Abstract
The importance of long-chain alkanes and free fatty acids present in leaf surface waxes of two Commelinaceae rice-field weeds, Commelina benghalensis L. and Murdannia nudiflora (L.) Brenan, was evaluated as short-range attractant and oviposition stimulant in the Lema praeusta (Fab.) (Coleoptera: Chrysomelidae). Surface waxes were extracted by dipping leaves in n-hexane for 1 min at 27 ± 1 °C. Thin-layer chromatography, gas chromatography–mass spectrometry, and gas chromatography–flame ionization detection analyses of n-hexane extracts revealed 20 n-alkanes from C14 to C36 and 13 free fatty acids from C12:0 to C22:0. Pentacosane and palmitoleic acid were predominant among n-alkanes and free fatty acids, respectively. Females showed attraction to one leaf equivalent surface wax of both weeds against the control solvent (petroleum ether) in Y-tube olfactometer bioassays. However, the insect could not discriminate between one leaf equivalent surface waxes of two weeds, suggesting that both weeds were equally attractive to females. Among all identified alkanes and fatty acids, females showed attraction towards individual docosane, tricosane, pentacosane and heptacosane, and tridecanoic acid, palmitoleic acid, linoleic acid, and arachidic acid, resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis and M. nudiflora, respectively. A synthetic blend of either docosane, tricosane, pentacosane, and heptacosane, resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis, or tridecanoic acid, palmitoleic acid, linoleic acid, and arachidic acid, resembling in amounts as present in one leaf equivalent surface wax of M. nudiflora, served as short-range attractant and oviposition stimulant in L. praeusta.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The family Commelinaceae consists of 600 species and is common throughout the Caribbean, North and Latin America, Africa, Asia, Middle East, and parts of Oceania (Wilson 1981; Isaac et al. 2013). Commelina benghalensis L., commonly known as tropical spiderwort or Benghal dayflower, is a major weed in groundnut, cereal, maize, soya bean, and cotton (Holm et al. 1977; Wilson 1981; Caton et al. 2010; Ahmed et al. 2015); whereas Murdannia nudiflora (L.) Brenan, commonly known as dove weed, is a major weed in cocoa, rubber, sugarcane, coffee, cotton, soya bean, peanut, maize, and banana (Holm et al. 1977; Wilson 1981; Ahmed et al. 2015). Both C. benghalensis and M. nudiflora are also considered as major weeds of rice in Asia (Wilson 1981; Caton et al. 2010). In India, growth of C. benghalensis is tremendous during rainy season, but its above ground leaves die off during winter season and it again regenerates on the beginning of rainy season, while M. nudiflora is available throughout the year. The main strategy for controlling both weeds is the application of herbicides in India.
In a recent study, we demonstrated that the insect, Lema praeusta (Fab.) (Coleoptera: Chrysomelidae) feeds on Commelinaceae weeds such as C. benghalensis, C. obliqua Vahl, C. maculata Edgew., M. nudiflora, M. vaginata (L.) G. Brückn., M. spirata (L.) G. Brückn., Tradescantia zebrina (Schinz) D. R. Hunt, T. pallida (Rose) D. R. Hunt, T. spathacea Sw., and Cyanotis cristata (L.) D. Don., and L. praeusta serve as biocontrol agent against both C. benghalensis and M. nudiflora in rice fields, but no feeding occurred on rice (Das et al. 2018). Its larvae feed for 6–8 days through four instars to complete their larval development on C. benghalensis and M. nudiflora (Das et al. 2018). After pupation in soil (8–9 days), newly emerged adults feed for 95–110 and 65–80 days on C. benghalensis and M. nudiflora leaves, respectively (Das et al. 2018).
The first physical contact between an insect herbivore and plant occurs on the leaf surface. In addition, insect herbivores feed and lay eggs on leaves, suggesting that females employ sensory cues from leaf surface waxes of host plants to determine suitability of the host plant as feeding site and oviposition site (Harborne 1994; Schoonhoven et al. 2005). Thus, leaf surface waxes influence insect behavior as allelochemicals. Therefore, an understanding of the insect oviposition behavior and identification of surface wax compounds responsible for such a behavior could help to develop future biocontrol programs (Padovan et al. 2010; Piesik et al. 2012; Smith and Beck 2013; Wheeler and Schaffner 2013; Mitra et al. 2017). It is widely accepted that the amount and composition of leaf surface wax compounds such as long-chain alkanes, free fatty acids, alcohols, aldehydes, and acetates vary among plant species (Jetter et al. 2000; Sarkar et al. 2013; Karmakar et al. 2016; Mukherjee and Barik 2016; Mitra et al. 2017). It is well known that insect herbivores employ long-chain alkanes and free fatty acids, both are major components of cuticular waxes, as short-range volatile cues to find and recognize their hosts in their microhabitat (Eigenbrode and Espelie 1995; Müller and Hilker 2001; Schoonhoven et al. 2005; Müller 2006; Manosalva et al. 2011; Mukherjee et al. 2013; Sarkar and Barik 2014; Malik and Barik 2015; Sarkar and Barik 2015). Li and Ishikawa (2006) demonstrated that long-chain n-alkanes and free fatty acids from leaf surface waxes of the Japanese knotweed Fallopia japonica (Houtt.) Ronse Decr. served as oviposition stimulant in the European corn borer, Ostrinia latipennis Warren (Lepidoptera: Crambidae). In the Y-shaped glass tube olfactometer bioassays, Altica cyanea Weber (Coleoptera: Chrysomelidae) females showed attraction towards a synthetic blend of hexadecane, octadecane, eicosane, tricosane, palmitic acid, and alpha-linolenic acid as found in leaf surface wax of Ludwigia octovalvis (Jacq.) Raven (Mitra et al. 2017). The above-mentioned synthetic blend also acted as oviposition stimulant in A. cyanea females (Mitra et al. 2017). For this, we hypothesized that long-chain alkanes and free fatty acids present in leaf surface waxes of both weeds, C. benghalensis and M. nudiflora, could act as close-range cues in host finding and stimulant for egg laying in L. praeusta.
Thus, the aims of the study were to (1) identify and quantify n-alkanes and free fatty acids present in leaf surface waxes of C. benghalensis and M. nudiflora weeds by gas chromatography–mass spectrometry (GC–MS) and gas chromatography–flame ionization detection (GC–FID) analyses, respectively, to compare surface wax composition with other plants, (2) to evaluate behavioral responses of L. praeusta by a short Y-shaped glass tube olfactometer towards leaf surface waxes of both weeds, role of individual synthetic n-alkanes and free fatty acids, and blends of synthetic alkanes and fatty acids resembling in amounts as present in one leaf equivalent surface wax of both weeds to observe whether n-alkanes and free fatty acids could act as short-range olfactory cues to attract L. praeusta, and (3) to assess whether attractive synthetic blends (n-alkanes and free fatty acids), resembling in amounts as present in one leaf equivalent surface wax of both weeds, act as oviposition stimulant in L. praeusta. If alkanes and free fatty acids present in leaf surface waxes of both weeds might act as allelochemicals for short-range host finding and stimulant for egg laying by the biocontrol agent, then this approach will contribute to the evaluation of host plant specificity of the prospective biological control agent.
