Abstract
The Asian eulophid wasp Tetrastichus planipennisi is being released in North America as a biocontrol agent for the emerald ash borer (Agrilus planipennis), a very destructive invasive buprestid beetle that is devastating ash trees (Fraxinus spp.). We identified, synthesized, and tested a female-produced sex pheromone for the wasp. The key component eliciting behavioral responses from male wasps in flight tunnel bioassays was identified as (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene. Female specificity was demonstrated by gas chromatographic (GC) comparison of male and female volatile emissions and whole body extracts. The identification was aided by coupled gas chromatography/mass spectrometry analysis, microchemical reactions, NMR, GC analyses with a chiral stationary phase column, and matching GC retention times and mass spectra with those of synthetic standards. The tetramethyl-triene hydrocarbon was synthesized as a mixture of two enantiomeric pairs of diastereomers, and as the pure insect-produced stereoisomer. In flight-tunnel bioassays, males responded to both the natural pheromone and the chiral synthetic material by upwind flight and landing on the source. In contrast, the mixture of four stereoisomers was not attractive, indicating that one or more of the “unnatural” stereoisomers antagonized attraction. Field trials, using yellow pan traps baited with natural pheromone, captured significantly more male wasps than control traps over a four week trial. The identified pheromone could increase the efficiency and specificity of the current detection methods for Tetrastichus planipennisi and aid in the determination of parasitoid establishment at release sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The larval endoparasitoid Tetrastichus planipennisi Yang (Hymenoptera: Eulophidae) (Liu et al. 2003, 2007; Ulyshen et al. 2010; Yang et al. 2006) is being released in North America as a biocontrol agent for the invasive emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) (Bauer et al. 2014, 2015; Duan et al. 2018; Gould et al. 2015). Both pest and parasitoid are native to China (Bray et al. 2011; Liu et al. 2003). Since being detected in 2002 in the state of Michigan (Haack et al. 2002, 2015), EAB has spread to thirty-seven US states and five Canadian provinces, resulting in the loss of hundreds of millions of ash trees (Fraxinus spp.) (Emeraldashborer Info 2020). EAB’s widespread infestations in forested ecosystems make biological control with coevolved natural enemies one of the most promising management tools available. Successive releases of T. planipennisi in several states has led to successful establishment of the parasitoid in sixteen US states (Bauer et al. 2015; Duan et al. 2013, 2018; Mapbiocontrol 2020). Current practices for the determination of parasitoid establishment require laborious processes such as felling EAB-infested ash trees, using sentinel ash logs to assess larval parasitism of EAB, or trapping adult parasitoids in non-selective yellow pan traps (Abell et al. 2015; Bauer et al. 2012, 2016; Duan et al. 2013; Liu et al. 2007; USDA–APHIS/ARS/FS 2020). A monitoring system based on traps baited with a pheromone for T. planipennisi would have numerous advantages for detecting and monitoring the establishment of this biocontrol agent and might help in determining population densities and dispersal. We report here the identification, synthesis, and behavioral evaluation of the female-produced sex pheromone of T. planipennisi.
Materials and Methods
Insects
Virgin adult T. planipennisi used in this study were reared from small ash logs shipped from the USDA APHIS PPQ rearing facility in Brighton, Michigan. Upon arrival at the USDA ARS lab in Peoria, Illinois, the logs, which contained parasitized EAB larvae, were placed into wide-mouth Mason jars (9.9 cm wide × 16.8 cm height) covered with mesh screen. Adult T. planipennisi emerging from the ash logs were collected daily, sexed, and placed into 240 ml Teflon bottles (Thomas Scientific, Swedesboro, NJ), together with a moist dental wick and several drops of honey, and kept in a growth chamber at 25 °C under a 17 L:7D hr. photoperiod. Male and female insects were housed in different locations within the Peoria facility.
