Abstract
Larval Diaphania indica (Saunders) (Lepidoptera: Crambidae) cause complete defoliation of Trichosanthes anguina L. and reduce crop yield in India. Females lay eggs on the leaf surface, and therefore leaf surface waxes are potentially involved in host selection. Alkanes and free fatty acids are the major constituents of leaf surface waxes, so a study was conducted to determine whether these wax constituents from three T. anguina cultivars (MNSR-1, Baruipur Long, and Polo No.1) could act as short-range attractants and oviposition stimulants in D. indica females. Twenty n-alkanes from n-C14 to n-C36 and 13 free fatty acids from C12:0 to C21:0 were detected in the leaf surface waxes of these cultivars. Heptadecane and stearic acid were predominant among n-alkanes and free fatty acids, respectively, in these cultivars. Females showed attraction towards one leaf equivalent surface wax of each of these cultivars against solvent controls (petroleum ether) in Y-tube olfactometer bioassays. A synthetic blend of heptadecane, eicosane, hexacosane, and stearic acid, a synthetic blend of hexacosane and stearic acid, and a synthetic blend of pentadecane and stearic acid comparable to amounts present in one leaf equivalent surface wax of MNSR-1, Baruipur Long, and Polo No.1, respectively, were short-range attractants and oviposition stimulants in D. indica. Female egg laying responses were similar to each of these blends, providing information that could be used to developing baited traps in integrated pest management (IPM) programs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Trichosanthes anguina L. (Cucurbitaceae), commonly known as snake gourd or serpent gourd, is an important crop in India, Sri Lanka, Nepal, Australia, North America, Nigeria, and Pakistan (Kage et al. 2009; Singh et al. 2017; Yusuf et al. 2007). The fruit is generally consumed as a vegetable due to its high nutritive value. The plant is a good source of functional ingredients such as carotenoids, flavonoids, phenolic acids, and vitamins A and E, which makes the whole plant pharmacologically and therapeutically active (Ojiako and Igwe 2008; Yusuf et al. 2007). The plant has been shown to possess antidiabetic (Arawwawala et al. 2009), gastro protective (Arawwawala et al. 2010a), anti-inflammatory (Arawwawala et al. 2010b), lipid lowering properties (Arwwawala et al. 2011a), antioxidant (Arawwawala et al. 2011b), and antimicrobial activity (Ali et al. 2011).
The caterpillar, Diaphania indica (Saunders) (Lepidoptera: Crambidae), is a major pest of T. anguina that consumes leaves, flowers, and young fruits of the crop in India and Sri Lanka (Debnath et al. 2020; Roopa et al. 2014). Generally, young fruits are not the primary target, but when leaves are completely eaten then the larvae attack young fruits. The insect also consumes leaves of cucumber, melon, gherkin, bottle gourd, bitter gourd, luffa, little cucumber, and cotton (Clavijo et al. 1995; Hosseinzade et al. 2014; Pandey 1977; Tripathi and Pandy 1973). Larvae of D. indica feed on the leaves of T. anguina for 9–12 days through five instars to complete their larval development, and after pupation (8–10 days), newly emerged male and female adults live for 6–8 and 9–10 days, respectively (Debnath et al. 2020). The first and second instars consume the lower epidermis of leaves, while third to fifth instars gregariously consume whole leaf tissue. Severe infestation by the larvae of D. indica on T. anguina plants causes complete defoliation, which results in loss of crop yield.
The influence of plant leaf surface waxes on insect host plant acceptance is an area of interest because after reaching the plant, the first contact between a plant and an insect takes place on the leaf surface (Müller 2006; Müller and Hilker 2001). For lepidopterans, host plant oviposition on a suitable host is essential for first instar survival, so chemicals on or near the plant surface, including surface waxes are vital oviposition cues. Hence, leaf surface wax chemicals serve a vital role in host acceptance by herbivorous insects. Therefore an assessment of leaf surface wax chemicals stimulating oviposition in insect pests of economic significance is significant due to their potential use in the manipulation of insect behavior in a crop field.
The leaf surface waxes are mainly comprised of long-chain alkanes, free fatty acids, esters, aldehydes, and primary and secondary alcohols, which differ within plant species as well as cultivars of a plant species (Tomasi et al. 2018). Long-chain alkanes and free fatty acids, the major components of surface wax, can be short-range attractants (Manosalva et al. 2011; Schiestl et al. 1999) and stimulate oviposition in females (Li and Ishikawa 2006; Müller 2006). Long-chain alkanes such as heneicosane, docosane, tricosane, tetracosane, pentacosane, hexacosane, and heptacosane along with other alkane compounds from the labellum extracts of orchid flower, Ophrys sphegodes Miller elicited attraction in the solitary bee, Andrena nigroaenea (Kirby) (Schiestl et al. 1999). Manosalva et al. (2011) elucidated that long-chain free fatty acids present in the root surface waxes of young red clover (Trifolium pratense L.) served as short-range attractant in Hylastinus obscurus (Marsham) (Coleoptera: Curculionidae). Five long-chain alkanes – hexacosane, heptacosane, octacosane, nonacosane, and tritriacontane, present in the epicuticular wax of corn (Zea mays L.) leaves stimulated oviposition in Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) (Udayagiri and Mason 1997). In another study, Li and Ishikawa (2006) demonstrated that long-chain n-alkanes and free fatty acids present in the surface waxes of Japanese knotweed Fallopia japonica (Houtt.) Ronse Decr. stimulated oviposition in O. nubilalis. According to Jiang et al. (2015), the sugarcane and maize leaf surface waxes comprised of alkanes and other chemicals stimulated oviposition in O. furnacalis. However, no study has demonstrated the role of leaf surface waxes in D. indica.
This investigation is conducted to: (i) determine the composition of n-alkanes and free fatty acids in the leaf surface waxes of three T. anguina cultivars (cv. MNSR-1, Baruipur Long, and Polo No. 1, three cultivars commonly cultivated in West Bengal due to high yielding potential because they are well adapted to conditions there), (ii) observe whether the leaf surface waxes of these cultivars act as short-range attractants and stimulate oviposition in D. indica, (iii) study the behavioral responses of D. indica using Y-tube olfactometer bioassays towards the individual synthetic n-alkanes and fatty acids, and blends comparable to the amounts present in one leaf equivalent surface wax of these cultivars, and (iv) examine whether the most attractive synthetic blends (n-alkanes and free fatty acids) comparable to the amounts present in one leaf equivalent surface wax of these cultivars stimulate oviposition in D. indica.