Methods
Insects
Adults of L. praeusta were collected by light traps in rice fields adjacent to the University of Burdwan (23°16′N and 87°54′E), West Bengal, India, and maintained in 1 L glass jars, containing C. obliqua leaves as food source, and covered with fine-mesh nylon nets. They were kept at 27 ± 1 °C, 65 ± 10% relative humidity and 12 L:12 D photoperiod in a biological oxygen demand incubator (ADS instruments and Tech., Calcutta, India). Natural condition of leaves was maintained by attaching a moist piece of cotton around cut ends of C. obliqua leaves followed by wrapping with aluminum foil to prevent moisture loss, and fresh leaves were given daily by replacing the previous ones. Newly emerged F2 males and females started to mate after 1–2 days. Before mating, males and females were provided C. obliqua leaves as food source, but mated females were fed on 5% sucrose solution through a cotton ball in a small glass Petri dish (3 cm diameter). Between 4 and 6 days old (1–2 days after initial mating) mated females were used for olfactory bioassays.
Plant materials
Mature leaves (2–3 weeks old) of C. benghalensis and M. nudiflora were collected from rice fields adjacent to the University of Burdwan during July–August, 2017. Collected leaves were rinsed with distilled water and dried with Whatman No. 1 filter paper.
Extraction of leaf surface waxes
Seventy-five grams of C. benghalensis and M. nudiflora leaves were separately collected three times, and leaves were dipped into 1 L of n-hexane for a period of 1 min at 27 ± 1 °C for extraction of surface waxes, which yielded a light straw-colored extract without traces of chlorophyll (Sarkar et al. 2014; Mitra et al. 2017). Extracts were filtered through Whatman No. 41 filter paper and evaporated to dryness under fume hood. Each dried crude extract from 75 g leaves of either C. benghalensis or M. nudiflora was dissolved in 30 ml of n-hexane and divided into three equal aliquots [each 10 ml of crude part was equivalent to ca. 25 g leaves; number of leaves for 25 g of C. benghalensis and M. nudiflora were 209 ± 7 and 224 ± 4 (mean ± standard error), respectively]. The three aliquots obtained from each crude extract were used for (1) identification and quantification of alkanes, (2) identification and quantification of free fatty acids, and (3) olfactometer and oviposition assays, respectively.
Identification and quantification of alkanes
An aliquot of each crude extract was fractioned by Thin-Layer Chromatography (TLC) on silica gel G (Sigma St. Louis, MO, USA) layers (thickness 0.5 mm), which had been prepared using a Unoplan (Shandon, London) coating apparatus, with carbon tetrachloride as the mobile phase (Mitra et al. 2017). A faint yellow band appeared on the TLC plate, and the plate was air-dried under laboratory condition (27 ± 1 °C). The plate was placed in an iodine chamber (25 cm length × 25 cm height × 13 cm width) for 1 min, which produced a deep yellow band with Rf (Retardation factor) value of 0.86 (Supplementary Fig. 1). The Rf value (0.86) was compared with the Rf value of a blend of synthetic alkanes between n-C14 and n-C36 [100 µg of each alkane from n-C14 to n-C36 were mixed to prepare a blend of synthetic alkanes (Sigma-Aldrich, Germany)]. The single hydrocarbon band produced in each TLC plate was eluted from the silica gel layer with 25 ml of chloroform. The extraction process was repeated three times each from C. benghalensis and M. nudiflora, and purified alkanes were isolated from each crude extract using TLC plates. A total of six purified alkane samples (three alkane samples each from C. benghalensis and M. nudiflora) were prepared for identification and quantification by gas chromatography–mass spectrometry (GC–MS) and GC–flame ionization detection (GC–FID), respectively. Half portion of each alkane sample was used for GC–MS and the remainder for GC-FID. All solvents used were of analytical grade and purchased from E. Merck, India Pvt. Ltd.
For identification of alkanes, 1 µl sample was analyzed with a Clarus 690 GC coupled to an SQ8C Mass Selective Detector using an SE-30 column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness). The temperature of injector was 280 °C, and the oven temperature program was initially 170 °C, held for 1 min, then raised at 4 °C/min to 300 °C, and finally held for 15 min (Mitra et al. 2017). Helium was the carrier gas and flow rate was 1 ml/min. The MS parameters were: 280 °C at the interface, ionization energy 70 eV, scan rate 5 scans/s, and scanned over the mass range 40–600 mass units. The identity of compounds was confirmed by injection of a blend of synthetic n-alkanes (n-C14 to n-C36). Alkanes were verified by comparison of the diagnostic ions and GC retention times with those of respective authentic standards.
For quantification of compounds, three separate samples of either C. benghalensis or M. nudiflora were analyzed by a Techcomp GC (Em Macau, Rua De Pequim, Nos. 202A-246, Centro Financeiro F7, Hong Kong) model 7900 fitted with an SE-30 capillary column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness) and a flame ionization detector which was run under the same temperature conditions (the oven temperature program was initially 170 °C, held for 1 min, and then raised at 4 °C/min to 300 °C and finally held for 15 min) as mentioned in GC–MS analysis. The carrier gas was nitrogen with a total flow rate of 18.5 ml/min and column flow rate of 2.3 ml/min. The volume of the sample injected was 1 µl with a split ratio of 1:5. The peaks were identified by comparison of their retention times with those of synthetic n-alkanes from n-C14 to n-C36, and areas of all peaks were converted into quantities of n-alkanes based on internal standard [1 mg heneicosane (n-C21)] and internal response factor (IRF) (see supplementary material S1). All n-alkanes (> 99% purity) between n-C14 and n-C36 were purchased from Sigma-Aldrich, Germany.
Identification and quantification of free fatty acids
An aliquot of each crude extract from either C. benghalensis or M. nudiflora was mixed with diethyl ether and filtered through Whatman No. 41 filter paper (Sarkar and Barik 2015). The extract was purified by TLC with n-butanol: acetic acid: water (4:1:5; this mixture was shaken and water was separated from this mixture by a separating funnel and discarded) as the mobile phase (Mukherjee et al. 2014; Sarkar and Barik 2015). The band was eluted from the silica gel layer with diethyl ether, and diethyl ether was removed under a nitrogen flow to get purified free fatty acids. The purified free fatty acids were esterified with 1 ml BF3-Methanol followed by warming for 5 min in a hot water bath at 50–60 °C, and cooled (for esterification: reaction of a carboxylic acid with an alcohol in the presence of an acid catalyst is required. BF3-Methanol provides a convenient methanol-catalyst system which, when used in excess with heating, quickly, and quantitatively converts carboxylic acids to their methyl esters). Hexane (30 ml) was added to this mixture followed by washing with saturated NaCl twice in a separating funnel. The hexane fraction was passed through 50 g anhydrous Na2SO4 twice. Half portion of each esterified sample (hexane fraction) was used for GC–MS and another for GC-FID. The extraction of free fatty acids from each crude extract was separately repeated thrice followed by esterification, and a total of six samples (three fatty acid samples each from C. benghalensis and M. nudiflora) were prepared.
One portion of the each esterified fatty acid sample was analyzed with a Clarus 690 GC coupled to an SQ8C Mass Selective Detector with an SE-30 column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness). One µl sample was injected. The temperature of injector was 280 °C, and the oven temperature program was initially held at 160 °C for 2 min, then raised at the rate of 3 °C/min to 220 °C, and finally held at 220 °C for 18 min (Mukherjee et al. 2014; Sarkar and Barik 2015). Helium was the carrier gas and flow rate was 1 ml/min. Fatty acids were verified by comparison of the diagnostic ions and GC retention times with those of respective synthetic esterified fatty acids [methyl laurate (C12:0), methyl tridecanoate (C13:0), methyl pentadecanoate (C15:0), methyl palmitate (C16:0), methyl palmitoleate (C16:1), methyl heptadecanoate (C17:0), methyl stearate (C18:0), methyl oleate (C18:1), methyl linoleate (C18:2), methyl nonadecanoate (C19:0), methyl arachidate (C20:0), methyl heneicosanoate (C21:0), and methyl docosanoate (C22:0)]. All standard esterified fatty acids (fatty acid methyl esters, purity ≥ 99%) were purchased from Sigma-Aldrich, Germany.
