Abstract
Induction of plant defences can show various levels of localization, which can optimize their efficiency. Locally induced responses may be particularly important in large plants, such as trees, that show high variability in traits and herbivory rates across their canopies. We studied the branch-localized induction of polyphenols, volatiles (VOCs), and changes in leaf protein content in Carpinus betulus L., Quercus robur L., and Tilia cordata L. in a common garden experiment. To induce the trees, we treated ten individuals per species on one branch with methyl jasmonate. Five other individuals per species served as controls. We measured the traits in the treated branches, in control branches on treated trees, and in control trees. Additionally, we ran predation assays and caterpillar food-choice trials to assess the effects of our treatment on other trophic levels. Induced VOCs included mainly mono- and sesquiterpenes. Their production was strongly localized to the treated branches in all three tree species studied. Treated trees showed more predation events than control trees. The polyphenol levels and total protein content showed a limited response to the treatment. Yet, winter moth caterpillars preferred leaves from control branches over leaves from treated branches within C. betulus individuals and leaves from control Q. robur individuals over leaves from treated Q. robur individuals. Our results suggest that there is a significant level of localization in induction of VOCs and probably also in unknown traits with direct effects on herbivores. Such localization allows trees to upregulate defences wherever and whenever they are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants employ a bewildering diversity and variability of defence strategies. The efficiency of particular defences is highly dependent on the identity of herbivores and surrounding abiotic conditions (Defossez et al. 2018; Volf et al. 2018). Variability in defences and plasticity in their deployment thus help plants face a wide variety of challenges (Koricheva et al. 2004; Volf et al. 2019b). Induced defences are deployed in response to external stimuli, such as herbivory. They do not prevent the initial damage but can be upregulated when required, allowing plants to prioritize investment in defence over growth (Backmann et al. 2019). Such a strategy allows plants to respond to spatial and temporal changes in insect herbivore communities that require deploying specific defences whenever and wherever they are needed (Lämke and Unsicker 2018; Turlings and Erb 2018).
Induced defences involve several, partly complementary, mechanisms rendering them efficient against a broad spectrum of herbivores. After herbivore attack, plants can upregulate defences that are targeted directly at the herbivore. Direct defences involve increases in the levels of secondary metabolites, enzymes, trichome density or leaf toughness (Agrawal 1999; Barbehenn et al. 2009; Barton 2016). For example, induced increase of polyphenol oxidase (PPO) activity in mountain birch can promote resistance against Epirrita autumnata (Borkhausen) caterpillars (Ruuhola et al. 2008). Herbivory can also cause compensatory regrowth and shifts in leaf or stem nutrients, which makes the plant more or less attractive to herbivores (Utsumi and Ohgushi 2008). Such changes can be induced immediately or with a delay, which sometimes can be as long as the period until the following season (Rubert-Nason et al. 2015; Tuomi et al. 1988). In addition, plants can employ indirect defences, which attract predators and parasitoids. Indirect defences may involve the production of various volatile organic compounds (VOCs) (Clavijo McCormick et al. 2012; Dicke and Loon 2000; Turlings and Erb 2018). VOCs, such as terpenoids, or various green leaf volatiles including esters and alcohols, can be detectable by predators and parasitoids even in complex environments (Vet et al. 1991). VOCs thus may play a prominent role in complex environments such as tree canopies, as they may help predators and parasitoids navigate efficiently through the dense foliage towards their prey (Amo et al. 2013; Turlings and Erb 2018).
Induced defence responses can show various levels of localization, which helps to further optimize their efficiency (Eyles et al. 2010). After herbivore or pathogen attack, the strongest and fastest induced responses are commonly observed at the site of attack (Mason et al. 2017; Piggott et al. 2004; Tuomi et al. 1988). Mostly, plant defences are also induced at the level of the whole plant. Such a systemic response elevates defence levels in hitherto undamaged plant parts (Eyles et al. 2010; Kachroo and Robin 2013). The strength of the response at more distal plant parts depends on how they are connected to the phloem or on the distance from the attacked site if the induction relies on airborne signals (Heil and Ton 2008; Rubert-Nason et al. 2015; Viswanathan and Thaler 2004). Localized induced responses can be especially important in large plants, such as trees, as they can further help upregulate the defences where they are currently needed and can reduce resource investments in defence (Lämke and Unsicker 2018; Volf et al. 2020). Saving on the costs may be especially important in the case of energetically demanding chemical defences (Mason et al. 2017). Furthermore, as trees are large, they are more likely to experience localized herbivory (Mason et al. 2017), partly due to their complex architecture and spatial variation in their traits. Indeed, there is substantial variation in leaf traits, abiotic factors, and the number and types of biotic interactions among different sections of the canopy (Lämke and Unsicker 2018; Murakami et al. 2005; Rubert-Nason et al. 2015). For example, young and light-exposed leaves are more valuable for the plant because they are photosynthetically active. At the same time, such leaves are usually more attractive for herbivores due to their higher nutritive quality and lower lignin content (Schultz and Baldwin 1982). Allocating induction of defences primarily to parts of the canopy bearing young or photosynthetically active foliage can be beneficial for trees (Lämke and Unsicker 2018).
Induced responses in trees are highly variable among species, individuals and their parts (Lämke and Unsicker 2018; Neuvonen et al. 1987; Rubert-Nason et al. 2015). They range from being systemic to highly localized in individual leaves or needles (Bonello and Blodgett 2003; Eyles et al. 2010; Piggott et al. 2004; Rubert-Nason et al. 2015). Both systemic and highly localized induced responses may have direct and indirect effects on herbivores (Eyles et al. 2010; Lämke and Unsicker 2018). We hypothesize that localization of induced responses in individual branches (e.g. Tuomi et al. 1988) may be especially important in trees. First, it can help trees cope with the variation in abiotic and biotic factors within their canopy (Lämke and Unsicker 2018; Volf et al. 2020). Second, it combines the energy-saving benefits of localized upregulation of defences with the benefits of upregulating defences in large enough modules. The latter is important to protect the attacked canopy section against both sessile and mobile herbivores, while efficiently attracting their natural enemies to the damaged area.
In order to explore the extent and importance of branch-localized induction, we studied methyl jasmonate-induced responses in polyphenols, VOCs, and changes in nutritional value (leaf protein content) in a common garden experiment. We compared the responses among Carpinus betulus L. (European hornbeam), Quercus robur L. (common oak), and Tilia cordata Mill. (small-leaved lime) individuals to see if they are consistent among the three species. We supplemented the chemical analysis with predation assays, using clay caterpillars, and food-choice trials with generalist winter-moth caterpillars (Operophtera brumata). Combined, these experimental data allowed us to assess whether the induced responses had relevant ecological consequences. We expected (i) branch-localized induction in all traits we measured, (ii) increased predation rates on induced trees, and (iii) lower preference of caterpillars for leaves from induced branches.