Methods and Materials
Insects
Light traps were placed near a Cucumis sativus L. planting in the field to collect adults (males and females) of D. indica, and collected adults were kept on the same leaves for egg laying. Newly emerged first instars were fed on cucumber leaves, and they were reared at 27 ± 1 °C, 65 ± 10% relative humidity, and 12 L: 12 D photoperiod in a ‘BOD’ incubator. A moist piece of cotton was attached with the cut end of the petiole of each cucumber leaf and this was wrapped by aluminium foil to prevent water loss from the leaf. Fresh leaves were provided at 24 h intervals by replacing the previous ones. The second generation D. indica larvae were also fed on cucumber leaves. Gravid F2 females (2–3 days old, i.e., after emerging from pupa) were used for olfactory bioassays and oviposition assays. Gravid females were identified based on their swollen abdomens and slower movement of fusiform hairs at the end of the abdomen.
Plant Materials
Three cultivars of T. anguina seeds, MNSR-1, Baruipur Long, and Polo No. 1 (hereafter MNS, BAR, and POLO, respectively) were grown at the Crop Research Farm (CRF) [soil organic matter 5.3 ± 0.2% (± Standard Error), pH 7.7], University of Burdwan (23°16′ N & 87°54′ E), West Bengal, India during May, 2019. Mature leaves (six to seven-weeks old plants) were collected, and leaves were washed with deionised water followed by paper towelling. Mature leaves were collected because gravid females prefer to lay eggs on the abaxial surface of mature leaves than either young or senescent leaves.
Extraction of Leaf Surface Waxes
Seventy-five grams of leaves of each T. anguina cultivar were separately collected five times (5 × 75 g MNS, 5 × 75 g BAR, and 5 × 75 g POLO), and the gum arabic method of Jetter and Schäffer (2001) was used to isolate leaf surface waxes from an intact leaf. Before the use of gum arabic (Roth, Karlsruhe, Germany), the contaminants of gum arabic were separated by soxhlet extraction with hot chloroform. An aqueous solution of ca. 0.1 ml [50% (w/w)] gum arabic was applied per cm2 of leaf surface on the adaxial and abaxial surface of each leaf with a small paintbrush. After drying for 1 h, a thin whitish adhesive layer formed and was removed from each leaf with forceps, keeping the leaves as undamaged and intact (without damaging the epidermal and mesophyll tissue). The gum arabic fractions of adaxial and abaxial leaf surfaces were collected for each leaf and extracted with 10 ml each of water and chloroform (v/v). After vigorous agitation and phase separation, the organic solution was removed and the solvent was evaporated under reduced pressure.
Each dried crude extract from 75 g leaves of each T. anguina cultivar was then dissolved in 30 ml chloroform and divided into three equal crude fractions [each 10 ml crude fraction was equivalent to ca. 25 g of leaves; the number of leaves for 25 g MNS, BAR, and POLO were 14 ± 1, 16 ± 1, and 18 ± 1 (mean ± standard error), respectively]. One mg heneicosane (n-C21) was added as internal standard to the second fraction of each crude extract for identification and quantification of alkanes, while tricosanoic acid (C23:0, 1 mg) was added to the third fraction of each crude extract for identification and quantification of free fatty acids. Each fraction of crude extract was filtered through Whatman No. 41 filter paper and evaporated to dryness at room temperature. The first, second, and third fractions were used for (i) olfactometer and oviposition bioassays, (ii) identification and quantification of alkanes, and (iii) identification and quantification of free fatty acids, respectively.
Identification and Quantification of Alkanes
Alkanes were identified and quantified according to the protocol of Mitra et al. (2020a). The second fraction of each crude extract (equivalent to ca. 25 g of leaves) was fractioned by Thin Layer Chromatography (TLC) on silica gel G (Sigma St. Louis, MO, USA) layers (thickness 0.5 mm) with carbon tetrachloride as the mobile phase. A faint yellowish band appeared on the TLC plate, and the plate was air-dried under laboratory conditions. The single hydrocarbon band appeared in each TLC plate was eluted from the silica gel layer with chloroform. A total of 15 purified alkane samples (five alkane samples from each T. anguina cultivar) were prepared for gas chromatography-mass spectrometry (GC–MS) and GC-flame ionisation detector (GC-FID) for identification and quantification, respectively. Half portion of each sample was used for identification by GC–MS and the remainder for quantification of alkane compounds by GC-FID.
For identification of alkanes, the samples were analyzed with Clarus 690 GC coupled to a SQ8C Mass Selective Detector using a SE-30 column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness). The oven temperature program was initially 170 °C held for 1 min, then raised at 4 °C/min to 300 °C, and finally held for 15 min (Sarkar et al. 2014). Helium was the carrier gas. The MS parameters were 280 °C at the interface, ionization energy 70 eV, scan speed 5 scans/sec and scanned over the mass range 40–600 mass units. The identity of compounds was confirmed by injections of a mixture of synthetic n-alkanes (n-C14 to n-C36). All n-alkanes (≥ 99% purity) between n-C14 and n-C36 were purchased from Sigma Aldrich. Alkanes were verified by the comparison of diagnostic ions and GC retention times with those of respective authentic standards.
For quantification of compounds, five separate samples of each T. anguina cultivar (MNS, BAR, and POLO) were analyzed by a Techcomp GC (Em Macau, Rua De Pequim, Nos. 202A-246, Centro Financeiro F7, Hong Kong) model 7900 fitted with a SE-30 capillary column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness) and a flame ionization detector which was run under the same temperature conditions as mentioned in GC–MS analysis. The carrier gas was nitrogen with a flow rate of 18.5 ml/min. The volume of sample injected was 1 µl with a split ratio of 1:5. The amount of individual alkanes was computed from the GC peak areas and the areas of each peak were converted into quantities of n-alkanes based on internal standard heneicosane (n-C21).
Identification and Quantification of Free Fatty Acids
Free fatty acids were identified and quantified according to the protocol of Mitra et al. (2020a). The third fraction of each crude extract obtained from each T. anguina cultivar (MNS, BAR, and POLO) (equivalent to ca. 25 g of leaves) was mixed with diethyl ether and filtered through Whatman No. 41 filter paper. The extract was purified by TLC on silica gel G layers (thickness 0.5 mm) with n-butanol: acetic acid: water (4:1:5; this mixture was shaken, and water was separated from this mixture by a separating funnel and discarded) as the mobile phase (Das et al. 2019). The band was eluted from the silica gel layer with diethyl ether, and diethyl ether was removed under reduced pressure leaving purified free fatty acids. The purified free fatty acids were esterified with 3 ml BF3-methanol followed by warming for 5 min in a hot water bath at 50–60 °C and cooled. Hexane (30 ml) was added to this mixture followed by washing twice with saturated NaCl in a separating funnel. The aqueous layer of each sample was discarded, and the hexane fraction was passed through 50 g anhydrous Na2SO4 twice. One portion of each esterified sample (hexane fraction) was used for GC–MS and another for GC-FID. The extraction of free fatty acids from each crude extract was separately repeated five times followed by esterification, and a total of 15 samples were prepared.