Collection of Insect-Produced Volatiles and Preparation of Cuticular Extracts
Volatiles were collected from individuals and groups of ca. 150, 1–4 d-old virgin female wasps, in 240 ml Teflon bottle collection chambers. Insects had access to a moist dental wick and drops of honey. Control chambers without insects also had water and drops of honey added. Each collection chamber had two Teflon tubes inserted through small holes in the cap, each holding an adsorbent trap (6 × 0.4 cm ID) with 100 mg of HayeSep-Q (Restek, Bellefonte, PA). The inlet trap filtered incoming air (250 ml.min−1) pulled through the chamber by a vacuum pump (Air Cadet, Fisher Scientific, Waltham, MA). The second trap captured the volatiles emitted within the chamber. Collection duration was 1–3 d, and collected volatiles were recovered by rinsing the outlet trap’s HayeSep-Q filter with 400 μl of hexane. Collection chambers were kept in an incubator during collection at 27 °C and ca. 50% RH. Light was provided by eight 40 W fluorescent tubes set ca. 0.5 m above and behind the collection bottles, under a 17 L:7D photoperiod. Females remained alive and produced pheromone for at least one week under these conditions. Over the course of 6 months, volatiles were collected from 17 cohorts of 100–200 females per bottle, as well as from individual females, accumulating volatiles from roughly 2500 virgin females. Cuticular extracts were obtained from females (ca. 7–10 d old) that were removed from the collection chambers and from approximately twenty 1–3 d-old males. Insects were killed by freezing and then soaked for 5 min in 0.1–1 ml hexane.
Analysis and Purification of Pheromone
Collections of headspace volatiles, solvent extracts, liquid chromatography (LC) and HPLC fractions, were analyzed by gas chromatography with flame ionization detection (GC-FID) and coupled GC/mass spectrometry (GC/MS). Samples were injected in splitless mode using a Hewlett Packard 6890 GC, interfaced to a Hewlett Packard 5973 mass selective detector. For most analyses, a 30 m DB-5 capillary column (0.25 mm ID, 0.25 μm film thickness, J&W Scientific, Folsom, CA) was used. The temperature program was 50 °C for 1 min, then increased to 280 °C at 10 °C.min−1, and held for 5 min. The inlet temperature was 250 °C, and the transfer line temperature 280 °C. The Wiley (Wiley 2007) and National Institute of Standards and Technology (NIST 2017) mass spectral libraries were installed on the data system.
GC/MS analyses with a chiral stationary phase GC column were conducted using a 30 m β-DEX 120 column (0.25 mm ID, 0.25 μm film thickness, Supelco, Bellefonte, PA). The temperature program was 50 °C for 1 min, then increased at 30 °C.min−1 to 190 °C, and held for 60 min. All GC analyses used helium as carrier gas, at constant pressure (41.4 kPa).
Hexane extracts of headspace volatiles were fractionated by LC on silica gel (70–230 mesh, Fisher Scientific, Pittsburgh, PA, USA) to determine compound polarity. A column (Pasteur pipette; 0.5 ID × 1 cm) was eluted with 2.5 ml each of hexane, then 5, 10, 15, 25, and 100% ether in hexane. Fractions were stored at −20 °C, before analysis by GC-FID and GC/MS.
Female-derived pheromone was purified (96% by GC-FID) by HPLC using a Waters 515 pump (flow rate 1 ml.min−1), and a Waters R401 differential refractometer detector. The impure compound was injected onto a Supelcosil LC-SI silica column (25 cm, 0.46 cm ID, 5 μm particle size, Supelco, Bellefonte, PA) treated with silver nitrate as described by Heath and Sonnet (1980) for separation of unsaturated compounds. The pheromone was eluted from the HPLC column with 2% 1-hexene in hexane.
Female pheromone emissions were quantified by 24 h collections of volatiles from individual 3–5 d-old virgin females. Quantifications of pheromone emissions and dosing solutions used in the flight tunnel bioassays and field trapping were performed by GC-FID with nonadecane as an internal standard.
Nuclear magnetic resonance (NMR) spectra of the purified pheromone were acquired on an Avance 500-MHz instrument (Bruker, Billerica, MA, USA). Samples were dissolved in CDCl3. Experiments provided 1H, 13C, and COSY spectra.