Methods and Materials
The experiment was conducted between 10 May and 11 June 2018 in the Research Arboretum Großpösna (51°15’41"N, 12°29’55"E, ca. 160 m a.s.l.) on an experimental plot that was set up by Ohse (2018). The plot includes 24 tree species with 32 replicates per species. The trees were planted in the winter of 2013/14 as two-years old saplings in a randomized grid design with 1.0 m gaps between individual plants. We selected 15 individuals of Carpinus betulus L. (average height (h) = 3.4 m, average diameter at breast height (DBH) = 1.6 cm), 15 individuals of Quercus robur L. (h = 3.0 m, DBH = 1.6 cm) and 15 individuals of Tilia cordata Mill. (h = 3.5 m, DBH = 1.9 cm) for our experiment. The closest distance between the selected neighbouring trees was 1.0–4.0 m. We avoided neighbouring conspecific trees and trees growing directly at the margins of the plot to avoid edge effects. All trees were treated with Promanal insecticide (Progema, Aerzen, Germany) on 15 March 2018 and with Spruzit insecticide (Neudorff, Emmerthal, Germany) on 25 April 2018 to minimize variation between the experimental trees due to naturally occurring herbivory prior to the experiment.
Induction Experiment
We randomly selected 5 individuals per tree species as controls and 10 individuals per species for the induction treatment. We selected three major branches in each tree (Fig. 1). The middle branches on the treated trees were used for an induction treatment (hereafter referred to as treated branches on treated trees - TT). The other two branches on the treated trees and all three branches on the control trees were used as two types of control (hereafter referred to as control branches on treated trees - CT and control branches on control trees - CC). This experimental set-up allowed us to distinguish between localized (upregulation in TT only) and systemic induction (upregulation in both TT and CT). The selected branches were 68–201 cm above ground level and were shaded by surrounding trees. In each branch, we selected its terminal part to be used in the experiments and for measurements of induced responses. The terminal parts were ca. 50 cm long and had similar numbers of leaves when compared among conspecifics (C. betulus – 45 ± 17 leaves, Q. robur – 25 ± 14 leaves, T. cordata – 31 ± 10 leaves, values are mean ± SD). We measured the shortest distance between the studied branches (as the distance between their closest leaves on a straight line) and their distance over the trunk (as their distance over trunk and basal parts of the respective branches).
Experimental design and timeline. We selected 15 trees per species. Ten were subjected to our treatment, five were used as controls. In each tree, we selected three major branches. The middle branches on the treated trees were treated with MeJA (TT branches). The other two branches on the treated trees and all three branches on the control trees were used as two types of control (CT and CC branches). The example of our timeline gives an overview of the first nine days of our experiment. The experiment started with an induction treatment followed by the first VOC sampling. After that, we repeated the induction treatment before placing clay caterpillars to measure predation rates. The caterpillars were placed for 48 hours. Then they were checked for predation marks, the induction was repeated, and the caterpillars were placed again
We treated TT branches with methyl jasmonate (MeJA). MeJA is commonly used to simulate herbivory damage by chewing insects in ecological experiments (e.g. Mrazova and Sam 2018). MeJA typically induces a broad and largely unspecific spectrum of responses. While it does not provide the same level of specificity and intensity of induction as a treatment with real herbivory, it allows for relative comparisons between plants or their parts. It also allows to induce plant responses in a standardized manner across a large number of branches and can reduce potential variation in induced responses due to naturally occurring herbivory (Klimm et al. 2020). Here, we use it as a proxy for comparing general inducibility and the level of localization in induction in the three tree species and their branches.
We enclosed TT branches in clear 45 × 55 cm polyamide bags (Studio Cook BV, Zeewolde, NL) and put 9 µl of MeJA (Purity 95% Sigma-Aldrich, St. Louis, Missouri, US) on a cotton ball (Hartmann, Heidenheim, DE) inside. CT and CC branches received bags with cotton without MeJA. We left the branches enclosed in the bags for 24 hours. We then removed the bags and measured volatiles or placed clay caterpillars for predation experiments (Fig. 1). The induction treatment was repeated 11 times, typically at three-day intervals to allow enough time for the predation experiment and to explore changes over time. Only after the first VOC sampling, we repeated the induction at a shorter interval before we placed clay caterpillars on the branches (Fig. 1).
The experiment started on 10 May and was carried out until 11 June 2018. By that time, we observed that some of the trees tend to suffer from severe water stress because of the drought that affected the region in 2018. Therefore, we selected 8 tree individuals per species without obvious drought stress symptoms (5 treated and 3 control individuals), re-measured their volatile production, and stopped all experimental work in the field to avoid the effects of drought on our results.
VOC Sampling and Analysis
We passively sampled VOCs using polydimethylsiloxane (PDMS) tubes (Carl Roth GmbH, Karlsruhe, Germany) from all studied branches following Kallenbach et al. (2014) to compare their production. We placed two clean 1.5 cm PDMS cuttings (technical replicates) on a stainless-steel wire, attached them to the measured branch and enclosed the branch and tubes in a 45 × 55 cm polyamide bag. The VOCs were passively adsorbed to the PDMS cuttings from the headspace for 24 hours. We carried out the sampling twice: after the first induction treatment (initial sampling; all trees sampled) and approx. one month later after 11 induction treatments (final sampling; eight trees per species sampled) once the experiment came to a halt because of the drought. In both cases, we waited ca. 30 minutes after removing the bags used for the induction treatment before we put new bags on the branches to sample VOCs.
We performed gas chromatography to quantify the sampled volatiles. The PDMS cuttings were analysed by a thermal desorption-gas chromatograph-mass spectrometer (TD-GC-MS). The TD-GC-MS consisted of a thermodesorption unit (MARKES, Unity 2, Llantrisant, UK) and an autosampler (MARKES, Ultra 50/50). PDMS cuttings were transferred to empty stainless steel tubes (MARKES), and then desorbed with helium as carrier gas and a flow path temperature of 160 °C using the following conditions: Dry Purge 5 min at 20 ml/min and Pre Purge 2 min at 10 ml/min to remove remaining water, Desorption 8 min at 200 °C with 60 ml/min, Pre Trap fire purge 1 min at 60 ml/min, Trap heated from 0 to 300 °C at maximum speed and hold for 4 min. The VOCs were separated on a gas chromatograph (Bruker, GC-456, Bremen, Germany) connected to a triple-quad mass spectrometer (Bruker, SCION) equipped with a DB-WAX column: (30 m x 0.25 mm inner diameter x 0.25um film thickness, Restek). The temperature program was set to the following: 60 °C (hold 2 min), 30 °C/min to 150 °C, 10 °C/min to 200 °C and 30 °C/min to 230 °C (hold 5 min). Helium was used as carrier gas at a constant flow rate of 1 ml/mi. MS conditions were set to 40 °C for the manifold, 240 °C at the transfer line and 220 °C for the ion source. The scan-range was 33 − 500 m/z for a full scan and scan-time was 250 ms. We selected the most prominent peaks in the chromatograms and set signal to noise ratio to > 10. Peaks that were also present in the chromatograms of empty stainless-steel tubes were regarded as systemic contamination and were excluded from further analysis. VOCs that responded to our treatment were tentatively identified by comparison to the NIST database and comparison to retention indices from the literature. The peak areas of these compounds were calculated using the Bruker Workstation software (v8.0.1).