The remaining portion of the each esterified fatty acid sample (three separate samples each from C. benghalensis and M. nudiflora) were analyzed using a Techcomp Gas Chromatograph model 7900 fitted with an SE-30 capillary column (Agilent, USA; length: 30 m × 0.32 mm × 0.25-μm film thickness) and a flame ionization detector which was run under same temperature conditions as mentioned in GC–MS analysis. The injector port temperature was 280 °C. The carrier gas was nitrogen with a total flow rate of 20 ml/min and column flow rate of 2.5 ml/min (Mukherjee et al. 2014; Sarkar and Barik 2015). At a split ratio of 1:5, 1 µl of a sample was injected. The peaks were identified by comparison of their retention times with those of standard esterified fatty acids. The amount of individual free fatty acids was computed from the GC peak areas and the areas of all peaks were converted into quantities of fatty acids based on internal standard methyl tricosanoate (one mg) and internal response factor. All solvents used were of analytical grade and purchased from E. Merck, India Pvt. Ltd. For olfactory and oviposition bioassays, all standard fatty acids (purity ≥ 99%) were purchased from Sigma-Aldrich, Germany.
Olfactometer bioassays
At the beginning of experiment, two filter papers were placed in glass vials (1 cm radius × 3 cm long) without test stimuli to observe whether there was any intrinsic bias of L. praeusta within the Y-tube olfactometer. Behavioral responses of 90 males to one leaf equivalent surface wax of either C. benghalensis or M. nudiflora were tested against the control solvent. Similarly, behavioral responses of 90 mated females to one leaf equivalent surface wax of either C. benghalensis or M. nudiflora were conducted against the control solvent to observe whether there are differences in behavioral responses of males or mated females towards surface waxes. All next bioassays were carried out using mated females, as females employ olfactory cues both for feeding and egg laying (Schoonhoven et al. 2005). Mated females (4–6 days old) were provisioned with water and starved for 12 h prior to use in olfactory bioassays. The attractiveness of L. praeusta was assessed using a horizontal Y-shaped glass tube olfactometer (internal radius of 0.6 cm; length of common arm is 5 cm and each arm is 5 cm; two lateral arms at an angle of 45°) (Supplementary Fig. 2). Ends of arms of the Y-tube were connected to two glass-made micro-kit adapters having attached with glass vials (1 cm radius × 3 cm long), each containing a piece (2 × 2 cm2) of Whatman No. 41 filter paper. Each adapter contained two entrances: one air inlet tube for pushing air into the glass vial and another one as outlet tube connecting to one arm of the olfactometer (Supplementary Fig. 2). One glass vial contained a piece (2 × 2 cm2) of Whatman No. 41 filter paper moistened with 1 ml of a test sample, while the other glass vial contained a filter paper of the same size moistened with one ml of the control solvent (petroleum ether). Charcoal-filtered air was pushed into the system at 200 ml min−1. Connections between different parts of the set-up consisted of Teflon tubing.
The olfactometer bioassays were conducted in the laboratory at 27 ± 1 °C, 70 ± 5% relative humidity (RH) and light intensity 150 lx. One ml of a test sample and the control solvent were applied to filter paper pieces and allowed to evaporate the solvent under fume hood. These filter papers were introduced into glass vials before the first insect was released into the olfactometer, for each experiment tested as a sample against the control solvent. A single mated female was introduced into a porous glass vial (1 cm radius × 3 cm long), which was then attached with the common arm of the olfactometer and exposed to a particular odor, consisting of 1 ml of the control solvent (petroleum ether) in one glass vial and 1 ml of a test sample (leaf surface waxes, individual synthetic alkanes, and fatty acids or synthetic blends consisting of alkanes and fatty acids) in another glass vial. Behavior of insects, i.e., olfactory responses of insects towards crude surface wax odor air flowing through one arm and control solvent air flowing through other arm, was studied in a Y-tube olfactometer for 20 min in preliminary bioassays, and subsequently, it was observed that olfactory responses of the insects towards either crude surface wax odor-loaded arm or control solvent-loaded arm at 2 min and 20 min were the same. Therefore, behavior of each female was observed for 2 min in the Y-tube in further bioassays. A female was considered to have made a choice in case of reaching the end of one arm, the insect was removed from the Y-tube, and the choice of the insect was recorded as a positive (showed attraction to test samples) or negative (showed repellence to test samples) response, respectively. However, when two samples (each sample was attractive to the insect against the control solvent) were tested against each other, then behavior of each insect was recorded as a positive response towards each sample. A female was discarded not having made a choice within 2 min (non-responding: the insect remained in main arm of the Y-tube or did not show any movement until the end of observation period), and was replaced by a new one (Mukherjee et al. 2015; Sarkar et al. 2015). Each experiment with a test sample was conducted until a total of 90 female insects had responded (each insect was used once throughout olfactory bioassays); and after testing five insects, the olfactometer set-up was cleaned with petroleum ether followed by acetone, and the position of the two arms was turned 180° to avoid any positional effects.
Dual choice bioassays with L. praeusta females
Behavioral responses of females towards one leaf equivalent surface wax (crude extract) of either C. benghalensis or M. nudiflora were tested against the control solvent (Supplementary material Table 1a and 1b). Furthermore, behavioral responses of L. praeusta towards one leaf equivalent surface wax of C. benghalensis and M. nudiflora were tested against each other (Supplementary material Table 1b).
Responses of L. praeusta to individual synthetic compounds, resembling in amounts of individual compounds as present in one leaf equivalent surface wax of either C. benghalensis or M. nudiflora, were dissolved in 1 ml of petroleum ether, were tested against 1 ml of control solvent to find role of the individual compounds on the insect. Responses of L. praeusta to synthetic blends (consisting of those synthetic compounds to which L. praeusta showed behavioral responses or showed clear attraction), resembling in amounts as they were found in one leaf equivalent surface wax of either C. benghalensis or M. nudiflora, were tested against the control solvent (Supplementary material Tables 1b, 2 and 3).
One leaf equivalent surface wax of either C. benghalensis or M. nudiflora was tested against individual or synthetic blends resembling in amounts as they were found in one leaf equivalent surface wax of either C. benghalensis or M. nudiflora (Supplementary material Table 1b).
Dose responses of individual synthetic compounds were done to find the lowest and highest doses, where the insect started to produce response and showed the highest attraction, respectively. Lema praeusta was attractive to docosane, tricosane, pentacosane, and heptacosane, resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis; whereas the insect was attracted to tridecanoic acid, palmitoleic acid, linoleic acid, and arachidic acid resembling in amounts as present in one leaf equivalent surface wax of M. nudiflora. Therefore, dose–response bioassays of L. praeusta to the above eight compounds were tested against the control solvent [docosane: 8, 16, and 32 µg/ml petroleum ether; tricosane: 5, 10, and 20 µg/ml petroleum ether; pentacosane: 10, 20, and 40 µg/ml petroleum ether; heptacosane or tridecanoic acid or palmitoleic acid: 2.50, 5, and 10 µg/ml petroleum ether; linoleic acid: 2, 4, and 8 µg/ml petroleum ether, and arachidic acid: 1.5, 3, and 6 µg/ml petroleum ether] (for preparation of different doses see Supplementary material S2).
