ (2018) Crop domestication: anthropogenic effects on insect–plant interactions in agroecosystems. Curr Opin Insect Sci 29:56–63
ChunYu Z, YaDong L, XueSen C et al (2009) GC/MS analysis of volatile components in highbush blueberry cultivars. Acta Hortic Sin 36:187–194
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectol 65:149–160
Cipollini ML (2000) Secondary metabolites of vertebrate-dispersed fruits: evidence for adaptive functions. Rev Chil Hist Nat 73:421–440
Cipollini ML, Stiles EW (1993) Fruit rot, antifungal defense, and palatability of fleshy fruits for frugivorous birds. Ecology 74:751–762
Cloonan KR, Abraham J, Angeli S et al (2018) Advances in the chemical ecology of the spotted wing drosophila (Drosophila suzukii) and its applications. J Chem Ecol 44:922–939
Cloonan KR, Hernández-Cumplido J, De Sousa ALV et al (2019) Laboratory and field evaluation of host-related foraging odor-cue combinations to attract Drosophila suzukii (Diptera: Drosophilidae). J Econ Entomol 112:2850–2860
Dalton DT, Walton VM, Shearer PW et al (2011) Laboratory survival of Drosophila suzukii under simulated winter conditions of the Pacific Northwest and seasonal field trapping in five primary regions of small and stone fruit production in the United States. Pest Manag Sci 67:1368–1374
Darwin C (1868) The variation of animals and plants under domestication. London: John Murray. First edition, first issue. Volume 1
de Lange ES, Farnier K, Gaudillat B, Turlings TCJ (2016) Comparing the attraction of two parasitoids to herbivore-induced volatiles of maize and its wild ancestors, the teosintes. Chemoecology 26:33–44
Diamond J (2002) Evolution, consequences and future of plant and animal domestication. Nature 418:700–707
Du X (2014) Aroma active volatiles in four southern highbush blueberry. J Agric Food Chem 62:4537–4543
Du X, Qian M (2010) Flavor chemistry of small fruits: blackberry, raspberry, and blueberry. In: Qian M, Rimando A (ed) Flavor and health benefits of small fruits. American Chemical Society, pp 3–27
Du X, Plotto A, Song M et al (2011) Volatile composition of four southern highbush blueberry cultivars and effect of growing location and harvest date. J Agric Food Chem 59:8347–8357
Eck P, Childers N (1966) Bluebberry culture. Rutgers University Press, New Brunswick, New Jersey, USA
Ehlenfeldt MK (2009) Domestication of the highbush blueberry at Whitesbog, New Jersey, 1911–1916. Acta Hortic 810:147–152
Farneti B, Khomenko I, Grisenti M et al (2017) Exploring blueberry aroma complexity by chromatographic and direct-injection spectrometric techniques. Front Plant Sci 8:1–19
Faucher CP, Hilker M, de Bruyne M (2013) Interactions of carbon dioxide and food odours in drosophila: Olfactory hedonics and sensory neuron properties. PLoS ONE 8:e5636
Feeny PP (1973) Biochemical coevolution between plants and their insect herbivores. In: Raven EL, Gilbert PH (eds) Coevolution of animals and plants. University of Texas Press, Austin, Texas, USA, pp 3–19
Feng Y, Bruton R, Park A, Zhang A (2018) Identification of attractive blend for spotted wing drosophila, Drosophila suzukii, from apple juice. J Pest Sci 91:1251–1267
Fuller DQ, Allaby RG, Stevens C (2010) Domestication as innovation: the entanglement of techniques, technology and chance in the domestication of cereal crops. World Archaeol 42:13–28
Fuller DQ, Denham T, Arroyo-Kalin M et al (2014) Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record. Proc Natl Acad Sci 111:6147–6152
Gallardo RK, Zhang Q, Dossett M et al (2018) Breeding trait priorities of the blueberry industry in the United States and Canada. HortScience 53:1021–1028
Goff SA, Klee HJ (2006) Plant volatile compounds: sensory cues for health and nutritional value? Science 311:815–819
Hamby KA, Hernández A, Boundy-Mills K, Zalom FG (2012) Associations of yeasts with spotted-wing Drosophila (Drosophila suzukii; Diptera: Drosophilidae) in cherries and raspberries. Appl Environ Microbiol 78:4869–4873