Microchemical Reactions
Hydrogenation of the pheromone was used to confirm the number of carbon-carbon double bonds. A sample of headspace volatiles (100 μl) was concentrated just to dryness under a stream of nitrogen and resuspended in 100 μl methylene chloride, to which was added ~0.5 mg of PtO2 (Adam’s catalyst). Reduction was accomplished by bubbling a gentle steam of hydrogen through the sample for 5 min at room temperature. The reduced sample was filtered and analyzed by GC/MS.
Carbon Skeleton Determination
A Wittig-Horner condensation between (2E,4E,6E)-2,4,6-trimethyl-nonatrienal and (3E)-pent-3-en-2-yl triphenylphosphonium bromide (both compounds were available from earlier research, Bartelt 1999) was used to synthesize a sample of the tetramethyl-pentaene shown in Scheme 1. The tetramethyl-pentaene was hydrogenated over PtO2 as described above. The resulting blend of saturated hydrocarbons was analyzed by GC/MS and the spectra and retention times were compared with the hydrogenation products from the female-derived pheromone.
Chemicals
The 4-component blend of (6RS,10RS)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene and the single pure enantiomer (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene were synthesized as shown in Schemes 2 and 3. The experimental details and spectral data are provided in the supporting online information.
Flight Tunnel Bioassays
Olfactory stimuli were released in a flight tunnel (0.6 × 0.6 × 1.35 m) at a linear air flow of 0.3 m.sec−1 at 25 °C and 40–60% RH (Bartelt et al. 1990). The flight tunnel was lit by four 40-W fluorescent tubes mounted 10 cm from the top and parallel with the wind direction. Individually housed males were transferred to glass tubes (5 × 1.2 cm I.D.) covered with wire mesh at one end and a removable cap at the other.
Extracts of headspace volatiles, body washes, and synthetic compounds were released from a piezo-electric sprayer (El-Sayed et al. 1999) from the upwind portion of the tunnel. Solutions in hexane were delivered (10 μl/min) by a motor-driven (CMA/Microdialysis, North Chelmsford, MA) syringe into a glass capillary suspended 0.5 m above the tunnel floor. Vibration of the capillary by a piezo-ceramic disc at ca. 122 kHz dispersed the solution into micro-droplets, which evaporated within a few centimeters. Virgin male wasps (1–3 d old) were released individually at the downwind end of the tunnel from a platform 0.5 m above the tunnel floor. Batches of 10–20 insects were flown on different days. The wasps were scored for plume-oriented upwind flight over at least 50 cm and for landing on a paper disc surrounding the sprayer capillary. The response time limit was set at 3 min. Wasps that dropped to the floor or flew to the ceiling within the 3 min response limit were returned to the release platform and allowed an additional 3 min response time.
Field Test
To test the attractiveness of the natural female-derived pheromone, we conducted a field experiment over four weeks in August–September 2013. The experiment was conducted in a wooded area along the Grand River within the 540 acre William M. Burchfield Park in Holt, Ingham County Park, Michigan. The site consisted of an early successional bottomland forest of mixed hardwoods dominated by green ash (Fraxinus pennsylvanica) with an established infestation of EAB. Biological control releases of T. planipennisi were conducted during the peak EAB infestation from to 2007–09. By 2013, the EAB infestation level had declined and the T. planipennisi population was well established (Duan et al. 2013, 2017). Ten matched pairs of ash trees were selected for the experiment. Trees within each pair were spaced ca. 20 m apart and were randomly assigned one of two treatments: pheromone or control. Yellow pan traps (Bauer et al. 2016; USDA–APHIS/ARS/FS 2019) were attached to the trunks of ash trees at chest height and baited with red rubber septa (11 mm, Wheaton Scientific, Millville, NJ) impregnated (Zilkowski et al. 2006) with a methylene chloride solution of natural purified pheromone (99% pure by GC-FID) at 5 μg/septum or with solvent only, which served as a control. A small batch of pheromone-loaded septa (N = 3; aged for 1 d at room temperature in a fume hood) was tested in the flight tunnel prior to the field test; male T. planipennisi (N = 10) exhibited plume-oriented flights and were able to locate the pheromone source. Pan traps were filled with a 20% solution of food-grade propylene glycol (ChemWorld, Taylor, MI). Captured insects were collected weekly by straining trap contents through a paint strainer and storing in labeled zip-sealed plastic bags, which were kept frozen until examined in the laboratory. Septa were replaced after 2 weeks.