Oviposition assays
Ten square glass chambers (15 × 15 cm2) were used for oviposition assays, and coarse grade emery papers were placed along sides and bottom of each glass jar to prevent egg laying on the wall and floor of glass jar. During oviposition assays, females were without leaves, but a cotton piece soaked with sucrose solution was provided in a Petri dish (3 cm diameter). Filter papers (Whatman No. 41) of 2 × 2 cm2 sizes were used for oviposition assays. Initially, females did not lay eggs on filter papers without or with the control solvent (petroleum ether). One ml of a test sample and the control solvent were applied to separate filter paper pieces and allowed to evaporate the solvent under fume hood, and these filter papers were separately placed in two round Petri dishes (each Petri dish 3 cm diameter). One filter paper containing a test sample in a Petri dish and the other filter paper containing the control solvent in another Petri dish were placed with a gap of 8 cm inside an experimental glass jar (15 × 15 cm2), and a mated female was released in the experimental glass jar. At least, ten mated females were separately used for each experiment. Mated females lay 2–6 eggs on a leaf for the first time between 24 h and 32 h after mating (personal observation). After mating, each female was separately kept for 24 h in a glass jar (8 cm diameter × 10 cm length) followed by placing of the individual in an experimental glass chamber (15 × 15 cm2) for 8 h, and when a mated female laid eggs for the first time, then the insect was discarded (personal observation). If a female did not lay eggs between 24 h and 32 h after mating, the insect was also discarded. The following combinations were tested during oviposition assays.
-
(1)
A single leaf of C. benghalensis versus a single dewaxed leaf of C. benghalensis, and a single leaf of M. nudiflora versus a single dewaxed leaf of M. nudiflora (for dewaxing of leaves, a single leaf was dipped in 30 ml of n-hexane for 1 min at room temperature for extraction of surface waxes) were tested to find whether leaf surface waxes of both weeds were acting as stimulant for egg laying by females.
-
(2)
One leaf equivalent surface wax (crude extract) of either C. benghalensis or M. nudiflora versus the control solvent (petroleum ether) were tested to find whether crude surface waxes from leaves of both weeds were acting as oviposition stimulant in L. praeusta.
-
(3)
One leaf equivalent surface wax of C. benghalensis versus one leaf equivalent surface wax of M. nudiflora was tested to find whether leaf surface wax of a particular weed was more preferred by L. praeusta for oviposition.
-
(4)
To find the role of synthetic blends as oviposition stimulant in L. praeusta, a synthetic blend of four compounds (14.54 µg docosane + 11.02 µg tricosane + 17.99 µg pentacosane + 5.92 µg heptacosane was dissolved in 1 ml of petroleum ether, as the insect showed the highest attraction to the above-mentioned synthetic blend of four compounds against the control solvent in the Y-tube olfactometer bioassay) resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis was tested against the control solvent.
Another synthetic blend of four compounds (4.38 µg tridecanoic acid + 4.83 µg palmitoleic acid + 3.44 µg linoleic acid + 2.31 µg arachidic acid were dissolved in 1 ml of petroleum ether, as the insect showed the highest attraction to the above-mentioned synthetic blend of four compounds against the control solvent in the Y-tube olfactometer bioassay) resembling in amounts as present in one leaf equivalent surface wax of M. nudiflora was tested against the control solvent.
-
(5)
To find whether synthetic blends could act as oviposition stimulant in L. praeusta, one leaf equivalent surface wax of C. benghalensis or M. nudiflora versus a synthetic blend resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis or M. nudiflora (C. benghalensis: 14.54 µg docosane + 11.02 µg tricosane + 17.99 µg pentacosane + 5.92 µg heptacosane dissolved in 1 ml of petroleum ether or M. nudiflora: 4.38 µg tridecanoic acid + 4.83 µg palmitoleic acid + 3.44 µg linoleic acid + 2.31 µg arachidic acid dissolved in 1 ml of petroleum ether) were tested.
Statistical analyses
Data on total amounts of surface waxes, alkanes and free fatty acids, and amounts of individual alkanes and free fatty acids present in C. benghalensis and M. nudiflora leaves were normally distributed as determined by Levene’s test for homogeneity of variance (Supplementary Table 4). Data on total amounts of surface waxes, alkanes, and free fatty acids were subjected to Student’s t test. The principal component analysis (PCA) was conducted to show differences between the chemical composition of C. benghalensis and M. nudiflora using individual alkanes and free fatty acids as variables (XLSTAT version 13). Data on behavioral responses of 90 L. praeusta females (number of insects showed a positive or negative choice) to a test sample were analyzed based on the null hypothesis that the probability of scores for the test compound(s) or control solvent is equal to 50%, i.e., Chi square analysis (H0: P = 50%) (Adhikary et al. 2015; Sarkar et al. 2017; Karmakar et al. 2018). Insects that did not respond by selection either arm of the olfactometer were excluded from the analyses.
Results
Composition of n-alkanes and free fatty acids in leaf surface waxes of C. benghalensis and M. nudiflora weeds
The n-hexane extracts of mature C. benghalensis and M. nudiflora leaves yielded 1.53 ± 0.02 and 1.39 ± 0.02 mg/g leaf fresh weight (mean ± SE) surface waxes, respectively, which was significantly higher in C. benghalensis compared to M. nudiflora (t = 6.105, P < 0.05). Among the total amounts of leaf surface waxes, alkanes represented for 70.81% (1.09 ± 0.06 mg/g fresh weight, mean ± SE) and 58.70% (0.82 ± 0.05 mg/g fresh weight, mean ± SE) in C. benghalensis and M. nudiflora, respectively, while free fatty acids accounted for 6.81% (0.10 ± 0.01 mg/g fresh weight, mean ± SE) and 16.34% (0.23 ± 0.01 mg/g fresh weight, mean ± SE) in C. benghalensis and M. nudiflora leaves, respectively, with the balance [C. benghalensis: 22.38% (0.34 ± 0.04 mg/g fresh weight, mean ± SE); M. nudiflora: 24.96% (0.35 ± 0.03 mg/g fresh weight, mean ± SE)] consisting of unidentified surface wax compounds.
For C. benghalensis, the first two principal components, PC1 and PC2, represented for 51.17% and 48.83% of the total variance, respectively (Fig. 1a); whereas, for M. nudiflora, PC1 and PC2, represented for 53.82% and 46.18% of the total variance, respectively (Fig. 1b). Except for hexadecane (n-C16), docosane (n-C22) and heptacosane (n-C27), where no differences in terms of their relative abundance were observed in biplots between C. benghalensis and M. nudiflora, rest of the alkanes and free fatty acids were different in terms of their relative abundances between C. benghalensis and M. nudiflora (Fig. 1a, b).