One portion of the esterified fatty acids was analyzed with an Clarus 690 GC coupled to a SQ8C Mass Selective Detector with a SE-30 column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness). The oven temperature program was initially held at 160 °C for 2 min, then raised at the rate of 3 °C/min to 220 °C and finally held at 220 °C for 18 min (Mukherjee et al. 2014; Sarkar and Barik 2015). Helium was the carrier gas. The MS temperature parameter was 280 °C at the interface, ionization energy 70 eV, scan speed 5 scans/sec, and scanned over the mass range 40–600 mass units. Fatty acids were verified by comparing the diagnostic ions and GC retention times with those of respective standard esterified fatty acids [methyl laurate (C12:0), methyl tridecanoate (C13:0), methyl tetradecanoate (C14:0), methyl pentadecanoate (C15:0), methyl palmitate (C16:0), methyl palmitoleate (C16:1), methyl heptadecanoate (C17:0), methyl stearate (C18:0), methyl linoleate (C18:2), methyl linolinate (C18:3), methyl nonadecanoate (C19:0), methyl arachidate (C20:0), and methyl heneicosanoate (C21:0)]. All standard esterified fatty acids (fatty acid methyl esters, ≥ 99% purity) were purchased from Sigma-Aldrich, Germany.
The remaining portion of esterified fatty acids (five separate samples of each T. anguina cultivar) were analyzed using a Techcomp Gas Chromatograph model 7900 fitted with a SE-30 capillary column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness) and a flame ionization detector, which was run under the same temperature conditions as described for GC–MS analysis. The injector port temperature was 280 °C. The carrier gas was nitrogen with a flow rate of 20 ml/ min. The volume of the sample injected was 1 µl with a split ratio of 1:5. The amount of individual free fatty acids was computed from the GC peak areas and the areas of each peak were converted into quantities of fatty acids based on reference standard methyl tricosanoate (C23:0).
Olfactometer Bioassays
A glass Y-tube (internal diameter: 3 cm; common arm: 10 cm and each arm 10 cm; two lateral arms at an angle of 45°) was used to perform the olfactometer bioassays of gravid D. indica females (Supplementary Fig. 1). A glass adapter was attached with the end of each arm of the Y tube. Each adapter was fitted with a glass vial (3 cm diameter × 3 cm long). Chracoal filtered air (70 ml/min) was pushed through an inlet tube of each adapter, so the purified air could enter the vial and then exit through an outlet tube directed to an arm of the Y tube. One glass vial held a piece (2 × 2 cm2) of Whatman No. 41 filter paper moistened with 1 ml of the test samples, while the other glass vial held a filter paper of the same size moistened with 1 ml of the control solvent (petroleum ether). All the connections between different parts of the set-up consisted of teflon tubing.
Bioassays were performed in the laboratory at 27 ± 1ºC, 65 ± 10% relative humidity (RH), and 150 lx light intensity. In preliminary bioassays, the behavioral response of gravid females to the control solvent (petroleum ether) was neutral. One ml of the test sample and the control solvent was applied to separate filter paper pieces and the solvent was allowed to evaporate, and these filter papers were introduced into the glass vials before the first insect was released into olfactometer, for each experiment tested as a sample against control. Individual females were released into a porous glass vial (3 cm diameter × 3 cm long), which was then attached with the common arm of glass Y and exposed to a particular odor, consisting of 1 ml of the control solvent (petroleum ether) in one glass vial and 1 ml of the test sample (leaf surface waxes, individual synthetic alkanes, and fatty acids or synthetic blends comprised of alkanes and fatty acids compounds) in another glass vial. The behavioral response of each gravid female was observed for 3 min. A choice was recorded when a gravid female reached the far end of one arm, and the choice of insect was recorded as a positive (showed attraction to test samples) or negative (did not show attraction to test samples) response, respectively, and subsequently, the female was discarded. Gravid females that did not show any choice within 3 min or remained in the stem of the Y-tube were discarded and recorded as no response (Mitra et al. 2020b). For each bioassay, 60 naïve females were tested excluding those who did not respond. Each gravid female was tested once. After five insects had been tested, the olfactometer set-up was cleaned with petroleum ether followed by acetone, left to dry, and subsequently, the odor sources were switched between left and right arms to avoid positional bias.
Dual Choice Bioassays with Gravid D. indica Females Towards Crude Surface Waxes
Bioassay 1: Behavioral responses of females towards one leaf equivalent surface wax (crude extract) of each T. anguina cultivar (MNS, BAR, and POLO) were tested against the control solvent (petroleum ether) to observe whether leaf surface waxes from each T. anguina cultivar attracted D. indica (Table 1, Supplementary Table 1).
Bioassay 2: Behavioral responses of females towards one leaf equivalent surface wax of T. anguina cultivars were tested against each other (MNS vs. BAR, MNS vs. POLO, and BAR vs. POLO) to observe whether the leaf surface wax of a particular T. anguina cultivar was more attractive to the test insect among three T. anguina cultivars (Supplementary Table 1).
Dual Choice Bioassays with Gravid D. indica Females Towards Individual Synthetic Compounds and Synthetic Blends
Bioassay 3: Behavioral responses of females towards individual synthetic compounds, comparable to the amounts of individual compounds present in one leaf equivalent surface wax of each T. anguina cultivar (MNS, BAR, and POLO) dissolved in 1 ml petroleum ether and tested against 1 ml control solvent to observe the role of individual compounds in D. indica (Supplementary Table 2a and 2b). Those compounds eliciting a response individually were then combined for bioassay in proportions representing those present on waxes of MNS (MNS blend 4), BAR (BAR blend 2), and POLO (POLO blend 2) and were tested against the control solvent (Supplementary Table 3).
Bioassay 4: One leaf equivalent surface wax of each T. anguina cultivar (MNS, BAR, and POLO) was tested against individual or synthetic blends comparable to the amounts present in one leaf equivalent surface wax of each T. anguina cultivar (MNS, BAR, and POLO) to observe whether crude surface wax and synthetic blends were equally attractive to the test insect (Supplementary Table 1).
Bioassay 5: Each of the three blends, MNS blend 4, BAR blend 2, and POLO blend 2 were tested against each of the other two (Supplementary Table 1). The above bioassays were conducted to observe whether a particular synthetic blend comparable to one leaf equivalent surface wax of a particular T. anguina cultivar was most attractive to the test insect compared to the other two synthetic blends.