Statistics
Data analyses were performed using JMP (10.0.0) for Windows software (SAS Institute Inc., Cary, NC). A Wilcoxon test with Bonferroni correction (α = 0.0125) was used to compare flight tunnel responses (upwind flight and landing) for each release rate (0, 0.2, 2, and 20 pg/min), and to compare responses to synthetic versus natural pheromone. Field data were not normally distributed and the number of T. planipennisi captured in treatment pairs were analyzed with a Wilcoxon Signed-Rank test.
Results
Identification of the Female-Specific Compound
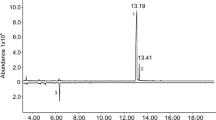
GC-FID and GC/MS analyses of headspace volatiles revealed that females emitted a compound that was absent from volatiles from males or control collections (Fig. 1). Collections of headspace volatiles from individual females showed that a single female emitted 129.1 ng/day (N = 5, ± 19.2 SD) of the female-specific compound. The 6 month cumulative collections and cuticular extracts of approx. 2500 virgin female wasps yielded, after purification, approximately 200 μg of the compound, which was used in the identification and behavioral studies.
The electron impact mass spectrum of the compound (Fig. 2a) showed m/z 234 as a possible molecular ion, with a dominant m/z 109 fragment. The spectrum was not found in either the commercial US National Institute of Standards and Technology (NIST 2017) or the Wiley (Wiley 2007) mass spectral libraries. The compound eluted from silica gel with hexane, suggesting a hydrocarbon with a possible molecular formula of C17H30, which would require 3 double bonds and/or rings. Following hydrogenation, the molecular weight increased to 240 amu, indicating the presence of 3 double bonds, and the GC/MS profile showed at least 5 isomeric peaks with identical mass spectra (Fig. 2b). Those spectra showed some similarities with library spectra of n-alkanes with several methyl groups. Verification of the carbon skeleton was obtained by matches of the GC retention times and mass spectra of several of the peaks of the isomeric mixture with those of the model compound 4,6,8,10-tetramethyltridecane, prepared as shown in Scheme 1.
The NMR data of the purified pheromone are listed in Table 1. Interpretation of the NMR spectra, in combination with the knowledge of the carbon skeleton, provided information about the placement and geometry of the double bonds. Specifically, there were six methyl groups, three of which were allylic, and three of which were attached to saturated carbons, indicating that the three double bonds were all within the carbon chain. The chemical shift (1.76 ppm) and coupling of the terminal methyl group at C1 to the alkene proton at 5.55 ppm on C2 established that the first double bond was between C2 and C3. Furthermore, the large coupling constant (15.5 Hz) between the protons on C2 and C3 established that this was a trans-1,2-disubstituted alkene. The chemical shifts of the two alkene protons suggested that this double bond was conjugated to a second C=C double bond between C4 and C5. Thus, from the carbon skeleton, C4 had to have a methyl group attached, and C5 a single proton, identified as a doublet at 5.12 ppm. This proton was in turn coupled to the allylic proton at 2.65 ppm on C6, and the chemical shift (0.92 ppm) of the protons of C15, the methyl group attached to C6, indicated that the C15 methyl was attached to a sp3 carbon, ruling out the possibility of the third double bond being between C6 and C7. This was corroborated by the identification of the two C7 protons at 1.94 ppm, coupled to the proton on C6. Furthermore, the chemical shift of the C7 protons indicated that they were allylic, thus placing the third double bond between C8 and C9, with the last alkene proton (4.86 ppm) being on C9. The remaining C10-C13 saturated alkane structure was trivial based on knowledge of the carbon skeleton. These data confirmed the general structure of the pheromone to be (2E,4E/Z,8E/Z)-4,6,8,10-tetramethyltrideca-2,4,8-triene as the female-specific compound. Furthermore, the GC retention time and mass spectrum of the natural pheromone matched that of one of the two diastereomers of synthetic (6RS,10RS)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene, strongly suggesting that the double bonds in the natural pheromone had the (2E,4E,8E) configuration. Thus, the only missing piece of information was the absolute configurations at C6 and C10.