The total amount of alkanes was 1.33-fold higher in leaf surface waxes of C. benghalensis compared to M. nudiflora (t = 3.499, P < 0.05). The identified n-alkanes in leaf surface waxes of C. benghalensis and M. nudiflora represented for 93.19% (1.01 ± 0.06 mg/g leaf fresh weight, mean ± SE) and 97.42% (0.79 ± 0.05 mg/g leaf fresh weight, mean ± SE) of total alkanes, respectively (Table 1), while the balance consisted of unidentified branched-chain alkanes [C. benghalensis: 6.81% (0.07 ± 0.003 mg/g leaf fresh weight, mean ± SE); M. nudiflora: 2.58% (0.02 ± 0.001 mg/g leaf fresh weight (mean ± SE)]. Twenty n-alkanes between n-C14 and n-C36 were detected in leaf surface waxes of both weeds (Table 1). Pentacosane (n-C25) was predominant among alkanes in both weeds, representing for 13.86% and 14.14% of total alkanes in leaf surface waxes of C. benghalensis and M. nudiflora, respectively. Eicosane (n-C20), the second most abundant alkane, represented for 12.55% and 12.82% of total alkanes in leaf surface waxes of C. benghalensis and M. nudiflora, respectively. However, amounts of n-C25 and n-C20 were 1.3-fold higher in leaf surface waxes of C. benghalensis compared to M. nudiflora. Hexacosane (n-C26) was identified in the least amount (0.22%) in C. benghalensis, but it was 0.55-fold higher in leaf surface waxes of C. benghalensis compared to M. nudiflora. Octacosane (n-C28) was at the lowest level (0.24%) in M. nudiflora, but it was 1.35-fold higher in leaf surface waxes of C. benghalensis compared to M. nudiflora.
The total amount of free fatty acids was 2.18-fold higher in leaf surface waxes of M. nudiflora compared to C. benghalensis (t = − 7.705, P < 0.05). Thirteen free fatty acids between C12:0 and C22:0 were identified in leaf surface waxes of C. benghalensis and M. nudiflora (Table 2). Palmitoleic acid (C16:1) was the most abundant among free fatty acids in both weeds, accounting for 19.23% and 19.05% of total free fatty acids in leaf surface waxes of C. benghalensis and M. nudiflora, respectively. However, the amount of C16:1 fatty acid was 2.16-fold higher in leaf surface waxes of M. nudiflora compared to C. benghalensis. The amounts of lauric acid (C12:0), tridecanoic acid (C13:0), pentadecanoic acid (C15:0), palmitic acid (C16:0), heptadecanoic acid (C17:0), linoleic acid (C18:2), oleic acid (C18:1), arachidic acid (C20:0), heneicosanoic acid (C21:0), and docosanoic acid (C22:0) were 2.21-, 2.08-, 2.13-, 2-, 2.25-, 2.30-, 2.02-, 2.40-, 2.07-, and 2.32-fold higher in leaf surface waxes of M. nudiflora compared to C. benghalensis. Heptadecanoic acid (C17:0) was the least abundant, accounting for 0.03% of total free fatty acids in leaf surface waxes of both C. benghalensis and M. nudiflora.
Dual choice bioassays with L. praeusta females
Lema praeusta females did not show any bias towards filter papers (χ2 = 0.04; df = 1; P = 0.8339), suggesting that the insect was neutral to the control solvent (petroleum ether).
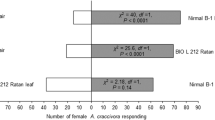
Both males and mated females were equally attracted towards one leaf equivalent surface wax of C. benghalensis against the control solvent (χ2 = 16.04; df = 1; P < 0.0001) (Fig. 2). Similarly, both males and mated females were equally attracted towards one leaf equivalent surface wax of M. nudiflora against the control solvent (χ2 = 12.84; df = 1; P < 0.001) (Fig. 2). These findings suggested that behavioral responses of males and mated females were similar. Therefore, rest bioassays were conducted with mated females.
Behavioral responses Lema praeusta females to one leaf equivalent surface wax of Commelina benghalensis or Murdannia nudiflora against the control solvent (petroleum ether), and C. benghalensis versus M. nudiflora in the Y-tube olfactometer bioassay. Numbers in brackets are the number of insects that did not respond to either treatment
Among all identified alkanes and free fatty acids in leaf surface waxes of C. benghalensis, the insect showed behavioral responses to eight individual synthetic compounds [eicosane (χ2 = 3.6; df = 1; P = 0.0578), docosane (χ2 = 7.5; df = 1; P = 0.0061), tricosane (χ2 = 6.4; df = 1; P = 0.0114), pentacosane (χ2 = 6.4; df = 1; P = 0.0114), heptacosane (χ2 = 8.71; df = 1; P = 0.0032), hentriacontane (χ2 = 1.6; df = 1; P = 0.2059), palmitoleic acid (χ2 = 0.04; df = 1; P = 0.8339), and stearic acid (χ2 = 0.18; df = 1; P = 0.6733)] resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis against the control solvent. This observation revealed that females showed attraction towards individual docosane, tricosane, pentacosane, and heptacosane against the control solvent control, and for this, a synthetic blend of these four compounds was tested. Females displayed attraction towards a synthetic blend of above eight compounds (χ2 = 14.4; df = 1; P = 0.0002) or a synthetic blend of four compounds (docosane, tricosane, pentacosane, and heptacosane) (χ2 = 11.38; df = 1; P = 0.0007) against the control solvent (Table 3).
Among all detected alkanes and free fatty acids in leaf surface waxes of M. nudiflora, the insect showed behavioral responses to 12 individual synthetic compounds [eicosane (χ2 = 0.4; df = 1; P = 0.5271), docosane (χ2 = 0.71; df = 1; P = 0.3991), tricosane (χ2 = 1.6; df = 1; P = 0.2059), pentacosane (χ2 = 2.18; df = 1; P = 0.14), heptacosane (χ2 = 0.18; df = 1; P = 0.6733), hentriacontane (χ2 = 0.04; df = 1; P = 0.8339), lauric acid (χ2 = 0.4; df = 1; P = 0.5271), tridecanoic acid (χ2 = 6.4; df = 1; P = 0.0114), palmitoleic acid (χ2 = 8.71; df = 1; P = 0.0032), linoleic acid (χ2 = 7.51; df = 1; P = 0.0061), stearic acid (χ2 = 0.18; df = 1; P = 0.6733), and arachidic acid (χ2 = 5.38; df = 1; P = 0.0204)] resembling in amounts as present in one leaf equivalent surface wax of M. nudiflora against the control solvent. This observation demonstrated that the insect was attractive to individual tridecanoic acid, palmitoleic acid, linoleic acid, and arachidic acid, and for this, a synthetic blend of these four compounds were tested. The insects were attracted towards a synthetic blend of 12 compounds (χ2 = 11.38; df = 1; P = 0.0007) or a synthetic blend of four compounds (tridecanoic acid, palmitoleic acid, linoleic acid, and arachidic acid) (χ2 = 10; df = 1; P = 0.0016) against the control solvent (Table 3).
The insect could not distinguish between one leaf equivalent surface wax of C. benghalensis and a synthetic blend of eight compounds (eicosane, docosane, tricosane, pentacosane, heptacosane, hentriacontane, palmitoleic acid, and stearic acid) (χ2 = 0.04; df = 1; P = 0.8339) (Table 4). Furthermore, the insect could not distinguish between one leaf equivalent surface wax of C. benghalensis and a synthetic blend of four compounds (docosane, tricosane, pentacosane, and heptacosane) (χ2 = 0.4; df = 1; P = 0.5271) (Table 4).