Measurements of Polyphenols and Protein Content
We sampled leaves from the studied branches to quantify induced changes in polyphenols, protein content, and leaf palatability to caterpillars (see Caterpillar choice assays below). Using a scalpel to minimize the wound, we sampled the first two youngest fully developed leaves from all TT branches, upper CT branches, and middle CC branches (a single leaf per branch was sampled in the case of Q. robur, which had sufficiently large leaves). The leaves were sampled after four (T. cordata) or five (C. betulus and Q. robur) induction treatments (10 and 14 days after the first treatment, respectively) in order to time their sampling with the caterpillar hatching in our colony.
Half of the sampled leaf material (avoiding the central vein) was freeze-dried and homogenized to analyse polyphenol profiles and protein content. Polyphenols were extracted from ca. 20 mg (in 0.01 mg accuracy) of homogenized material using 80:20 (v/v) acetone/water solvent as described in detail in Malisch et al. (2016). We ran two separate sets of assays to analyse polyphenols. Firstly, we quantified total content for each of the main polyphenol sub-groups (in mg/g) by UPLC-QqQ-MS/MS with the methods of Engström et al. (2014; 2015) as described in e.g. Malisch et al. (2016). With these methods polyphenols are first separated by UPLC and then each polyphenol is fragmented in the MS ion source to produce compound group-specific fragments that are detected by the group-specific MS/MS methods created for each polyphenol group separately (see Salminen (2018) for further details). This technology enables even the determination of the sub-unit composition (procyanidin and prodelphinidin units) and molecular size (mean degree of polymerization) of polymeric proanthocyanidins. The measured polyphenol sub-groups included (1) galloyl and hexahydroxydiphenoyl (ellagitannins, HHDP) units found in hydrolysable tannins, (2) procyanidin and prodelphinidin units found in proanthocyanidins, (3) kaempferol, quercetin and myricetin units found in flavonol glycosides, and (4) quinic acid units found in quinic acid derivatives such as caffeoyl and coumaroyl quinic acids. Compound groups were quantified by using pentagalloylglucose (galloyl units), tellimagrandin I (HHDP units), kaempferol-3-O-glucoside (kaempferol units), quercetin-3-O-glucoside (quercetin units), myricetin-3-O-rhamnoside (myricetin units), purified procyanidin-rich proanthocyanidin fraction (procyanidin units), purified prodelphinidin-rich proanthocyanidin fraction (prodelphinidin units), and chlorogenic acid (quinic acid units) as external standards. Secondly, we ran two activity assays to quantify the two major functions of polyphenols in anti-herbivore protection – oxidative activity and protein precipitation capacity. Polyphenol oxidative activity was measured following Salminen & Karonen (2011) using gallic acid as the standard. Protein precipitation capacity was measured following Hagerman’s radial diffusion assay (Hagerman and Butler 1978) using pentagalloylglucose as the standard. Both assays gave activities in the unit of mg/g.
The protein content was measured with Bradford colorimetric assay (Kruger 2009). We extracted the proteins from 3 to 6 mg of the dried leaf material with TCA/acetone solution following Jorge et al. (2005). We dissolved the protein in 200 µl 8M Urea/2% Chaps (Carl Roth GmbH, Karlsruhe, Germany) and added 34 µl of this solution to 1 ml of Bradford reagent solution and measured the protein content using V-630 UV-Vis spectrophotometer (Jasco, Easton, MD, USA).
Predation Levels
We used clay caterpillars to estimate changes in predation rates in response to the MeJA treatment. This method is widely used to estimate predation rates by both vertebrate and invertebrate predators. Although it has some limitations (i.e. plasticine caterpillars are immobile, they do not produce the same cues as real lepidopteran larvae etc.) and may inflate real absolute predation rates, this method provides conservative estimates of relative predation pressure comparable across treatments (Howe et al. 2009). It allows placing large number of replicates and leaving them in the field for several days, which is particularly important in temperate habitats where the predation rates are generally low. We prepared the caterpillars (3 mm in diameter, 3 cm in length) using non-toxic Newplast plasticine (Newclay Products, Newton Abbot, UK). Within each tree, we attached 5 caterpillars to twigs with Loctite 401 super glue (Henkel, Düsseldorf, DE) (1 to each experimental branch and 2 to additional branches; 225 clay caterpillars placed in total) following each induction treatment. The caterpillars were first placed on 13 May. They were exposed to predators for 48 hours, checked for predation marks before the next induction treatment following Howe et al. (2009). Damaged caterpillars were either replaced or fixed. The last checking was on 9 June. We classified the predation marks as bird or invertebrate predation. Marks caused by surrounding twigs and leaves or by invertebrates which do not prey on caterpillars (i.e. snails) were excluded.
We noticed that predation rates inside our experimental plot, where the canopy density reduced light availability, were low. Therefore, we placed 75 additional clay caterpillars on the branches at the margin of the plot for a comparison. The caterpillars were placed in ca. 2 m intervals, 1.5 m above ground level on randomly selected trees of various species. The clay caterpillars were checked in the same intervals and on the same days as the caterpillars inside the plot. As the decision to place these caterpillars was made directly before the experiment started, we did not have a chance to treat these trees with insecticides. We thus avoided placing the clay caterpillars on trees showing high herbivory damage to lower possible effects of naturally occurring herbivory. We compared the light intensity between the trees inside the plot and on the margin at 1.2 m height above ground using Quantum Sensor LI-190 (LI-COR, Lincoln, Nebraska, USA) on 6 June 2018 between 9:30 and 10:15 when the sky was clear.
We temporally ceased both the induction treatment and clay caterpillar exposure for a period of three days (22– 24 May 2018) to conduct the food-choice trials with O. brumata caterpillars.
Caterpillar Choice Assays
We used the second half of the sampled leaf material for food-choice trials to explore the effects of induction treatment on the leaf palatability to winter moth caterpillars (Operophtera brumata L.). O. brumata is a polyphagous species feeding on all three species studied and it commonly occurs in the region. O. brumata females were obtained in November 2017 near České Budějovice, CZ. After oviposition, the eggs were stored at 2 °C to overwinter. The eggs were transferred to 14 °C 14:10 light conditions at the end of April. The larvae fed on T. cordata leaves until their last instar. Since their previous experience with non-treated T. cordata leaves could affect their preference, we removed T. cordata from the food-choice trials and used C. betulus and Q. robur only. To run the two-choice trials with O. brumata caterpillars, we prepared leaf discs of 1.5 cm in diameter from the sampled leaves, while avoiding the mid vein. In a ‘branch comparison’, each larva was offered a pair of discs from a TT branch and a CT branch from the same tree. We repeated food-choice trials for each treated Carpinus and Quercus individual twice (40 last-instar larvae used in total). In a ‘tree comparison’, each larva was offered a pair of discs from a TT branch and a CC branch on a control tree. Each control tree was compared to four randomly selected treated trees (40 last-instar larvae used in total). All larvae were allowed to feed for 7 hours. Then the leaf discs were photographed and the remaining leaf area was measured in imageJ (Abràmoff et al. 2004).