Bioassay 6: Dose response bioassays of D. indica towards individual synthetic compounds were conducted to determine the lowest and highest doses where the female responded initially and showed the highest attraction. These five compounds were tested against the control solvent because in preliminary tests females showed responsiveness (pentadecane: 30, 60, and 120 µg/ml petroleum ether; heptadecane: 150, 300, and 600 µg/ml petroleum ether; eicosane: 200, 400, and 800 µg/ml petroleum ether; hexacosane: 75, 150, and 300 µg/ml petroleum ether; and stearic acid: 45, 90, and 180 µg/ml petroleum ether).
Oviposition Assays
Gravid females were used for oviposition assays. Glass chambers (20 × 20 cm2) were used for oviposition assays. To prevent egg laying on the floor and bottom of the glass chamber, coarse grade emery papers were placed along sides and bottom of each glass chamber. Filter papers (Whatman No. 41) of 2 × 2 cm2 sizes were used for oviposition assays. In preliminary assays, females did not lay eggs on filter papers with or without the control solvent (petroleum ether). A test sample (1 ml) and the control solvent (1 ml) were applied to separate filter paper pieces, and allowed to evaporate the solvent. These filter papers were separately kept in two Petri dishes (1 cm diameter × 3 cm long), and these two Petri dishes were placed in the glass chamber with a gap of 10 cm, and subsequently, a gravid female was released in a glass chamber. Ten gravid females were separately tested for each experiment apart from the insects that did not react. Each female was observed continuously for 6 h after releasing in a square glass chamber, and when a female laid eggs for the first time, eggs were counted and this female was discarded (Sambaraju et al. 2016). If a female did not lay eggs within 6 h, this female was also discarded.
Oviposition Assays of Gravid D. indica Females Towards Leaf Surface Waxes
Oviposition assay 1: A single leaf of each T. anguina cultivar (MNS, BAR, and POLO) was tested against the dewaxed leaf of the same cultivar to find whether leaf surface waxes from each T. anguina cultivar stimulated females to lay eggs (Supplementary Table 4).
Oviposition assay 2: One leaf equivalent surface wax (crude wax) of each T. anguina cultivar (MNS, BAR, and POLO) vs. the control solvent (petroleum ether) was tested on filter papers to observe whether crude leaf surface waxes from each T. anguina cultivar stimulated females to lay eggs on filter papers (Supplementary Table 4).
Oviposition assay 3: A single leaf of each T. anguina cultivar was tested against each other (MNS vs. BAR, MNS vs. POLO, and BAR vs. POLO) to observe whether the leaf surface wax of a particular cultivar stimulated females to lay more eggs compared to the other two cultivars (Supplementary Table 4).
Oviposition assay 4: One leaf equivalent surface wax (crude wax) of each T. anguina cultivar was tested against each other (MNS vs. BAR, MNS vs. POLO, and BAR vs. POLO) on filter papers to observe whether leaf surface wax of a particular cultivar stimulated females to lay more eggs compared to the other two cultivars (Supplementary Table 4).
Oviposition Assays of Gravid D. indica Females Towards Synthetic Blends
Oviposition assay 5: MNS blend 4 or BAR blend 2 or POLO blend 2 vs. the control solvent were conducted on filter papers to observe whether synthetic blends resembling one leaf equivalent surface wax of each T. anguina cultivar stimulated females to lay eggs on filter papers (Supplementary Table 4). Previously, it was observed whether one leaf equivalent surface wax from these cultivars could serve as oviposition stimulant in females on filter papers.
Oviposition assay 6: One leaf equivalent surface wax of MNS vs. MNS blend 4, one leaf equivalent surface wax of BAR vs. BAR blend 2, and one leaf equivalent surface wax of POLO vs. POLO blend 2 were performed on filter papers to observe whether crude surface waxes and synthetic blends were equally acting as stimulants in females to lay eggs on filter papers (Supplementary Table 4).
Oviposition assay 7: MNS blend 4 vs. BAR blend 2 or POLO blend 2, and BAR blend 2 vs. POLO blend 2 were conducted on filter papers to conclude whether females preferred a particular synthetic blend for oviposition on filter papers (Supplementary Table 4).
Statistical Analyses
Levene’s test for homogeneity of variance was applied on the data of total crude surface waxes, and individual as well as total amounts of alkanes and free fatty acids present in the leaf surface waxes of three T. anguina cultivars (MNS, BAR, and POLO), and the test confirmed that the data conformed to ANOVA assumptions so the Discriminant function analysis (DA) and ANOVA were conducted with untransformed data. The surface wax profiles (n-alkanes and free fatty acids) of the three T. anguina cultivars were compared using DA (XLSTAT version 13). One-way ANOVA followed by post hoc Tukey test were performed using SPSS software (version 16.0) to compare the treatment effects on individuals, and total alkanes and free fatty acids (Zar 1999). Data from Y-tube olfactometer bioassays and oviposition assays were analyzed based on the null hypothesis that the probability of scores for the test compound(s) or control solvent is equal to 50%, i.e., by a Chi-square test (H0: P = 50%) (Karmakar et al. 2018).
Results
Leaf Surface Wax in T. anguina Cultivars
The DA revealed that the first factor F1 explained more than 75% of the variation, and the biplot represented the spatial orientation of response variables, MNS, BAR, and POLO cultivars against the two extracted factors (Fig. 1). As shown through the Fisher’s distance, three cultivars varied significantly with respect to the leaf surface wax compounds (Fig. 1, Supplementary Table 5). Based on canonical correlations and the eigen values, F1 and F2 explained 100% of variation among the three cultivars (Supplementary Table 5). Discrimination by DA was significant (Wilk’s λ < 0.001, F8, 18 = 190.275, P < 0.001).
Discriminant function analysis (DA) using three Trichosanthes anguina cultivars, MNSR-1 (MNS), Baruipur Long (BAR), and Polo No.1 (POLO) as the response variables against the data set of leaf surface wax chemicals (long-chain alkanes and free fatty acids) obtained from three T. anguina cultivars as explanatory variables
The amount of leaf surface wax was the highest in MNS (42.28 ± 1.32 mg) followed by BAR (36.18 ± 1.13 mg) and the lowest in POLO (31.46 ± 1.07 mg) (F = 21.772; df = 2, 12; P < 0.001). Among the total amount of leaf surface waxes, alkanes represented for 25.72 ± 0.31, 22.45 ± 0.44, and 18.54 ± 0.34 mg (mean ± SE) in MNS, BAR, and POLO, respectively; whereas free fatty acids accounted for 5.63 ± 0.18, 3.82 ± 0.21, and 4.06 ± 0.17 mg (mean ± SE) in MNS, BAR, and POLO, respectively, with the balance consisting of unidentified surface wax compounds.