The insect could not discriminate between one leaf equivalent surface wax of M. nudiflora and a synthetic blend of 12 compounds (eicosane, docosane, tricosane, pentacosane, heptacosane, hentriacontane, lauric acid, tridecanoic acid, palmitoleic acid, linoleic acid, stearic acid, and arachidic acid) (χ2 = 0.18; df = 1; P = 0.6733) (Table 4). In addition, the insect could not differentiate between one leaf equivalent surface wax of M. nudiflora and a synthetic blend of four compounds (tridecanoic acid, palmitoleic acid, linoleic acid, and arachidic acid) (χ2 = 0.71; df = 1; P = 0.3991) (Table 4).
In dose-dependent bioassays, females were attracted to docosane at the minimal concentration of 16 µg/ml and, subsequently, showed the highest attraction at 32 µg/ml (Fig. 3a). Females showed gradual increase in attraction to tricosane from 10 to 20 µg/ml (Fig. 3b). Females displayed attraction to pentacosane between 20 and 40 µg/ml (Fig. 3c). Females were attracted to heptacosane, tridecanoic acid, and palmitoleic acid at the lowest concentration of 5 µg/ml, and showed the highest attraction at 10 µg/ml (Fig. 3d–f). Linoleic acid was attractive to the females between 4 and 8 µg/ml (Fig. 3g), while arachidic acid was attractive between 3 and 6 µg/ml (Fig. 3h).
Behavioral responses of Lema praeusta females to synthetic. a Docosane, b tricosane, c pentacosane, d heptacosane, e tridecanoic acid, f palmitoleic acid, g linoleic acid, and h arachidic acid against the control solvent (petroleum ether) in the Y-tube olfactometer bioassay. Numbers in brackets are the number of insects that did not respond to either treatment
Oviposition assays
-
(1)
Ten females laid 3.4 ± 0.43 eggs (mean ± SE) on a single leaf of C. benghalensis when tested against a single dewaxed C. benghalensis leaf (females did not lay egg on the dewaxed leaf); whereas ten females laid 3.1 ± 0.28 eggs (mean ± SE) on a single M. nudiflora leaf when tested against a single dewaxed M. nudiflora leaf (females did not lay eggs on the dewaxed leaf). The above observation suggested that females laid significantly more eggs on leaves containing surface waxes of both weeds (C. benghalensis: χ2 = 34.0, df = 1, P = 0.0001; M. nudiflora: χ2 = 31, df = 1, P = 0.0001) against dewaxed leaves, implicating that leaf surface waxes of both weeds acted as stimulant for egg laying in females.
-
(2)
Among ten mated females, seven females laid 2.5 ± 0.64 eggs (mean ± SE) on filter papers containing one leaf equivalent surface wax of C. benghalensis when tested against filter papers containing the control solvent (three females laid 0.8 ± 0.42 eggs, mean ± SE); whereas eight mated females laid 2.3 ± 0.52 eggs (mean ± SE) on filter papers containing one leaf equivalent surface wax of M. nudiflora when tested against filter papers containing the control solvent (two females laid 0.6 ± 0.43 eggs, mean ± SE). This study revealed that females laid significantly more eggs on crude surface waxes from leaves of both weeds (C. benghalensis: χ2 = 8.76, df = 1, P = 0.003; M. nudiflora: χ2 = 9.97, df = 1, P = 0.0016) compared to the control solvent.
-
(3)
Among ten mated females, five females laid 2.4 ± 0.88 eggs (mean ± SE) on filter papers containing one leaf equivalent surface wax of C. benghalensis when tested against filter papers containing one leaf equivalent surface wax of M. nudiflora (five females laid 2.1 ± 0.77 eggs, mean ± SE). This study revealed that females did not show significant preference for egg laying on crude surface wax of a particular weed when compared between crude leaf surface waxes of C. benghalensis and M. nudiflora (χ2 = 0.2, df = 1, P = 0.6547). This observation suggested that leaf surface waxes of both C. benghalensis and M. nudiflora weeds were equally acting as oviposition stimulant in L. praeusta.
-
(4)
Among ten mated females, seven females laid 2.2 ± 0.55 eggs (mean ± SE) on filter papers containing a synthetic blend of four compounds (14.54 µg docosane + 11.02 µg tricosane + 17.99 µg pentacosane + 5.92 µg heptacosane, as the insect showed attraction to the blend in the Y-tube olfactometer bioassay) resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis when tested against filter papers containing the control solvent (three females laid 0.6 ± 0.34 eggs, mean ± SE); whereas seven mated females laid 2.1 ± 0.59 eggs (mean ± SE) on filter papers containing a synthetic blend of four compounds (4.38 µg tridecanoic acid + 4.83 µg palmitoleic acid + 3.44 µg linoleic acid + 2.31 µg arachidic acid, as the insect showed attraction to the blend in the Y-tube olfactometer bioassay) resembling in amounts as present in one leaf equivalent surface wax of M. nudiflora when tested against filter papers containing the control solvent (three mated females laid 0.6 ± 0.31 eggs, mean ± SE). This study revealed that females laid significantly more eggs on synthetic blends resembling in amounts as present in one leaf equivalent surface wax of both weeds (C. benghalensis: χ2 = 9.14, df = 1, P = 0.002; M. nudiflora: χ2 = 8.33, df = 1, P = 0.004) compared to the control solvent.
-
(5)
In two choice assays, six mated females laid 1.9 ± 0.48 eggs (mean ± SE) on filter papers containing one leaf equivalent surface wax of C. benghalensis when tested against a synthetic blend of four compounds (14.54 µg docosane + 11.02 µg tricosane + 17.99 µg pentacosane + 5.92 µg heptacosane) resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis (four females laid 1.7 ± 0.3 eggs, mean ± SE). This study revealed that females did not show significant preference for egg laying between one leaf equivalent surface wax of C. benghalensis and a synthetic blend of four compounds (χ2 = 0.11, df = 1, P = 0.7389).
In two choice assays, five mated females laid 1.7 ± 0.62 eggs (mean ± SE) on filter papers containing one leaf equivalent surface wax of M. nudiflora when tested against a synthetic blend of four compounds (4.38 µg tridecanoic acid + 4.83 µg palmitoleic acid + 3.44 µg linoleic acid + 2.31 µg arachidic acid) resembling in amounts as present in one leaf equivalent surface wax of M. nudiflora (five females laid 1.4 ± 0.50 eggs, mean ± SE). This study revealed that females did not show significant preference for egg laying between one leaf equivalent surface wax of M. nudiflora and a synthetic blend of four compounds (χ2 = 0.2903, df = 1, P = 0.59).
Discussion
The present study demonstrated the presence of 20 n-alkanes from C14 to C36 and 13 free fatty acids from C12:0 to C22:0 in leaf surface waxes of both C. benghalensis and M. nudiflora weeds. n-Alkanes with chain lengths from C15 to C36 and free fatty acids from C12:0 to C22:0 were the most abundant components in leaf surface waxes of plants (Li and Ishikawa, 2006; Sarkar et al. 2014; Malik and Barik 2015; Malik et al. 2017; Mitra et al. 2017). This study revealed that n-alkane and free fatty acid compositions in leaf surface waxes of both C. benghalensis and M. nudiflora are overall similar with other plants. However, several studies indicated that different alkanes and free fatty acids were dominant in leaf surface waxes (van Maarseveen and Jetter 2009; Sarkar et al. 2013, 2014; Koukos et al. 2015; Karmakar et al. 2016; Malik et al. 2017). Tricosane and palmitic acid were predominant alkanes and free fatty acids, respectively, in surface waxes of L. octovalvis mature leaves (Mitra et al. 2017). Nonacosane (n-C29) and hexadecanoic acid were the most abundant n-alkanes and free fatty acids, respectively, in surface waxes of F. japonica mature leaves (Li and Ishikawa 2006). Pentacosane was dominant in leaf surface waxes of L. adscendens (L.) Hara (Barik et al. 2004). Palmitoleic acid was the most abundant in flower surface waxes of Polygonum orientale L. (Malik and Barik 2016). However, in the present investigation, pentacosane and palmitoleic acid were predominant alkane and free fatty acid in leaf surface waxes, respectively, in both C. benghalensis and M. nudiflora. The present study supports the hypothesis that the variation in the composition of leaf surface wax compounds might occur between plant species (Eigenbrode and Espelie 1995; Jetter et al. 2000; Piasentier et al. 2000; Dodoš et al. 2015).