Statistical Analysis
First, we analysed the effect of our induction treatment on the production of VOCs during the initial and final sampling for each tree species separately. We used Principal Component Analysis (PCA) to explore the variation in the overall VOC profiles among the studied branches. We then tested the effect of our treatment on the VOC profiles with Redundancy Analysis (RDA). We used the treatment (TT, CT, and CC) as explanatory variable and tree individual as a covariable defining the permutation blocks. The significance of all canonical axes was tested using 9999 permutations and the explained variability was adjusted following ter Braak and Smilauer (2012). The multivariate analyses were performed in CANOCO 5 (ter Braak and Smilauer 2012). Furthermore, we used Linear Mixed Effect Models (LMEs) in R 3.6.1 (R Core Team 2019) using ‘lme4’ package (Bates et al. 2015) to confirm the trends in individual volatiles. We used the treatment as a fixed effect and tree individual as a random factor. Due to the high number of individual compounds involved in the comparisons, we applied Bonferroni correction to the results of LME models comparing production of individual VOCs across the treatments. We used log-transformed peak areas representing individual compounds in all analyses. In case we found a significant treatment effect, we performed a post-hoc test using the function ‘difflsmeans’ in ‘lmerTest’ R package (Kuznetsova et al. 2017), specifically looking for locally or systemically induced compounds. We used the following definitions of locally and systemically induced compounds based on their production by the studied branches:
Furthermore, we used LMEs to test if the total VOC production in CT branches was correlated with their distance from TT branches. We used the shortest distance between the branches and their distance over trunk as a fixed effects and tree identity as a random factor in our LMEs.
Second, we analysed if the total polyphenol content, polyphenol diversity (measured as Shannon-index based on the content of individual sub-groups), production of individual polyphenol sub-groups, polyphenol protein precipitation capacity, polyphenol oxidative activity, and total protein content differed between CC, CT, and TT branches using LMEs. We performed the analysis for each tree species separately and used tree individual as a random factor. In case we found a significant effect of the treatment, we performed a post-hoc test using the function ‘difflsmeans’ (Kuznetsova et al. 2017). We used log-transformation to normalize polyphenol content and activity data (in mg/g). We used arcsine transformation to normalize the relative share of procyanid and prodelphinidin units of proanthocyanidins (in %).
Third, we tested if the treatment had any effect on predation rates. We compared the total number of predated clay caterpillars between the treated and control trees within our plot and between CT and TT branches using linear models and LMEs, respectively. When comparing the treated and control trees, we summarized the total number of predation events from all branches in individual trees and log-transformed it. We then created a null model including the tree species (to account for its effect) and compared it with a model including both the tree species and induction treatment as explanatory variables based on their AIC. When comparing CT and TT branches with LMEs, we compared a null model including the tree species as a fixed effect with a model including both the tree species and treatment as fixed effects based on their AIC. Both the null and tested model included the tree individual as a random factor. Furthermore, we calculated the average predation rates over 48 hours for caterpillars placed inside our plot and on its margin to see if there was any difference in predation rates.
Fourth, we analysed the effects of the treatment on caterpillar preference in the food-choice trials. For each leaf disc, we quantified the proportion of its area eaten and normalized it with arcsine transformation. The proportion of disc area eaten was compared among the treatments using LMEs. In ‘branch comparison’, we used branch treatment (TT vs. CT) as a fixed effect, and the tree individual and trial (to link the discs from same trials) as nested random factors. In ‘tree comparison’, we used tree treatment (TT vs. CC) as a fixed effect and the control tree individual involved in the particular comparison and trial as nested random factors. We analysed the results for C. betulus and Q. robur separately.
Results
All together, we analysed 27 volatiles in Carpinus betulus, 59 volatiles in Quercus robur, and 46 volatiles in Tilia cordata (Table S1). Most of the classified volatiles belonged to the mono- and sesquiterpenes, although other classes were also recorded.
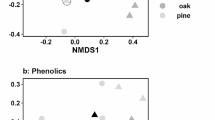
In our initial sampling directly after the first MeJA treatment, PCA analysis only revealed a limited separation between CC (control branches on control trees), CT (control branches on treated trees), and TT (treated branches on treated trees) in terms of their volatile profiles (Fig. 2). RDA showed that MeJA treatment had a significant effect in the case of C. betulus (pseudo-F = 7.6, p = 0.001, 18.1% of adjusted variability explained) and Q. robur (pseudo-F = 3.1, p = 0.001, 7.1% of adjusted variability explained). Its effect on T. cordata was not significant (pseudo-F = 1.8, p = 0.182, 2.7% of adjusted variability explained). Four individual compounds, two mono- and two sesquiterpenes, responded to the MeJA treatment in C. betulus when analysed with LMEs and applying Bonferroni correction. All four showed a localized induction. One monoterpene, trans-ß- ocimene, increased significantly upon MeJA treatment in Q. robur, showing a localized induction. There was no compound showing a significant response to the MeJA treatment in T. cordata (Table S2).
Variation in volatile organic compound (VOC) profiles between the studied branches as visualized by PCA. In the case of initial sampling, PCA analysis revealed only a limited separation in VOC profiles between treated and control branches in Carpinus betulus (A, first two unconstrained axes explained 63.3% of the variation in VOCs), Quercus robur (B, first two unconstrained axes explained 47.4% of variation), and Tilia cordata (C, first two unconstrained axes explained 61.2% of the variation in VOCs), although the effect of treatment was significant in C. betulus and Q. robur when subsequently analysed by RDA. In the case of the final sampling, treated branches on treated trees showed clearly different VOC profiles, suggesting a strongly localized induction in C. betulus (D, first two unconstrained axes explained 78.6% of the variation in VOCs), Quercus (E, first two unconstrained axes explained 70.4% of the variation in VOCs), and Tilia (F, first two unconstrained axes explained 77.3% of the variation in VOCs) that was also confirmed by the subsequent RDA. Branches are shown as circles and the treatment is colour coded. VOCs are shown as arrows. Black arrows indicate VOCs showing individual significant response to the treatment when analysed with LMEs. Other VOCs are in grey
For the VOCs collected at our final sampling after 11 MeJA treatments, PCA analysis revealed a strong separation in volatile profiles between CC and CT branches on the one side and TT branches on the other (Fig. 2). RDA showed that the treatment had a significant effect in the case of C. betulus (pseudo-F = 10.8, p = 0.007, 38.0% of adjusted variability explained), Q. robur (pseudo-F = 32.0, p = 0.005, 66.0% of adjusted variability explained), and T. cordata (pseudo-F = 17.6, p = 0.013, 52.6% of adjusted variability explained). Ten individual compounds responded to MeJA treatment in C. betulus when analysed with LMEs and applying Bonferroni correction. In addition to the four terpenoids induced after one treatment, the emission of six more sesquiterpenes significantly increased in MeJA treated branches. Depending on the compound, the emission on TT branches was 17-1000 times more compared to that of CC branches (Fig. 3). In Q. robur, 45 compounds responded to the MeJA treatment. The majority of compounds that could be identified were terpenes, four mono- and 21 sesquiterpenes or derivatives thereof. But we also found an aldehyde (Q3), an alkatetraene (Q10), two alcohols (Q18, Q47) and an ester (Q41) that were strongly induced by MeJA. Out of these, 36 showed localized induction, one showed systemic induction, and eight showed other trends. In T. cordata, 27 compounds responded to the treatment (Table S2). The ones we identified included eight mono- and 12 sesquiterpenes or derivatives thereof, and one benzoate (T45). Out of the compounds showing a significant response to the treatment in T. cordata, 21 showed localized induction, one showed systemic induction, and five showed other trends. Considering all three tree species, sesquiterpenes were most frequently induced in TT branches when compared to CC branches (Fig. 3). Whereas TT branches of C. betulus and T. cordata produced only one new compound after 11 MeJA treatments, we found 15 compounds in the VOC profiles of Q. robur TT branches that were produced de novo (Fig. 3).