The four possible stereoisomers of (6RS,10RS)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene were resolved into three distinct peaks on the chiral stationary phase GC column (Fig. 3). One diastereomeric pair of enantiomers showed almost baseline separation, whereas the other diastereomeric pair eluted as a single peak. This separation afforded an opportunity to determine the exact stereochemistry of the natural compound. Synthesized (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene matched the GC retention time and mass spectrum of the natural material exactly, completing the identification of the pheromone. The synthetic (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene sample contained 3.8% of another diastereomer.
Gas chromatography analysis of (a) natural (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene, (b) synthetic (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene, and (c) the mixture of four stereoisomers of (2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene on a chiral stationary phase β-DEX 120 column (190 °C isothermal)
Synthesis of the Pheromone
The pheromone was synthesized in two stages. In the first stage, the pheromone was synthesized as a mixture of the (6RS,10RS)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene isomers; i.e., with the double bond geometries fixed but both chiral centers variable. Having verified that one of the two resulting diastereomeric pairs of enantiomers matched the retention time of the insect-produced compound on an achiral GC column, confirming the geometries of the three double bonds, we then developed a stereoselective synthesis for one of the four possible stereoisomers. As a starting point, we chose the (6S,10S)-enantiomer, based on the strong similarity between the structures of this pheromone and a pheromone component of another parasitic wasp, Trichogramma turkestanica (Tröger et al. 2014), which produces (6S,8S,10S)-(2E,4E)-4,6,8,10-tetramethyltridecadien-1-ol and the corresponding hydrocarbon. Note, that the carbon skeletons of these compounds differ from that of the T. planipennisi pheromone only by the absence of the double bond between C8 and C9. Fortunately, this “educated guess” turned out to be correct.
To make the (6RS,10RS)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene mixture (Scheme 2), 2-methylpentanal 1 was subjected to Wittig olefination with (carbethoxyethylidene)triphenylphosphorane to give ethyl (2E)-2,4-dimethylhept-2-enoate 2. Lithium aluminum hydride (LAH) reduction of 2 gave allylic alcohol 3, which was converted to the corresponding bromide 4 via the mesylate in one pot (Jin et al. 2007). The enolate of methyl propanoate was alkylated with 4 in THF/DMPU to give methyl (4E)-2,4,6-trimethylnon-4-enoate 5. LAH reduction of 5 followed by Swern oxidation gave aldehyde 6, which was subjected to Wittig olefination with (carbethoxyethylidene)triphenylphosphorane to afford α,β-unsaturated ester 7. LAH reduction and Swern oxidation gave aldehyde 8, which underwent trans-selective Wittig olefination with ethyltriphenylphosphonium bromide and phenyllithium under the Schlosser conditions (Wang et al. 2003) to give (2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene 9 as two enantiomeric pairs of diastereomers.
The non-stereoselective route to prepare triene 9 as two enantiomeric pairs of diastereomers, described above, was readily adapted to prepare (6S,10S)-triene 24 (Scheme 3). Both the (6S)- and (10S)- chiral centers were established with Evans’ chiral auxiliary (Evans et al. 1982). Thus, (S)-oxazolidinone 10 was acylated with pentanoyl chloride and then selectively alkylated with MeI. The methylation afforded a 12:1 mixture (~ 85% de) which was separated by flash chromatography to give 12, establishing what would become the (10S)-chiral center in the final product 24. LAH reduction of 12 gave alcohol 13. Swern oxidation to the aldehyde followed by in situ Wittig reaction afforded α,β-unsaturated ester 14 (Kasun et al. 2015; Lister and Perkins 2004). LAH reduction of 14 gave allylic alcohol 15, which was converted to allylic bromide 16 by treatment with mesyl chloride and Et3N, then LiBr. (R)-Oxazolidinone 17 was acylated with propanoyl chloride, and then stereoselectively alkylated with 16 to give 19, establishing what would become the (6S)-chiral center in the final product 24. After reductive removal of the chiral auxiliary, the resulting allylic alcohol 20 was converted to the final product (6S,10S)-triene 24 following the same reaction sequences as used in preparation of triene 9.