Alkanes in Leaf Surface Waxes of Three T. anguina Cultivars
Total amounts of alkanes differed significantly among the leaf surface waxes of these cultivars, which were the highest in MNS, intermediate in BAR, and the lowest in POLO. Among the total amount of alkanes, the unidentified branched-chain alkanes represented for 0.03 ± 0.001, 0.01 ± 0.001, and 0.01 ± 0.001 mg (mean ± SE) in MNS, BAR, and POLO, respectively. Twenty n-alkanes from n-C14 to n-C36 were detected in the leaf surface waxes of these cultivars (Supplementary Fig. 2, Table 2). Heptadecane (n-C17) and pentacosane (n-C25) were the predominant and least abundant in the leaf surface waxes of these cultivars.
Free Fatty acids in Leaf Surface Waxes of T. anguina Cultivars
Total amounts of free fatty acids were higher in MNS compared to BAR and POLO, but there was no significant difference between BAR and POLO (Table 3). Thirteen free fatty acids from C12:0 to C21:0 were detected in the leaf surface waxes of these cultivars (Supplementary Fig. 3, Table 3). Stearic acid (C18:0) was the most abundant in the leaf surface waxes of these cultivars. Tetradecanoic acid (C14:0), palmitoleic acid (C16:1), and heneicosanoic acid (C21:0) were the least abundant among the free fatty acids in MNS, BAR, and POLO, respectively (Table 3).
Olfactometer Bioassays with Gravid D. indica Females Towards Crude Surface Waxes
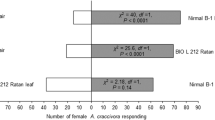
More females were attracted towards one leaf equivalent of surface wax from each T. anguina cultivar against solvent controls (Fig. 2). Females did not show preference towards the leaf surface wax of a particular T. anguina cultivar when the leaf surface waxes of three cultivars were tested against each other (Fig. 3).
Behavioral responses of Diaphania indica females towards one leaf equivalent surface wax of three Trichosanthes anguina cultivars (cv. MNSR-1, Baruipur Long, and Polo No.1) against solvent controls (petroleum ether) in the Y-tube olfactometer bioassay. Numbers in brackets are the number of insects that did not respond to either treatment
Behavioral responses of Diaphania indica females towards one leaf equivalent surface wax of three Trichosanthes anguina cultivars (cv. MNSR-1, Baruipur Long, and Polo No.1) were tested against each other in the Y-tube olfactometer bioassay. Numbers in brackets are the number of insects that did not respond to either treatment
Olfactometer Bioassays with Gravid D. indica Females Towards Synthetic Blends
Among all the identified alkanes and free fatty acids present in the leaf surface wax of three T. anguina cultivars, females showed responses towards 10 individual synthetic compounds (pentadecane, heptadecane, eicosane, pentacosane, hexacosane, heptacosane, nonacosane, palmitoleic acid, palmitic acid, and stearic acid) comparable to the amounts present in one leaf equivalent surface wax of each T. anguina cultivar against solvent controls (Table 4). Females were attracted towards a synthetic blend of above 10 compounds comparable to the amounts present in one leaf equivalent surface wax of each T. anguina cultivar against solvent controls (Table 4).
The insect showed attraction towards four individual synthetic compounds (heptadecane, eicosane, hexacosane, and stearic acid) comparable to the amounts present in one leaf equivalent surface wax of MNS against solvent controls. MNS blend 4 was attractive to the females against solvent controls (Table 4). Females were attracted towards two individual synthetic compounds (hexacosane and stearic acid) comparable to the amounts present in one leaf equivalent surface wax of BAR against solvent controls. BAR blend 2 was attractive to the females against solvent controls (Table 4). Females elicited attraction towards two individual synthetic compounds (pentadecane and stearic acid) comparable to the amounts present in one leaf equivalent surface wax of POLO against solvent controls. POLO blend 2 attracted females against solvent controls (Table 4).
Females could not differentiate between one leaf equivalent surface wax of each T. anguina cultivar and a synthetic blend of 10 compounds (pentadecane, heptadecane, eicosane, pentacosane, hexacosane, heptacosane, nonacosane, palmitoleic acid, palmitic acid, and stearic acid) comparable to the amounts present in one leaf equivalent surface wax of each T. anguina cultivar (Table 5). Females could not distinguish between one leaf equivalent surface wax of MNS and MNS blend 4 (Table 5). Females could not discriminate between one leaf equivalent surface wax of BAR and BAR blend 2 (Table 5). Females could not make a distinction between one leaf equivalent surface wax of POLO and POLO blend 2 (Table 5). Females could not discriminate between MNS blend 4 and BAR blend 2 or POLO blend 2 (Fig. 4). Females also could not distinguish between BAR blend 2 and POLO blend 2 (Fig. 4).
Behavioral responses of Diaphania indica females towards a synthetic blend of four compounds (heptadecane, eicosane, hexacosane, and stearic acid) comparable to one leaf equivalent surface wax of MNSR-1 vs. a synthetic blend of two compounds (hexacosane and stearic acid) comparable to one leaf equivalent surface wax of Baruipur Long or a synthetic blend of two compounds (pentadecane and stearic acid) comparable to one leaf equivalent surface wax of Polo No. 1, and a synthetic blend of two compounds (hexacosane and stearic acid) comparable to one leaf equivalent surface wax of Baruipur Long vs. a synthetic blend of two compounds (pentadecane and stearic acid) comparable to one leaf equivalent surface wax of Polo No. 1 were tested against each other in the Y-tube olfactometer bioassay. Numbers in brackets are the number of insects that did not respond to either treatment
In dose response bioassays, females started to demonstrate attraction towards pentadecane at 60 µg/ml petroleum ether and showed the highest attraction at 120 µg/ml petroleum ether (Table 6). Females were attracted towards heptadecane at 300 µg/ml petroleum ether and displayed the highest attraction at 600 µg/ml petroleum ether (Table 6). Females started to display attraction towards eicosane at 400 µg/ml petroleum ether and showed the highest attraction at 800 µg/ml petroleum ether (Table 6). Females started to show attraction towards hexacosane at 150 µg/ml petroleum ether and showed the highest attraction at 300 µg/ml petroleum ether (Table 6). Females started to display positive responses towards stearic acid at 90 µg/ml petroleum ether and showed the highest attraction at 180 µg/ml petroleum ether (Table 6).