Increase in volatile organic compound (VOC) emissions in TT branches (TT – treated branches on treated trees) in comparison to CC branches (CC – control branches on control trees) in Carpinus betulus (A, D), Quercus robur (B, E), and Tilia cordata (C, F) after the initial (upper row) and final induction (lower row). The bars show the number of VOCs from the recorded groups that increased 1-10x, 11-100x, 101-1,000x, 1,001–10,000x or appeared in the samples from TT branches only (marked as “New”). Only the compounds showing statistically significant localized or systemic induction are shown (Table S2)
The VOC production in CT branches was generally not correlated to their distance to TT branches. The only two exceptions were (i) a negative correlation between the total VOC production in CT branches and their shortest distance to TT branches in the case of our initial sampling from Q. robur (χ2(1) = 4.07, p = 0.044) and (ii) a positive correlation between the total VOC production in CT branches and their trunk distance to TT in the case of our final sampling from C. betulus (χ2(1) = 5.97, p = 0.015).
The polyphenol profiles, polyphenol activities, or total protein content generally did not show a significant response to the MeJA treatment (Table S3). The only exception was the percentage of procyanidin units in the total proanthocyanidin content that was significantly different between the control branches on treated trees and treated branches on treated trees in Q. robur (χ2(2) = 6.25, p = 0.044). In T. cordata, the content of kaempferols showed a marginally significant response to the treatment (χ2(2) = 5.33, p = 0.0696). In C. betulus, neither polyphenols nor protein content showed a significant response to the treatment.
We recorded 98 predation events inside the plot over the entire period (Fig. S1). Only eight predation events could be attributed to birds. Other clay caterpillars were either predated on by invertebrates or it was not possible to distinguish whether the mark was caused by a bird or by an invertebrate. The average number of predation events was higher on the treated trees than on the control trees (F1 = 5.04, p = 0.030; Fig. 4). There was no difference between predation on CT and TT branches (χ2(1) = 0.94, p = 0.333). Due to low predation rates inside the plot, we also set up an additional experiment to assess predation rates at the margins over 48 hours. Inside the plot, 5.45% (0.44% by birds) of the clay caterpillars showed predation marks. At the plot margin, the overall predation rate was 13.77% (3.39% by birds). The light intensity measured at individual branches inside the plot (18.7 ± 29.8 µmol s-1 m-2 per µA) was ca. 2.3% of the levels on its margin (798.7 ± 555.9 µmol s-1 m-2 per µA).
Average number of predation events on treated and control tree individuals. There were more clay caterpillars predated on trees treated with methyl jasmonate (F1 = 5.04, p = 0.030). The graph shows results combined for all three tree species studied. The boxes show the first to third quartile with the medians as horizontal lines, the whiskers show range. Significant differences are marked with asterisks
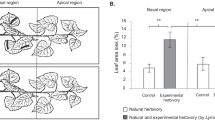
The MeJA treatment affected caterpillar preference in the food-choice trials (Fig. 5). In the case of C. betulus, the caterpillars preferred the discs from CC branches in ‘tree comparison’ (χ2(1) = 4.22, p = 0.040) and the discs from CT branches in ‘branch comparison’ (χ2(1) = 6.36, p = 0.012) over the discs from TT branches. In the case of Q. robur, the caterpillars preferred the discs from CC branches over the discs from TT branches in ‘tree comparison’ (χ2(1) = 5.93, p = 0.015). However, they did not differentiate between the leaf discs from CT and TT branches in the ‘branch comparison’(χ2(1) = 1.14, p = 0.286). In fact, they consumed very little of either disc category (Fig. 5).
Preference of Operophtera brumata caterpillars for leaves from Carpinus betulus (A) and Quercus robur (B) in ‘tree and branch comparisonsʼ. In ‘branch comparisonsʼ, each larva was offered a pair of discs from a TT branch (TT – treated branches on treated trees) and a CT branch (CT – control branches on treated trees) from the same treated tree. In ‘tree comparisonsʼ, each larva was offered a pair of discs from a TT branch and a CC branch (CC – control branches on control trees). In C. betulus, the caterpillars preferred the discs from CC branches in ‘tree comparisonʼ (χ2(1) = 4.22, p = 0.0401) and the discs from CT branches in ‘branch comparisonʼ (χ2(1) = 6.36, p = 0.0117). In the case of Q. robur, the caterpillars preferred the discs from CC branches in ‘tree comparisonʼ (χ2(1) = 5.93, p = 0.0149) whereas they did not differentiate between the leaf discs in ‘branch comparisonʼ (χ2(1) = 1.14, p = 0.2855). The boxes show the first to third quartile with the medians as horizontal lines, the whiskers show range. Significant differences are marked with asterisks
Discussion
We analysed induction of several responses with possible direct and indirect effects on herbivores and their localization to individual branches in three different tree species. Our results show that various tree traits differ in their inducibility and level of localization (Clavijo McCormick et al. 2014; Mason et al. 2017). When induced with MeJA, which we used as a proxy for induction by chewing herbivore damage, the induction of VOCs was strongly localized to individual branches. In contrast, we detected much weaker or no response in leaf polyphenols and protein content.