Long-chain alkanes and free fatty acids present in leaf surface waxes serve important role in plant–insect interactions such as short-range attractant (Manosalva et al. 2011; Sarkar et al. 2013; Mukherjee et al. 2013, 2014; Malik and Barik 2015; Karmakar et al. 2016; Malik et al. 2017) and oviposition stimulant (Udayagiri and Mason, 1997; Parr et al. 1998; Grant et al. 2000; Li and Ishikawa 2006; Mitra et al. 2017). In the current study, olfactometer and oviposition bioassay results demonstrated that L. praeusta females were attracted towards one leaf equivalent surface wax of C. benghalensis and M. nudiflora against the control solvent (petroleum ether). However, the insect could not distinguish between one leaf equivalent surface wax of C. benghalensis and M. nudiflora, suggesting that one leaf equivalent surface wax of both weeds are equally attractive to the insect. Hence, this study suggests that host plant suitability as oviposition site in L. praeusta females involve the reception of long-chain alkanes and free fatty acids in leaf surface waxes of both C. benghalensis and M. nudiflora. Lema praeusta females employ a synthetic blend of docosane, tricosane, pentacosane, and heptacosane, resembling in amounts as present in one leaf equivalent surface wax of C. benghalensis for oviposition; whereas females employ a synthetic blend of tridecanoic acid, palmitoleic acid, linoleic acid, and arachidic acid, resembling in amounts as present in one leaf equivalent surface wax of M. nudiflora for oviposition. These compounds are found in leaf surface waxes of numerous plant species and contribute to the host suitability as short-range attractant and oviposition stimulant (Schoonhoven et al. 2005). Five long-chain alkanes, hexacosane, heptacosane, octacosane, nonacosane, and tritriacontane, resembling in amounts as present in waxes of corn leaves, acted as oviposition stimulant in Ostrinia nubilalis (Hübner) (Udayagiri and Mason 1997). Epilachna dodecastigma (Coleoptera: Coccinellidae) females displayed short-range attraction to a synthetic blend of nonadecane, eicosane, heneicosane, pentacosane, heptacosane, octacosane, nonacosane, hentriacontane, and tritriacontane, resembling in amounts as present in surface waxes of Momordica charantia L. leaves (Sarkar et al. 2013). Long-chain alkanes and free fatty acids from surface waxes resembling in amounts as present in khesari seed coats acted as short-range attractant to Callosobruchus maculatus (Fabricius) (Coleoptera: Bruchidae) (Adhikary et al. 2014, 2016). According to Parr et al. (1998), chickpea is less acceptable host for oviposition in C. maculatus compared to mung bean seeds due to lower amount of fatty acids in surface waxes of chickpea seeds, and subsequently, suggested that an appropriate mixture of fatty acids in epicuticular waxes stimulated oviposition in C. maculatus. Affixed natural ratio of different compounds in leaf surface waxes is important in chemical communication between plant and insect, and oviposition of the insect could disappear when the ratios of key compounds were replaced by different ratios of the same compounds (Udayagiri and Mason 1997; Parr et al. 1998; Grant et al. 2000; Li and Ishikawa 2006; Mitra et al. 2017). Hence, this study suggested that L. praeusta could recognize C. benghalensis and M. nudiflora leaves by ratios of different compounds present in leaf surface waxes of both weeds.
The current study concludes that one leaf equivalent surface waxes of C. benghalensis and M. nudiflora were attractive to the insect. A synthetic blend of either 14.54, 11.02, 17.99, and 5.92 µg/ml of docosane, tricosane, pentacosane and heptacosane, respectively, or 4.38, 4.83, 3.44, and 2.31 µg/ml of tridecanoic acid, palmitoleic acid, linoleic acid, and arachidic acid, respectively, could serve as close-range olfactory cues both for host recognition and acceptance process as well as for oviposition in L. praeusta females. This information could be used to effectively screen surface wax profile of other non-target plants for their susceptibility towards L. praeusta. Further investigations on behavioral responses of L. praeusta females to leaf surface waxes of other plants in the ecological context should be a topic for future research.
References
Adhikary P, Mukherjee A, Barik A (2014) Role of surface wax alkanes from Lathyrus sativus L. seeds for attraction of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J Stored Prod Res 59:113–119
Adhikary P, Mukherjee A, Barik A (2015) Attraction of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) to four varieties of Lathyrus sativus L. seed volatiles. Bull Entomol Res 105:187–201
Adhikary P, Mukherjee A, Barik A (2016) Free fatty acids from Lathyrus sativus seed coats acting as short-range attractants to Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). J Stored Prod Res 67:56–62
Ahmed S, Opena JL, Chauhan BS (2015) Seed germination ecology of doveweed (Murdannia nudiflora) and its implication for management in dry-seeded rice. Weed Sci 63:491–501
Barik A, Bhattacharya B, Laskar S, Banerjee TC (2004) The determination of n-alkanes in the cuticular wax of leaves of Ludwgia adscendens L. Phytochem Anal 15:109–111
Caton BP, Mortimer M, Hill JE, Johnson DE (2010) A practical field guide to weeds of rice in Asia. International Rice Research Institute, Philippines
Das S, Koner A, Barik A (2018) Biology and life history of Lema praeusta (Fab.) (Coleoptera: Chrysomelidae), a biocontrol agent of two Commelinaceae weeds, Commelina benghalensis and Murdannia nudiflora. Bull Entomol Res. https://doi.org/10.1017/S0007485318000731
Dodoš T, Rajčević N, Tešević V, Matevski V, Janaćković P, Marin PD (2015) Composition of leaf n-alkanes in three Satureja montana L. subspecies from the Balkan peninsula: ecological and taxonomic aspects. Chem Biodivers 12:157–169
Eigenbrode SD, Espelie KE (1995) Effects of plant epicuticular lipids on insect herbivores. Annu Rev Entomol 40:171–194
Grant GG, Zhao B, Langevin D (2000) Oviposition response of spruce budworm (Lepidoptera: Tortricidae) to aliphatic carboxylic acids. Environ Entomol 29:164–170
Harborne JB (1994) Introduction to ecological biochemistry. Academic Press, London
Holm LG, Plucknett DL, Pancho JV, Herberger JP (1977) The world’s worst weeds: distribution and biology. University Press of Hawaii, Honolulu
Isaac W-A, Gao Z, Li M (2013) Managing Commelina species: prospects and limitations. In: Price AJ, Kelton JA (eds) Herbicides-current research and case studies in use. Intech, Croatia, pp 543–562
Jetter R, Schaffer S, Riederer M (2000) Leaf cuticular waxes are arranged in chemically and mechanically distinct layers: evidence from Prunus laurocerasus L. Plant Cell Environ 23:619–628