Flight Tunnel Bioassay
Males responded in a dose-dependent manner to the female-produced pheromone when it was dispensed in the flight tunnel by a piezo-electric sprayer (Fig. 4). Males responded to the plume with upwind flight, with responses significantly higher at lower release rates, and exhibited no responses to the control. The frequency of upwind flight in response to 0.2 and 2 pg/min was greater than to the control (Z = 3.51, P = 0.0004 and Z = 3.15, P = 0.0016, respectively), and the response to 0.2 pg/min was greater than to 20 pg/min (Z = -2.75, P = 0.006). No other comparisons were different. Additionally, there was no trend in dose-response for landing on the pheromone source. Females did not show any plume-oriented flights or land on the source when presented with the female-produced pheromone (2 pg/min release rate, N = 34). Most of the females failed to respond at all or flew directly to the ceiling of the wind tunnel. Females (N = 10) also failed to respond or flew directly to the ceiling of the wind tunnel when presented with only solvent control.
Flight tunnel responses (upwind flight and landing on source) of male Tetrastichus planipennisi wasps in response to solvent control and different dosages (pg/min) of the natural female-specific sex pheromone. Percentages with different letters indicate significance (Wilcoxon test, α = 0.0125), ns denotes no significance
Males showed no plume-oriented upwind flight or landing on the source when presented with the 4-component synthetic mixture of stereoisomers [i.e., (6RS,10RS)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene, 8 pg/min release rate]. Most of the males did not take flight or flew directly to the ceiling of the wind tunnel (N = 20). In contrast, there were no differences in upwind flight of males in response to synthetic (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene (2 pg/min release rate, N = 63) and the isolated natural pheromone (2 pg/min, N = 183, Z = -1.75, P = 0.08). However, more males landed on the natural pheromone stimulus than on the synthetic (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene stimulus (Z = -3.18, P = 0.0015).
Field Tests
A total of 39 males were caught in traps baited with the natural pheromone during the four-week test period (Table 2). There was a treatment effect, with more males caught in the pheromone traps compared to the solvent-baited control traps (S = −68, P < 0.0001). No such treatment effect was found for females (S = 0, P = 1). Significantly more males were attracted to the natural pheromone compared to females (S = −63, P = 0.0003).
Discussion
The flight tunnel and field trapping studies demonstrated that female T. planipennisi emit a sex pheromone attractive to conspecific males. We identified (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene as a key compound in this sex pheromone, obtaining exact retention times and mass spectral match between the insect-produced compound and the synthetic standard. Flight tunnel bioassays showed that male wasps were highly sensitive to the pheromone, responding to release rates of 0.2 pg/min, with some indication that increasing release rates might actually result in decreased responses. This sensitivity to the natural pheromone was also demonstrated in flight tunnel bioassays and field tests, in which male wasps were attracted to a slow release rate formulation of 5 μg of pheromone impregnated into a rubber septum.
Flight tunnel tests also showed that male wasps did not respond to the mixture of (6RS,10RS)-stereoisomers, indicating that presence of the additional stereoisomers in the mixture antagonized behavioral responses. Although plume-oriented upwind flight responses were similar between the natural and the (6S,10S)-synthetic pheromone, as described above, the landing rates on the source were lower for the synthetic pheromone than the insect-produced compound. The chiral stationary phase GC analysis revealed that the synthetic sample of (6S,10S)-(2E,4E,8E)-4,6,8,10-tetramethyltrideca-2,4,8-triene contained ca. 4% of another diastereomer, and it is possible that this relatively small impurity may have been responsible for the decreased landing rate.