VOC production varies substantially among tree species, tree individuals or even their parts (Clavijo McCormick et al. 2014; Lämke and Unsicker 2018). Generally, inducibility should be higher in early successional or fast growing plants (Rasmann et al. 2011). Here, we recorded the highest diversity and inducibility of VOCs in Q. robur, a light demanding and long-lived pioneer species. High VOC diversity and intraspecific variation has been shown to reduce herbivory damage in patches of shrubs such as Piper (Salazar et al. 2016), possibly by confounding cues to specialized herbivores or providing new ones to predators. High diversity and variation of VOCs across canopies of large and long-lived trees, such as oaks, that harbour diverse insect assemblages could theoretically play a similar role (Lämke and Unsicker 2018; Volf et al. 2020). We recorded a systemic upregulation in two VOCs, suggesting a signal transfer by phloem or by airborne signals (Heil and Ton 2008; Viswanathan and Thaler 2004). However, most of the compounds were upregulated in treated branches only. With one exception, we also did not detect a negative correlation between the overall VOC production in control branches and their distance to the treated branches, i.e. the control branches close to the treated ones did not generally produce more VOCs.
Terpenoids, in particular sequiterpenes, were the most frequently locally induced compounds, some also detected de novo in treated branches. The largely localized upregulation of terpenoid VOCs is similar to the results of Clavijo McCormick et al. (2014) who recovered similar trends in young poplars induced with herbivory. A highly localized induction of monoterpenes and sesquiterpenes has been recorded also in red pine phloem. This observation, together with the results presented here, suggest an important role of short-distance signalling for eliciting biosynthesis of terpenoids in trees (Mason et al. 2017). In general, such short-term signaling and localized induction responses allow individual tree modules to respond to steep environmental gradients within canopies, maximizing their functioning at the whole-canopy level.
Terpenoids are commonly induced by herbivore feeding in a wide range of plant species, including trees. The quantity and type of terpenoids that are induced, may depend on the herbivore species that are feeding or the type of damage they cause (Danner et al. 2018; Unsicker et al. 2015). As such, herbivore-induced terpenoids are important infochemicals to a wide range of predators and parasitoids that use them as cues to identify their specific prey or host. In particular, de novo synthesized terpenoids are reliable cues as they are produced only when the leaves are actually damaged (Vet et al. 1991). For example, blends of sesquiterpenes can attract birds even in the absence of visual cues or when emitted by individual branches (Amo et al. 2013; Mäntylä et al. 2017). Our results suggest that upregulation of VOCs localized into a single branch could contribute to predator attraction in the system we studied. In large trees, such a localized attraction can facilitate a faster herbivore removal by predators, especially in case of patchy and aggregated herbivore distribution across the canopy (Travis and Palmer 2005). However, studying an increase in predation rates following an induction event in large mature trees would be required to reveal the level of localization in predation across tree canopies. Indeed, here the predators extended their search for prey to the whole canopies of our relatively small trees where the closest distance between branches was ca. 45 cm, resulting into no difference in predation rates between CT and TT branches.
It was proposed that VOCs can serve an important role in complex environments with limited visibility and help natural enemies to navigate towards herbivores (Vet et al. 1991). We observed increased predation on treated trees in our densely vegetated plot where the light intensity was less than 3% of the levels of light intensity on its margin. This increase in predation rates provides some support for the hypothesis. However, the overall predation rates inside the plot were less than half that on its margin, although no trees on the margin were induced. The trend was even more pronounced when bird predation was considered separately as we observed ca. 8x more predation events by birds on the plot margin. The difference in predation rates between the plot and its margin illustrates that abiotic factors such as light intensity, temperature, or habitat openness can strongly modify predation rates (Posa et al. 2007; Seifert et al. 2016). Additionally, the canopy structure and abiotic conditions can modify how VOCs spread, affecting their efficiency (Douma et al. 2019). While VOCs thus can serve their role as indirect defences, even in a dense jungle of foliage, their ecological relevance depends on factors affecting predators and the physical structure and accessibility of the habitat. The efficiency may also differ between VOC types (Douma et al. 2019). Here, we recorded primarily terpenoid based VOCs. Other types of VOCs such as green leaf volatiles, including esters and alcohols that were only marginally represented in our samples, can also play an important role in other systems (Clavijo McCormick et al. 2012).
In contrast to the strong induction of VOCs, our induction treatment elicited only a limited change in proanthocyanidins and no changes in other polyphenols or in the protein content. Proanthocyanidins show low protein precipitation and oxidative activities in the caterpillar mid-gut (Salminen and Karonen 2011). Therefore, the shift in proanthocyanidin composition we recorded in Q. robur has relatively low potential to serve as a form of induced resistance against caterpillars or similar herbivores. Still, winter moth caterpillars preferred leaf discs of untreated leaves of Q. robur and also of C. betulus over those punched from induced leaves, suggesting either systemic (Q. robur) or branch-localized (C. betulus) differences in leaf quality. Therefore, there was probably a trait, or a combination of traits, other than polyphenols primarily responsible for the patterns in caterpillar food-choice in our study. We recorded a significant upregulation of various mono- and sesquiterpene VOCs in the treated branches. Possibly, the production of other bioactive terpenoids with higher molecular mass that are contained in the leaves of studied species (Frédérich et al. 2009) could be upregulated as well, explaining the trends in caterpillar preference we observed. Additionally, induced responses in plants may involve a number of changes, including changes in physical traits, such as changes in trichome density, that may have further contributed to the trends observed (Barton 2016).
The absence of a strong response in polyphenols to our induction treatment can result from the relatively short period between the treatment and the time they were measured. The induction of responses with direct effects on herbivores in herbaceous plants can be relatively rapid, leading to an upregulation of defensive metabolites over a course of several days (van Dam et al. 2004). While a relatively rapid upregulation of polyphenol-based defences has been recorded in trees (Rubert-Nason et al. 2015; Ruuhola et al. 2008), other studies on trees also reported an upregulation of polyphenols spanning over several months or seasons (Tuomi et al. 1988). Such a delayed induced resistance can impose negative effects on older instars of herbivores with long developmental times, herbivores occurring later in the season or possibly next generation of the same species in the following year (Eyles et al. 2010; Roden and Mattson 2008; Tuomi et al. 1988). However, in temperate regions, herbivore abundance on trees shows strong seasonality, with many herbivore species undergoing a rapid development in spring. The spring peak in caterpillar abundance can last only two weeks (Volf et al. 2019a). Combining the relatively slow induction of direct defences with a fast induction of VOCs attracting natural enemies of herbivores can thus be an important defensive strategy. Higher light intensity and canopy openness during leaf flushing can contribute towards the importance of VOCs during that period. Furthermore, releasing VOCs by damaged foliage can prime the surrounding branches and trees for a faster response to herbivory occurring later in the season (Kim and Felton 2013). Thus, there may be a seasonal shift in the relative importance of traits with direct and indirect effects on herbivores. More readily inducible VOCs may be more important early in the season when canopies are open and young leaves produce VOCs in higher quantitates (Rostás and Eggert 2008). On the other hand, some of the polyphenol sub-groups, such as proanthocyanidins, may become more important in the later season once they accumulate (Salminen et al. 2004).