Oviposition Assays with Gravid D. indica Females Towards Crude Surface Waxes
Females laid more eggs on filter paper treated with surface waxes from each T. anguina cultivar than on filter paper treated with solvent alone (Table 7). Oviposition assays revealed that wax extracts of each T. anguina cultivar elicited oviposition in D. indica, but there were no differences among the three cultivars (Table 7). Removal of waxes from each cultivar greatly reduced oviposition. Oviposition did not differ on leaves of each cultivar or extracted leaf waxes of each cultivar in dual choice bioassays (Table 7).
Oviposition Bioassays with Gravid D. indica Females Towards Synthetic Blends
Bioassays with synthetic blends (MNS blend 4, BAR blend 2, and POLO blend 2) showed that these stimulated oviposition similar to extracted waxes (Table 7). Synthetic blends of surface waxes of each cultivar elicited more oviposition on treated filter paper than solvent-treated controls (Table 7). Oviposition did not differ between filter paper treated with synthetic blends vs. filter paper treated with extracted surface waxes of each cultivar, nor did it differ between synthetic blends from each cultivar presented in dual choice bioassays (Table 7).
Discussion
The suitability of a host plant by monophagous or oligophagous insect herbivores depends on the chemical recognition of the host plant as an insect herbivore feeds and develops only on one or a few host plants within one plant family. Gravid lepidopteran females display a string of behavior to attain information from the epicuticular wax compounds of its host plant when a female touches on it, suggesting that sensory cues from the plant’s leaf surface play an essential role for host acceptance (Müller 2006). The dichloromethane extract of maize seedlings consisting of linoleic acid, oleic acid, and stearic acid attracted the western corn rootworm, Diabrotica virgifera virgifera LeConte (Hibbard et al. 1994). The olfactometer bioassay of D. indica demonstrated that leaf surface waxes from the three T. anguina cultivars attracted females from short-range for oviposition. The oviposition assays showed that females of D. indica prefer to lay eggs on intact leaves containing surface wax chemicals compared to leaves from which surface waxes have been removed. Further, this was corroborated by the result that females of D. indica preferred to lay eggs on filter paper substrates treated with leaf surface wax extracts. The experiments on short Y-tube olfactometer bioassays and oviposition assays suggest that additional work would be required to decide whether the clear preference by the gravid females is due to attraction or oviposition-stimulatory effects.
The surface wax composition of each T. anguina cultivar is comprised of alkanes (ca. 27%), acids (ca. 19%), ketones (ca. 19%), alcohols (ca. 12.5%), aldehydes (ca. 4%), amines (ca. 4%), alkenes (ca. 4%), glycosides and triterpenes (ca. 4%), and other compounds (ca. 8%) (unpublished data). But, when n-alkanes and free fatty acids were extracted separately, 20 n-alkanes from n-C14 to n-C36 and 13 free fatty acids from C12:0 to C21:0 were identified in the leaf surface waxes of each T. anguina cultivar. Different alkanes and free fatty acids were predominant in the leaf surface waxes of various plant species (Dodoš et al. 2015; Piasentier et al. 2000). The wax biosynthesis has been found to occur by multiple elongation systems, which are both sequential as well as parallel reactions. The three pathways ‒ decarbonylation, acyl-reduction, and β-ketoacyl-elongation are demonstrated as distinct and parallel pathways in the biosynthesis of wax. Through the decarbonylation pathway, aldehydes convert into odd chain alkanes followed by secondary alcohols and ketones (Post-Beittenmiller 1996). These three pathways occur in the epidermal tissue of most plants, but their relative proportions vary from organs to organs as well as species to species. In this study, low molecular weight compounds are abundant, indicating that odd chain alkanes are not so much transformed into secondary alcohols and ketones. Heptadecane and octanoic acid were the major wax components in pitted, ivyleaf, and palmleaf morningglory leaves (Chachalis et al. 2001). Among n-alkanes, heptadecane was predominant in the epicuticular waxes of Ficus glomerata leaves (Kundu and Sinhababu 2013). In the current investigation, heptadecane and stearic acid were the most abundant among alkanes and free fatty acids, respectively, in the three T. anguina cultivars. The current study revealed variations in the amounts of individual n-alkanes and free fatty acids in the leaf surface waxes of three T. anguina cultivars (MNS, BAR, and POLO). This study is in agreement with the hypothesis that variations in the compositions of leaf surface wax compounds might happen between plant species including within different cultivars of a plant species (Dodoš et al. 2015; Piasentier et al. 2000).
The present results obtained through olfactometer bioassays and oviposition assays suggested that the leaf surface waxes of three T. anguina cultivars played an important role in short-range attraction and stimulated females of D. indica to lay eggs. After coming close range of the host plants, leaf surface wax chemicals such as long-chain alkanes and free fatty acids serve a vital role in plant–insect interactions such as short-range attraction (Hibbard et al. 1994; Manosalva et al. 2011; Mitra et al. 2017; Schiestl et al. 1999) and stimulated females to lay eggs (Grant et al. 2000; Li and Ishikawa 2006; Parr et al. 1998; Phelan et al. 1991; Udayagiri and Mason 1997). This study also reveals that females of D. indica employ leaf surface wax chemicals such as alkanes and free fatty acids as olfactory cues, which stimulated females to lay eggs on the leaves of three T. anguina cultivars. But, females of D. indica could not make a distinction in olfactometer bioassays between MNS and BAR or POLO as well as BAR and POLO, and similarly, females did not prefer to lay eggs on a particular T. anguina cultivar, which is indicative of that one leaf equivalent surface wax of the three T. anguina cultivars is equally stimulated females of D. indica to lay eggs. However, females of D. indica laid eggs on a synthetic blend of four compounds – heptadecane, eicosane, hexacosane, and stearic acid comparable to the amounts present in one leaf equivalent surface wax of MNS or a synthetic blend of two compounds – hexacosane and stearic acid comparable to the amounts present in one leaf equivalent surface wax of BAR or a synthetic blend of two compounds – pentadecane and stearic acid comparable to the amounts present in one leaf equivalent surface wax of POLO, suggesting that the gravid D. indica females could recognize the three T. anguina cultivars mainly by both the qualitative (by discrete chemical compounds) and quantitative (by an exact amount of compounds) as contact cues to lay eggs. In the present study, it is interesting to observe that the volatility of n-alkanes from tetradecane (n-C14) to nonacosane (n-C29) differs by more than two orders of magnitude, yet the longer chain alkane hexacosane (n-C26) is as active for attraction as or more active than heptadecane (n-C17). Further, some of the long-chain alkanes and free fatty acids might be ubiquitous in the leaf surface waxes of various plants, but the specific combination and the amounts of n-alkanes and free fatty acids differ between plants, by which an insect can distinguish between leaf surface waxes of host plants and non-host plants (Li and Ishikawa 2006). The navel orangeworm, Amyelois transitella (Walker) (Lepidoptera: Pyralidae) employs long-chain fatty acids, particularly oleic acid and linoleic acid as ovipositional host-finding cues (Phelan et al. 1991). Fatty acids from C8 to C12, oleic acid (C18:1,) and linoleic acid (C18:2) comparable to the amounts present in the surface wax of Picea and Abies spp stimulated oviposition in spruce budworm, Choristoneura fumiferana (Clemens) (Lepidoptera: Tortricidae) (Grant et al. 2000). Further, a blend of pentacosane, heptacosane, nonacosane, hexatriacontane, palmitoleic acid, linolenic acid, and stearic acid, a synthetic blend of pentacosane, hexatriacontane, and stearic acid, and a synthetic blend of hexatriacontane, linolenic acid, and stearic acid comparable to the amounts present in one leaf equivalent surface wax of PDM 54, PUSA BAISAKHI, and SAMRAT cultivars of green gram, respectively, stimulated Spilosoma obliqua Walker (Lepidoptera: Arctiidae) to lay eggs (Mobarak et al. 2020). The current study suggested that insects could respond to a particular combination of compounds as host-plant acceptance process to lay eggs, and the cues for egg laying behavior by the gravid female could weaken when the crucial compounds were altered (Li and Ishikawa 2006; Parr et al. 1998).