Karmakar A, Malik U, Barik A (2016) Effects of leaf epicuticular wax compounds from Solena amplexicaulis (Lam.) Gandhi on olfactory responses of a generalist insect herbivore. Allelopathy J 37:253–272
Karmakar A, Mitra S, Barik A (2018) Systemically released volatiles from Solena amplexicaulis plant leaves with color cues influencing attraction of a generalist insect herbivore. Int J Pest Manag 64:210–220
Koukos D, Meletiou-Christou M-S, Rhizopoulou S (2015) Leaf surface wettability and fatty acid composition of Arbutus unedo and Arbutus andrachne grown under ambient conditions in a natural macchia. Acta Bot Gallica 162:225–232
Li G, Ishikawa Y (2006) Leaf epicuticular wax chemicals of the Japanese knotweed Fallopia japonica as oviposition stimulants for Ostrinia latipennis. J Chem Ecol 32:595–604
Malik U, Barik A (2015) Free fatty acids from the weed, Polygonum orientale leaves for attraction of the potential biocontrol agent, Galerucella placida (Coleoptera: Chrysomelidae). Biocontrol Sci Technol 25:593–607
Malik U, Barik A (2016) Volatiles and surface wax long-chain alkanes and free fatty acids from Polygonum orientale L. (Polygonaceae) flowers. Bot Lett 163:453–460
Malik U, Mitra S, Barik A (2017) Attraction of the biocontrol agent, Galerucella placida Baly (Coleoptera: Chrysomelidae) to the leaf surface alkanes of the weed, Polygonum orientale L. Allelopathy J 40:103–116
Manosalva L, Pardo F, Perich F, Mutis A, Parra L, Ortega F, Isaacs R, Quiroz A (2011) Behavioral responses of clover root borer to long-chain fatty acids from young red clover (Trifolium pratense) roots. Environ Entomol 40:399–404
Mitra S, Sarkar N, Barik A (2017) Long-chain alkanes and fatty acids from Ludwigia octovalvis weed leaf surface waxes as short-range attractant and ovipositional stimulant to Altica cyanea (Weber) (Coleoptera: Chrysomelidae). Bull Entomol Res 107:391–400
Mukherjee A, Barik A (2016) Long-chain primary alcohols in Momordica cochinchinensis Spreng leaf surface waxes. Bot Lett 163:61–66
Mukherjee A, Sarkar N, Barik A (2013) Alkanes in flower surface waxes of Momordica cochinchinensis influence attraction to Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae). Neotrop Entomol 42:366–371
Mukherjee A, Sarkar N, Barik A (2014) Long-chain free fatty acids from Momordica cochinchinensis leaves as attractants to its insect pest, Aulacophora foveicollis Lucas (Coleoptera: Chrysomelidae). J Asia Pac Entomol 17:229–234
Mukherjee A, Sarkar N, Barik A (2015) Momordica cochinchinensis (Cucurbitaceae) leaf volatiles: semiochemicals for host location by the insect pest, Aulacophora foveicollis (Coleoptera: Chrysomelidae). Chemoecology 25:93–104
Müller C (2006) Plant–insect interactions on cuticular surfaces. In: Riederer M, Muller C (eds) Biology of the plant cuticle. Blackwell Publishing, Oxford, pp 398–422
Müller C, Hilker M (2001) Host finding and oviposition behavior in a chrysomelid specialist—the importance of host plant surface waxes. J Chem Ecol 27:985–994
Padovan A, Keszei A, Köllner TG, Degenhardt J, Foley WJ (2010) The molecular basis of host plant selection in Melaleuca quinquenervia by a successful biological control agent. Phytochemistry 71:1237–1244
Parr MJ, Tran BMD, Simmonds MSJ, Kite GC, Credland PF (1998) Influence of some fatty acids on oviposition by the bruchid beetle, Callosobruchus maculatus. J Chem Ecol 24:1577–1593
Piasentier E, Bovolenta S, Malossini F (2000) The n-alkane concentrations in buds and leaves of browsed broadleaf trees. J Agric Sci 135:311–320
Piesik D, Wenda-Piesik A, Ligor M, Buszewski B, Delaney KJ (2012) Dock leaf beetle, Gastrophysa viridula Deg., herbivory on the mossy sorrel, Rumex confertus Willd: induced plant volatiles and beetle orientation responses. J Agric Sci 4:97–103
Sarkar N, Barik A (2014) Alkanes from bitter gourd as allelochemicals in olfactory responses of Epilachna dodecastigma (Wied.). Allelopathy J 33:43–51
Sarkar N, Barik A (2015) Free fatty acids from Momordica charantia L. flower surface waxes influencing attraction of Epilachna dodecastigma (Wied.) (Coleoptera: Coccinellidae). Int J Pest Manag 61:47–53
Sarkar N, Mukherjee A, Barik A (2013) Long-chain alkanes: allelochemicals for host location by the insect pest, Epilachna dodecastigma (Coleoptera: Coccinellidae). Appl Entomol Zool 48:171–179
Sarkar N, Malik U, Barik A (2014) n-Alkanes in epicuticular waxes of Vigna unguiculata (L.) Walp. leaves. Acta Bot Gallica 161:373–377
Sarkar N, Mukherjee A, Barik A (2015) Attraction of Epilachna dodecastigma (Coleoptera: Coccinellidae) to Momordica charantia (Cucurbitaceae) leaf volatiles. Can Entomol 147:169–180
Sarkar N, Mitra S, Barik A (2017) Momordica charantia L. (Cucurbitaceae) floral volatiles causing attraction of Epilachna dodecastigma (Coleoptera: Coccinellidae). Int J Pest Manag 63:138–145
Schoonhoven LM, van Loon JJA, Dicke M (2005) Insect-plant biology. Oxford University Press, Oxford
Smith L, Beck JJ (2013) Effect of mechanical damage on emission of volatile organic compounds from plant leaves and implications for evaluation of host plant specificity of prospective biological control agents of weeds. Biocontrol Sci Technol 23:880–907
Udayagiri S, Mason CE (1997) Epicuticular wax chemicals in Zea mays influence oviposition in Ostrinia nubilalis. J Chem Ecol 23:1675–1687
van Maarseveen C, Jetter R (2009) Composition of the epicuticular and intracuticular wax layers on Kalanchoe daigremontiana (Hamet et Perr. de la Bathie) leaves. Phytochemistry 70:899–906
Wheeler GS, Schaffner U (2013) Improved understanding of weed biological control safety and impact with chemical ecology: a review. Invasive Plant Sci Manag 6:16–29
Wilson AK (1981) Commelinaceae—a review of the distribution, biology and control of the important weeds belonging to this family. Trop Pest Manag 27:405–418
Acknowledgements
We thank anonymous reviewers for many helpful suggestions of earlier versions of the manuscript. We are thankful to Dr. Janakiraman Poorani, Principal Scientist, National Research Centre for Banana, Tamilnadu for identifying the insect, and Prof. Ambarish Mukherjee, Department of Botany, of this University for identification of these weeds. The financial assistance from the University of Burdwan as a JRF to Swati Das is gratefully acknowledged. We also thank DST PURSE Phase-II for providing necessary instrumental facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Marko Rohlfs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Das, S., Koner, A. & Barik, A. A beetle biocontrol agent of rice-field weeds recognizes its host plants by surface wax long-chain alkanes and free fatty acids. Chemoecology 29, 155–170 (2019). https://doi.org/10.1007/s00049-019-00285-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-019-00285-1