In conclusion, our results show that induction of VOCs can be localized to individual branches even in relatively small trees, thereby contributing to increased predation rates and herbivore removal. Additionally, our experiments suggest that localized changes in branches can also affect caterpillar preference in some tree species. In addition to helping trees to cope with steep environmental gradients across their canopies, such localized changes may have some potential to promote spatial chemical variation across the canopy (Lämke and Unsicker 2018; Rubert-Nason et al. 2015). Effects of this chemical variation can cascade to higher trophic levels, possibly promoting spatial variation in the associated communities of herbivores, predators, or parasitoids (Volf et al. 2020). Extending similar projects on localized induction to large canopy trees and studying them within and across seasons could bring insights not only into plant defensive strategies, but also into factors structuring diverse assemblages of canopy organisms.
Data Availability
The data used in this study are accessible at iDiv Data Repository: https://idata.idiv.de/ddm/Data/ShowData/1874. DOI: https://doi.org/10.25829/idiv.1874-3-3124.
References
Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with ImageJ. Biophotonics Int 11:36–42
Agrawal AA (1999) Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 80:1713–1723
Amo L, Jansen JJ, van Dam NM, Dicke M, Visser ME (2013) Birds exploit herbivore-induced plant volatiles to locate herbivorous prey. Ecol Lett 16:1348–1355
Backmann P, Grimm V, Jetschke G, Lin Y, Vos M, Baldwin IT, van Dam NM (2019) Delayed chemical defense: Timely expulsion of herbivores can reduce competition with neighboring plants. Am Nat 193:125–139
Barbehenn RV, Jaros A, Lee G, Mozola C, Weir Q, Salminen JP (2009) Tree resistance to Lymantria dispar caterpillars: importance and limitations of foliar tannin composition. Oecologia 159:777–788
Barton KE (2016) Tougher and thornier: general patterns in the induction of physical defence traits. Funct Ecol 30:181–187
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bonello P, Blodgett JT (2003) Pinus nigra–Sphaeropsis sapinea as a model pathosystem to investigate local and systemic effects of fungal infection of pines. Physiol Mol Plant Pathol 63:249–261
Clavijo McCormick A et al (2014) Herbivore-induced volatile emission in black poplar: regulation and role in attracting herbivore enemies. Plant Cell Environ 37:1909–1923
Clavijo McCormick A, Unsicker SB, Gershenzon J (2012) The specificity of herbivore-induced plant volatiles in attracting herbivore enemies. Trends Plant Sci 17:303–310
Danner H, Desurmont GA, Cristescu SM, van Dam NM (2018) Herbivore-induced plant volatiles accurately predict history of coexistence, diet breadth, and feeding mode of herbivores. New Phytol 220:726–738
Defossez E, Pellissier L, Rasmann S (2018) The unfolding of plant growth form-defence syndromes along elevation gradients. Ecol Lett 21:609–618
Dicke M, Loon JJ (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol Exp Appl 97:237–249
Douma JC, Ganzeveld LN, Unsicker SB, Boeckler GA, Dicke M (2019) What makes a volatile organic compound a reliable indicator of insect herbivory? . Plant Cell Environ 42:3308–3325
Engström MT, Pälijärvi M, Fryganas C, Grabber JH, Mueller-Harvey I, Salminen J-P (2014) Rapid qualitative and quantitative analyses of proanthocyanidin oligomers and polymers by UPLC-MS/MS. J Agric Food Chem 62:3390–3399
Engström MT, Pälijärvi M, Salminen J-P (2015) Rapid fingerprint analysis of plant extracts for ellagitannins, gallic acid, and quinic acid derivatives and quercetin-, kaempferol- and myricetin-based flavonol glycosides by UPLC-QqQ-MS/MS. J Agric Food Chem 63:4068–4079
Eyles A, Bonello P, Ganley R, Mohammed C (2010) Induced resistance to pests and pathogens in trees. New Phytol 185:893–908
Frédérich M, Marcowycz A, Cieckiewicz E, Mégalizzi V, Angenot L, Kiss R (2009) In vitro anticancer potential of tree extracts from the Walloon Region forest. Planta Med 75:1634–1637
Hagerman AE, Butler LG (1978) Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem 26:809–812
Heil M, Ton J (2008) Long-distance signalling in plant defence. Trends Plant Sci 13:264–272
Howe A, Lövei GL, Nachman G (2009) Dummy caterpillars as a simple method to assess predation rates on invertebrates in a tropical agroecosystem. Entomol Exp Appl 131:325–329
Jorge I, Navarro RM, Lenz C, Ariza D, Porras C, Jorrín J (2005) The Holm oak leaf proteome: Analytical and biological variability in the protein expression level assessed by 2-DE and protein identification tandem mass spectrometry de novo sequencing and sequence similarity searching. Proteomics 5:222–234
Kachroo A, Robin GP (2013) Systemic signaling during plant defense. Curr Opin Plant Biol 16:527–533
Kallenbach M, Oh Y, Eilers EJ, Veit D, Baldwin IT, Schuman MC (2014) A robust, simple, high-throughput technique for time‐resolved plant volatile analysis in field experiments. Plant J 78:1060–1072
Kim J, Felton GW (2013) Priming of antiherbivore defensive responses in plants. Insect Sci 20:273–285
Klimm FS, Weinhold A, Volf M (2020) Volatile production differs between oak leaves infested by leaf-miner Phyllonorycter harrisella (Lepidoptera: Gracillariidae) and galler Neuroterus quercusbaccarum (Hymenoptera: Cynipidae). Eur J Entomol 117:101–109
Koricheva J, Nykanen H, Gianoli E (2004) Meta-analysis of trade-offs among plant antiherbivore defenses: Are plants jacks-of-all-trades, masters of all? Am Nat 163:64–75
Kruger NJ (2009) The Bradford method for protein quantitation. In: Walker JM (ed) The protein protocols handbook. Springer Protocols Handbooks. Humana Press, Totowa, pp 17–24
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest package: Tests in linear mixed effects models. J Stat Softw 82:1–26
Lämke JS, Unsicker SB (2018) Phytochemical variation in treetops: causes and consequences for tree-insect herbivore interactions. Oecologia 187:377–388
Malisch CS, Salminen J-P, Kölliker R, Engström MT, Suter D, Studer B, Lüscher A (2016) Drought effects on proanthocyanidins in sainfoin (Onobrychis viciifolia Scop.) are dependent on the plant’s ontogenetic stage. J Agric Food Chem 64:9307–9316
Mäntylä E, Kleier S, Kipper S, Hilker M (2017) The attraction of insectivorous tit species to herbivore-damaged Scots pines. J Ornithol 158:479–491
Mason CJ, Villari C, Keefover-Ring K, Jagemann S, Zhu J, Bonello P, Raffa KF (2017) Spatial and temporal components of induced plant responses in the context of herbivore life history and impact on host. Funct Ecol 31:2034–2050
Mrazova A, Sam K (2018) Application of methyl jasmonate to grey willow (Salix cinerea) attracts insectivorous birds in nature. Arthropod-Plant Interactions 12:1–8