Hancock JF (2005) Contributions of domesticated plant studies to our understanding of plant evolution. Ann Bot 96:953–963
Hauser M (2011) A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Manag Sci 67:1352–1357
Hernandez-Cumplido J, Giusti MM, Zhou Y et al (2018) Testing the ‘plant domestication-reduced defense’ hypothesis in blueberries: the role of herbivore identity. Arthropod Plant Interact 12:483–493
Hirvi T, Honkanen E (1983) The aroma of blueberries. J Sci Food Agric 34:992–996
Horvat RJ, Senter SD, Dekazos ED (1983) GLC-MS analysis of volatile constituents in rabbiteye blueberries. J Food Sci 48:278–279
Huang CH, Yan FM, Byers JA et al (2009) Volatiles induced by the larvae of the Asian corn borer (Ostrinia furnacalis) in maize plants affect behavior of conspecific larvae and female adults. Insect Sci 16:311–320
Jaramillo SL, Mehlferber E, Moore PJ (2015) Life-history trade-offs under different larval diets in Drosophila suzukii (Diptera: Drosophilidae). Physiol Entomol 40:2–9
Johnson RA, Willson MF, Thompson JN, Bertin RI (1985) Nutritional values of wild fruits and consumption by migrant frugivorous birds. Ecology 66:819–827
Keesey IW, Knaden M, Hansson BS (2015) Olfactory specialization in Drosophila suzukii supports an ecological shift in host preference from rotten to fresh fruit. J Chem Ecol 41:121–128
Köllner TG, Held M, Lenk C et al (2008) A maize (E)-β-caryophyllene synthase implicated in indirect defense responses against herbivores is not expressed in most American maize varieties. Plant Cell 20:482–494
Lebreton S, Becher PG, Hansson BS, Witzgall P (2012) Attraction of Drosophila melanogaster males to food-related and fly odours. J Insect Physiol 58:125–129
Lee JC, Bruck DJ, Curry H et al (2011) The susceptibility of small fruits and cherries to the spotted-wing drosophila, Drosophila suzukii. Pest Manag Sci 67:1358–1367
Li T, Yang X, Yu Y et al (2018) Domestication of wild tomato is accelerated by genome editing. Nat Biotechnol 36:1160–1163
Linz J, Baschwitz A, Strutz A et al (2013) Host plant-driven sensory specialization in Drosophila erecta. Proc R Soc B Biol Sci 280:20130626
Liu Y, Dong W, Zhang F et al (2018) Identification of active components from volatiles of Chinese bayberry, Myrica rubra attractive to Drosophila suzukii. Arthropod Plant Interact 12:435–442
Liu F, Li S, Gao J et al (2019a) Changes of terpenoids and other volatiles during alcoholic fermentation of blueberry wines made from two southern highbush cultivars. LWT - Food Sci Technol 109:233–240
Liu S, Laaksonen O, Yang B (2019b) Volatile composition of bilberry wines fermented with non-Saccharomyces and Saccharomyces yeasts in pure, sequential and simultaneous inoculations. Food Microbiol 80:25–39
Lugemwa FN, Lwande W, Bentley MD et al (1989) Volatiles of wild blueberry, Vaccinium angustifolium: Possible attractants for the blueberry maggot fruit fly, Rhagoletis mendax. J Agric Food Chem 37:232–233
McCormick J (1979) The vegetation of the New Jersey Pine Barrens. In: Forman R (ed) Pine barrens: ecosystem and landscape. Academic Press Inc, New York, USA, pp 229–243
Meyer RS, Duval AE, Jensen HR (2012) Patterns and processes in crop domestication: an historical review and quantitative analysis of 203 global food crops. New Phytol 196:29–48
Minitab (2013) Minitab computer software. Minitab Inc., State College, Pennsylvania, USA
Moore JN (1965) Improving highbush blueberries by breeding and selection. Euphytica 14:39–48
Paudel S, Lin P-A, Foolad MR et al (2019) Induced plant defenses against herbivory in cultivated and wild tomato. J Chem Ecol 45:693–707
Purugganan MD, Fuller DQ (2009) The nature of selection during plant domestication. Nature 457:843–848
Revadi S, Vitagliano S, Rossi Stacconi MV et al (2015) Olfactory responses of Drosophila suzukii females to host plant volatiles. Physiol Entomol 40:54–64