The present study concludes that the leaf surface wax chemicals of three cultivars, i.e., MNS, BAR, and POLO of T. anguina contain oviposition stimulants for the gravid females of D. indica. Females of D. indica were attracted towards a synthetic blend of 305.30 µg heptadecane, 290.66 µg eicosane, 95.34 µg hexacosane and 78.85 µg stearic acid, a synthetic blend of 97.98 µg hexacosane and 56.52 µg stearic acid, and a synthetic blend of 63.61 µg pentadecane and 53.14 µg stearic acid comparable to the amounts present in one leaf equivalent surface wax of MNS, BAR, and POLO, respectively. This study propose that once volatile organic compounds (VOCs) from T. anguina plants causing long-range attraction of D. indica females have been determined then the above mentioned three synthetic blends along with the VOCs of leaves could be used as lures to developing baited traps in integrated pest management program (IPM) strategies.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
Code Availability
Not applicable.
References
Ali MA, Sayeed MA, Islam MS, Yeasmin MS, Khan GRMAM, Muhamad II (2011) Physicochemical and antimicrobial properties of Trichosanthes anguina and Swietenia mahagoni seeds. Bull Chem Soc Ethiop 25:427–436
Arawwawala M, Thabrew I, Arambewela L (2009) Antidiabetic activity of Trichosanthes cucumerina in normal and streptozotocin–induced diabetic rats. Int J Biol Chem Sci 3:287–296
Arawwawala LDAM, Thabrew MI, Arambewela LSR (2010a) Gastroprotective activity of Trichosanthes cucumerina in rats. J Ethnopharmacol 127:750–754
Arawwawala M, Thabrew I, Arambewela L, Handunnetti S (2010b) Anti-inflammatory activity of Trichosanthes cucumerina Linn. in rats. J Ethnopharmacol 131:538–543
Arawwawala LDAM, Thabrew MI, Arambewela LSR, Fernando N, Guruge LD (2011a) Antibacterial activity of Trichosanthes cucumerina Linn. extracts. Int J Pharm Biol Arch 2:808–812
Arawwawala M, Thabrew I, Arambewela L (2011b) In vitro and in vivo evaluation of antioxidant activity of Trichosanthes cucumerina aerial parts. Acta Biol Hung 62:235–243
Chachalis D, Reddy KN, Elmore CD, Steele ML (2001) Herbicide efficacy, leaf structure, and spray droplet contact angle among Ipomoea species and smallflower morningglory. Weed Sci 49:628–634
Clavijo AJ, Munroe E, Arias CQ (1995) The genus Diaphania Hübner (Lep.: Cramibidae); key to the economically important species. Agron Trop (Maracay) 45:347–358
Das S, Koner A, Barik A (2019) A beetle biocontrol agent of rice-field weeds recognizes its host plants by surface wax long-chain alkanes and free fatty acids. Chemoecology 29:155–170
Debnath R, Mobarak SH, Mitra P, Barik A (2020) Comparative performance and digestive physiology of Diaphania indica (Lepidoptera: Crambidae) on Trichosanthes anguina (Cucurbitaceae) cultivars. Bull Entomol Res 110:756–766
Dodoš T, Rajčević N, Tešević V, Matevski V, Janaćković P, Marin PD (2015) Composition of leaf n-alkanes in three Satureja montana L. subspecies from the Balkan peninsula: ecological and taxonomic aspects. Chem Biodivers 12:157–169
Grant GG, Zhao B, Langevin D (2000) Oviposition response of spruce budworm (Lepidoptera: Tortricidae) to aliphatic carboxylic acids. Environ Entomol 29:164–170
Hibbard BE, Bernklau EJ, Bjostad LB (1994) Long-chain free fatty acids: semiochemicals for host location by western corn rootworm larvae. J Chem Ecol 20:3335–3344
Hosseinzade S, Izadi H, Namvar P, Samih MA (2014) Biology, temperature thresholds, and degree-day requirements for development of the cucumber moth, Diaphania indica, under laboratory conditions. J Insect Sci 14:61. https://doi.org/10.1093/jis/14.1.61
Jetter R, Schäffer S (2001) Chemical composition of the Prunus laurocerasus leaf surface. Dynamic changes of the epicuticular wax film during leaf development. Plant Physiol 126:1725–1737
Jiang X-C, Dong W-X, Chen B, Xiao C, Gui F-R, Yan N-S, Qian L, Li Z-Y (2015) Electrophysiological and oviposition responses of Asian corn borer, Ostrinia furnacalis (Lepidoptera: Crambidae), to compounds rinsed from the surfaces of sugarcane and maize leaves. Eur J Entomol 112:295–301
Kage DN, Malashetty VB, Seetharam YN, Suresh P, Patil SB (2009) Effect of ethanol extract of whole plant of Trichosanthes cucumerina var. cucumerina L. on gonadotropins, ovarian follicular kinetics and estrous cycle for screening of antifertility activity in albino rats. Int J Morphol 27:173–182
Karmakar A, Mitra S, Barik A (2018) Systemically released volatiles from Solena amplexicaulis plant leaves with color cues influencing attraction of a generalist insect herbivore. Int J Pest Manag 64:210–220
Kundu S, Sinhababu A (2013) Analysis of n-alkanes in the cuticular wax of leaves of Ficus glomerata Roxb. J Appl Nat Sci 5:226–229
Li G, Ishikawa Y (2006) Leaf epicuticular wax chemicals of the Japanese knotweed Fallopia japonica as oviposition stimulants for Ostrinia latipennis. J Chem Ecol 32:595–604
Manosalva L, Pardo F, Perich F, Mutis A, Parra L, Ortega F, Isaacs R, Quiroz A (2011) Behavioral responses of clover root borer to long-chain fatty acids from young red clover (Trifolium pratense) roots. Environ Entomol 40:399–404
Mitra S, Sarkar N, Barik A (2017) Long-chain alkanes and fatty acids from Ludwigia octovalvis weed leaf surface waxes as short-range attractant and ovipositional stimulant to Altica cyanea (Weber) (Coleoptera: Chrysomelidae). Bull Entomol Res 107:391–400