Murakami M, Yoshida K, Hara H, Toda MJ (2005) Spatio-temporal variation in Lepidopteran larval assemblages associated with oak, Quercus crispula: the importance of leaf quality. Ecol Entomol 30:521–531
Neuvonen S, Haukioja E, Molarius A (1987) Delayed inducible resistance against a leaf-chewing insect in four deciduous tree species. Oecologia 74:363–369
Ohse B (2018) Mutual influences of tree saplings and mammalian herbivores in temperate mixed deciduous forest a functional biodiversity approach. PhD thesis, Universität Leipzig
Piggott N, Ekramoddoullah AK, Liu J-J, Yu X (2004) Gene cloning of a thaumatin-like (PR-5) protein of western white pine (Pinus monticola D. Don) and expression studies of members of the PR-5 group. Physiol Mol Plant Pathol 64:1–8
Posa MRC, Sodhi NS, Koh LP (2007) Predation on artificial nests and caterpillar models across a disturbance gradient in Subic Bay, Philippines. J Trop Ecol 23:27–33
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org, Vienna, Austria
Rasmann S, Bauerle TL, Poveda K, Vannette R (2011) Predicting root defence against herbivores during succession. Funct Ecol 25:368–379
Roden DB, Mattson WJ (2008) Rapid induced resistance and host species effects on gypsy moth, Lymantria dispar (L.): Implications for outbreaks on three tree species in the boreal forest. For Ecol Manag 255:1868–1873
Rostás M, Eggert K (2008) Ontogenetic and spatio-temporal patterns of induced volatiles in Glycine max in the light of the optimal defence hypothesis. Chemoecology 18:29–38
Rubert-Nason KF, Couture JJ, Major IT, Constabel CP, Lindroth RL (2015) Influence of genotype, environment, and gypsy moth herbivory on local and systemic chemical defenses in trembling aspen (Populus tremuloides). J Chem Ecol 41:651–661
Ruuhola T, Yang S, Ossipov V, Haukioja E (2008) Foliar oxidases as mediators of the rapidly induced resistance of mountain birch against Epirrita autumnata Oecologia 154:725–730
Salazar D, Jaramillo A, Marquis RJ (2016) The impact of plant chemical diversity on plant–herbivore interactions at the community level. Oecologia 181:1199–1208
Salminen J-P (2018) Two-dimensional tannin fingerprints by liquid chromatography tandem mass spectrometry offer a new dimension to plant tannin analyses and help to visualize the tannin diversity in plants. J Agric Food Chem 66:9162–9171
Salminen JP, Karonen M (2011) Chemical ecology of tannins and other phenolics: we need a change in approach. Funct Ecol 25:325–338
Salminen JP, Roslin T, Karonen M, Sinkkonen J, Pihlaja K, Pulkkinen P (2004) Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J Chem Ecol 30:1693–1711
Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149–151
Seifert CL, Schulze CH, Dreschke TC, Frötscher H, Fiedler K (2016) Day vs. night predation on artificial caterpillars in primary rainforest habitats–an experimental approach. Entomol Exp Appl 158:54–59
ter Braak CJ, Smilauer P (2012) Canoco reference manual and user’s guide: software for ordination (version 5.0). Microcomputer Power, Ithaca
Travis JMJ, Palmer SCF (2005) Spatial processes can determine the relationship between prey encounter rate and prey density. Biol Let 1:136–138
Tuomi J, Niemela P, Rousi M, Siren S, Vuorisalo T (1988) Induced accumulation of foliage phenols in mountain birch: branch response to defoliation? Am Nat 132:602–608
Turlings TC, Erb M (2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63:433–452
Unsicker SB, Gershenzon J, Köllner TG (2015) Beetle feeding induces a different volatile emission pattern from black poplar foliage than caterpillar herbivory. Plant Signal Behav 10:e987522
Utsumi S, Ohgushi T (2008) Host plant variation in plant-mediated indirect effects: moth boring‐induced susceptibility of willows to a specialist leaf beetle. Ecol Entomol 33:250–260
van Dam NM, Witjes L, Svatoš A (2004) Interactions between aboveground and belowground induction of glucosinolates in two wild Brassica species. New Phytol 161:801–810
Vet LE, Wäckers FL, Dicke M (1991) How to hunt for hiding hosts: the reliability-detectability problem in foraging parasitoids. Neth J Zool 41:202–213
Viswanathan D, Thaler J (2004) Plant vascular architecture and within-plant spatial patterns in resource quality following herbivory. J Chem Ecol 30:531–543
Volf M et al (2018) Community structure of insect herbivores is driven by conservatism, escalation and divergence of defensive traits in Ficus. Ecol Lett 21:83–92
Volf M et al (2019) Quantitative assessment of plant-arthropod interactions in forest canopies: A plot-based approach. PLoS One 14:e0222119
Volf M, Salminen J-P, Segar ST (2019) Evolution of defences in large tropical plant genera: perspectives for exploring insect diversity in a tri-trophic context. Curr Opin Insect Sci 32:91–97
Volf M, Wirth C, van Dam NM (2020) Localized defense induction in trees: a mosaic of leaf traits promoting variation in plant traits, predation, and communities of canopy arthropods? Am J Bot 107:1–4
Acknowledgements
We acknowledge EcoMetEoR – Ecometabolomics Platform for Ecology & Biodiversity Research that supported the VOC analysis. We thank Daniel Uhlig for his help with treating the trees with insecticides and their general maintenance and MIE group members for their support with the data collection. MV acknowledges funding by Alexander von Humboldt Foundation and the Federal Ministry for Education and Research Ref.3.3-CZE-1192673-HFST-P and Grant Agency of the Czech Republic 20-10543Y. AW, HU, CW and NMvD. thank the German Research Foundation for funding the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG– FZT 118, 202548816). We thank Bettina Ohse, Leuphana University, Germany, for setting up the experimental plot used in this study and Data & Code Unit (iBID) for help with uploading our data to iDiv Data Repository. Finally, we thank Jing Leong for language corrections and helpful comments on the manuscript.
Funding
Alexander von Humboldt Foundation and the Federal Ministry for Education and Research (Ref.3.3-CZE-1192673-HFST-P), Grant Agency of the Czech Republic (20-10543Y), German Research Foundation for funding the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG– FZT 118, 202548816).
Author information
Authors and Affiliations
Contributions
MV, AW, RR, CW, and NMvD designed the experimental approach; MV, CLS, and TH collected the data; MV, AW, HU, EA, J-P S conducted the chemical analysis, MV conducted the statistical analysis and wrote the first draft of the manuscript; all authors critically contributed to the final draft.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
We declare no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Rights and permissions
About this article
Cite this article
Volf, M., Weinhold, A., Seifert, C.L. et al. Branch-Localized Induction Promotes Efficacy of Volatile Defences and Herbivore Predation in Trees. J Chem Ecol 47, 99–111 (2021). https://doi.org/10.1007/s10886-020-01232-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01232-z