Rodríguez A, Alquézar B, Peña L (2013) Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytol 197:36–48
Rodriguez-Saona C, Cloonan KR, Sanchez-Pedraza F et al (2018) Differential susceptibility of wild and cultivated blueberries to an invasive frugivorous pest. J Chem Ecol 45:286–297
Rodriguez-Saona C, Vorsa N, Singh AP, Johnson-Cicalese J, Szendrei Z, Mescher MC, Frost CJ (2011) Tracing the history of plant traits under domestication in cranberries: potential consequences on anti-herbivore defences. J of Exp Bot 62(8):2633–2644
Rowen E, Kaplan I (2016) Eco-evolutionary factors drive induced plant volatiles: a meta-analysis. New Phytol 210:284–294
Scheidler NH, Liu C, Hamby KA et al (2015) Volatile codes: correlation of olfactory signals and reception in Drosophila-yeast chemical communication. Sci Rep 5:1–13
Spitaler U, Bianchi F, Eisenstecken D et al (2020) Yeast species affects feeding and fitness of Drosophila suzukii adults. J Pest Sci 93:1295–1309
SPSS (2015) IBM SPSS statistics for windows. IBM Corp., Armonk, New York, USA
Stensmyr MC, Giordano E, Balloi A et al (2003) Novel natural ligands for Drosophila olfactory receptor neurones. J Exp Biol 206:715–724
Stensmyr MC, Dweck HKM, Farhan A et al (2012) A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell 151:1345–1357
Tamiru A, Bruce TJA, Woodcock CM et al (2011) Maize landraces recruit egg and larval parasitoids in response to egg deposition by a herbivore. Ecol Lett 14:1075–1083
Ulrich D, Komes D, Olbricht K, Hoberg E (2007) Diversity of aroma patterns in wild and cultivated Fragaria accessions. Genet Resour Crop Evol 54:1185–1196
Urbaneja-Bernat P, Polk D, Sanchez-Pedraza F, et al (2020) Non-crop habitats serve as a potential source of spotted-wing drosophila (Diptera: Drosophilidae) to adjacent cultivated highbush blueberries (Ericaceae). Can Entomol 1–16
Wang X-G, Nadel H, Johnson MW et al (2009) Crop domestication relaxes both top-down and bottom-up effects on a specialist herbivore. Basic Appl Ecol 10:216–227
Whitehead SR, Turcotte MM, Poveda K (2017) Domestication impacts on plant-herbivore interactions: a meta-analysis. Philos Trans R Soc B Biol Sci 372:20160034
Zhang A, Linn C, Wright S et al (1999) Identification of a new blend of apple volatiles attractive to the apple maggot, Rhagoletis pomonella. J Chem Ecol 25:1221–1232
Zhang A, Amalin D, Shirali S et al (2004) Sex pheromone of the pink hibiscus mealybug, Maconellicoccus hirsutus, contains an unusual cyclobutanoid monoterpene. Proc Natl Acad Sci USA 101:9601–9606
Zhang X, Julien-David D, Miesch M et al (2005) Identification and quantitative analysis of β-sitosterol oxides in vegetable oils by capillary gas chromatography–mass spectrometry. Steroids 70:896–906
Acknowledgments
We thank Jennifer Frake, Nicolas Firbas, Kyra Huttinger, and Fernando Sanchez-Pedraza for laboratory and field assistance. Special thanks to Robert Holdcraft for assistance in volatile collection and analysis, Vera Kyryczenko-Roth for colony maintenance, and three anonymous reviewers for helpful comments on an earlier draft of this manuscript.
Funding
The study was partially supported by funding from the USDA NIFA Crop Protection and Pest Management (CPPM) program (award no. 2015-70006-24152), the USDA NIFA Specialty Crops Research Initiative (SCRI) program (award no. 2015-51181-24252), the New Jersey Blueberry Research Council, and the Hatch projects no. NJ08252 and NJ08140 to CR-S.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Communicated by Antonio Biondi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Urbaneja-Bernat, P., Cloonan, K., Zhang, A. et al. Fruit volatiles mediate differential attraction of Drosophila suzukii to wild and cultivated blueberries. J Pest Sci 94, 1249–1263 (2021). https://doi.org/10.1007/s10340-021-01332-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-021-01332-z