Mitra P, Das S, Barik A (2020a) Leaf waxes from Lathyrus sativus: short-range attractant and stimulant for nymph laying in a viviparous insect. Chemoecology 30:117–129
Mitra P, Mobarak SH, Debnath R, Barik A (2020b) The role of Lathyrus sativus flower surface wax in short-range attraction and stimulant for nymph laying by an adult viviparous aphid. Bull Entomol Res 110:231–241
Mobarak SH, Koner A, Mitra S, Mitra P, Barik A (2020) The importance of leaf surface wax as short-range attractant and oviposition stimulant in a generalist Lepidoptera. J Appl Entomol 144:616–631
Mukherjee A, Sarkar N, Barik A (2014) Long-chain free fatty acids from Momordica cochinchinensis leaves as attractants to its insect pest, Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae). J Asia-Pac Entomol 17:229–234
Müller C (2006) Plant–insect interactions on cuticular surfaces. In: Riederer M, Müller C (eds) Biology of the plant cuticle. Blackwell Publishing, Oxford, pp 398–422
Müller C, Hilker M (2001) Host finding and oviposition behavior in a chrysomelid specialist–the importance of host plant surface waxes. J Chem Ecol 27:985–994
Ojiako OA, Igwe CU (2008) The nutritive, anti-nutritive and hepatotoxic properties of Trichosanthes anguina (snake tomato) fruits from Nigeria. Pak J Nutr 7:85–89
Pandey PN (1977) Host preference and selection of Diaphania indica Saunders (Lep., Pyralidae). Dtsch Ent Z F 24:159–173
Parr MJ, Tran BMD, Simmonds MSJ, Kite GC, Credland PF (1998) Influence of some fatty acids on oviposition by the bruchid beetle, Callosobruchus maculatus. J Chem Ecol 24:1577–1593
Phelan PL, Roelofs CJ, Youngman RR, Baker TC (1991) Characterization of chemicals mediating ovipositional host-plant finding by Amyelois transitella females. J Chem Ecol 17:599–613
Piasentier E, Bovolenta S, Malossini F (2000) The n-alkane concentrations in buds and leaves of browsed broadleaf trees. J Agric Sci 135:311–320
Post-Beittenmiller D (1996) Biochemistry and molecular biology of wax production in plants. Annu Rev Plant Physiol Plant Mol Biol 47:405–430
Roopa HS, Rajashekara S, Ramakrishna S, Venkatesha MG (2014) Screening of bio-pesticides on Diaphania indica (Saunders) (Lepidoptera: Pyralidae), a pest of gherkins. Trends Life Sci 29:37–43
Sambaraju KR, Donelson SL, Bozic J, Phillips TW (2016) Oviposition by female Plodia interpunctella (Lepidoptera: Pyralidae): description and time budget analysis of behaviors in laboratory studies. Insects 7:4. https://doi.org/10.3390/insects7010004
Sarkar N, Barik A (2015) Free fatty acids from Momordica charantia L. flower surface waxes influencing attraction of Epilachna dodecastigma (Wied.) (Coleoptera: Coccinellidae). Int J Pest Manage 61:47–53
Sarkar N, Malik U, Barik A (2014) n–Alkanes in epicuticular waxes of Vigna unguiculata (L.) Walp. leaves. Acta Bot Gal 161:373–377
Schiestl FP, Ayasse M, Paulus HF, Lӧfstedt C, Hansson BS, Ibarra F, Francke W (1999) Orchid pollination by sexual swindle. Nature 399:421–422
Singh A, Singh R, Navneet (2017) Ethnomedicinal, pharmacological, antimicrobial potential and phytochemistry of Trichosanthes anguina Linn.- a review. Bull Pure Appl Sci 36b:82–90
Tomasi P, Dyer JM, Jenks MA, Abdel-Haleem H (2018) Characterization of leaf cuticular wax classes and constituents in a spring Camelina sativa diversity panel. Ind Crop Prod 112:247–251
Tripathi R, Pandy P (1973) A non cucurbitaceous food plant of Diaphania indica. J Sci Technol 11:80–86
Udayagiri S, Mason CE (1997) Epicuticular wax chemicals in Zea mays influence oviposition in Ostrinia nubilalis. J Chem Ecol 23:1675–1687
Yusuf AA, Folarin OM, Bamiro FO (2007) Chemical composition and functional properties of snake gourd (Trichosanthes cucumerina) seed flour. Nig Food J 25:36–45
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, New Jersey, p 663
Acknowledgements
We thank anonymous reviewers for many helpful suggestions of earlier versions of the manuscript. We thank Dr. M. Alma Solis, Research Entomologist, SEL, USDA, Smithsonian Institution, Washington for authenticating the insect. We also thank DST PURSE Phase-II for providing the necessary instrumental facilities.
Funding
The financial assistance from the UGC-JRF, New Delhi, Govt. of India to Rahul Debnath [F.No. 16-6(DEC. 2017)/2018(NET/CSIR)] is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
A.B. and R.D. designed experiments. R.D. and P.M. performed bioassays. R.D. and S.D. did chemical analyses. A.B. and R.D. analysed data. R.D. and P.M. made the Figs. A.B. wrote the manuscript. All authors edited the manuscript and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors agreed to the submission of the final manuscript.
Conflict of Interest/Competing Interests
The authors declare that they have no conflict of interest/competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Debnath, R., Mitra, P., Das, S. et al. Leaf Surface Wax Chemicals in Trichosanthes anguina (Cucurbitaceae) Cultivars Mediating Short-Range Attraction and Oviposition in Diaphania indica. J Chem Ecol 47, 664–679 (2021). https://doi.org/10.1007/s10886-021-01291-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01291-w