Long-range sex pheromones are now known for a number of parasitic Hymenoptera and are typically emitted by females to attract males (see reviews by Ayasse et al. 2001; Keeling et al. 2004). However, to our knowledge, this is the first species in the family Eulophidae for which a pheromone has been reported, although two structures similar to the T. planipennisi tetramethyl-triene have been reported as pheromone components for a trichogrammatid wasp (Geerdink et al. 2014; Tröger et al. 2014). In particular, female T. turkestanica were found to produce (6S,8S,10S)-(2E,4E)-4,6,8,10-tetramethyltrideca-2,4-diene, differing by only one double bond from the T. planipennisi structure. The EI mass spectrum of this diene (Tröger et al. 2014) showed a similar fragmentation pattern to that of the pheromone of T. planipennisi, which was helpful in the elucidation of the structure of the latter pheromone. Interestingly, the multiple methylated polyunsaturated hydrocarbon pheromones of unrelated Carpophilus spp. sap beetles (Coleoptera: Nitidulidae) also show strong structural similarities to the T. planipennisi pheromone (see review by Bartelt 1999). Most of the pheromone identifications of Carpophilus spp. were performed at the USDA ARS Peoria Laboratory, where several synthetic intermediates from that work were still available, and were used in the synthesis of a key model compound used to verify the carbon skeleton of the T. planipennisi pheromone, as described above.
As EAB continues to invade new areas of North America, the establishment of T. planipennisi as one of its biological control agents appears to be occurring in tandem. After the first releases of the wasp in Michigan in 2007 (Abell et al. 2015; Bauer et al. 2009, 2012) and subsequent establishment in years thereafter (Bauer et al. 2015; Duan et al. 2013, 2017, 2018), T. planipennisi has expanded its range to include at least sixteen states and provinces in northern regions of North America (Bauer et al. 2015; MapBioControl 2020).
Besides T. planipennisi, three additional EAB parasitoids have been approved for release in the US (Bauer et al. 2008; Federal Register 2007, 2015): the gregarious larval ectoparasitoids, Spathius agrili, Yang, S. galinea Belokobylskij and Strazenac (Hymenoptera: Braconidae), and an egg parasitoid Oobius agrili Zhang and Huang (Hymenoptera: Encyrtidae). Spathius agrili was recovered at sites in two US states, but establishment has not been documented (Duan et al. 2019; Hooie et al. 2015; Mapbiocontrol 2020). A three-component, male-produced, aggregation sex pheromone has been identified for S. agrili (Cooperband et al. 2013; Cossé et al. 2012). The second Spathius species, S. galinea, collected from the Russian Far East, has been recovered in several states (Mapbiocontrol 2020) and is established in at least Connecticut, New York, and Massachusetts (Duan et al. 2019). A preliminary study of the S. galinea pheromone indicated several male-specific compounds with similarities to the S. agrili pheromone (Cossé, unpublished). The egg parasitoid Oobius agrili has been released in at least 23 US states and two Canadian provinces and has been recovered in 13 US states (Mapbiocontrol 2020).
The determination of parasitoid establishment requires a variety of laborious methods that are carried out at least two years after the final releases (USDA–APHIS/ARS/FS 2019). The development of a monitoring system based on adults being attracted to the yellow color of pan traps (Abell et al. 2015; Bauer et al. 2012, 2016) is less laborious, but is far from species-specific. Baiting the yellow pan traps with T. planipennisi sex pheromone may increase trap specificity as well as the likelihood of trapping T. planipennisi to assess its establishment in the vicinity of release sites, as was demonstrated in the field trial reported here. Although synthesis of the pheromone may be expensive, because of the absolute necessity for material of high stereochemical purity, it may still be economically feasible because of the very small dosages (5 micrograms) required per lure. In this context, 1 g of synthetic pheromone would suffice for 200,000 lures.
References
Abell K, Poland T, Cossé A, Bauer L (2015) Trapping techniques for emerald ash borer and its introduced parasitoids, pp. 113–127, in Van Driesche RG, Reardon RC (eds.), Biology and Control of Emerald Ash Borer. U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team, Morgantown, WV. FHTET-2014-09. Available at: https://www.nrs.fs.fed.us/pubs/49295
Ayasse M, Paxton RJ, Tengo J (2001) Mating behavior and chemical communication in the order Hymenoptera. Annu Rev Entomol 46:31–78
Bartelt RJ (1999) Sap Beetles. In: Hardie J, Minks AK (eds) Pheromones of non-lepidopteran insects associated with agricultural plants. CABI Publishing, New York, pp 69–89
Bartelt RJ, Dowd PF, Plattner PD, Weisleder D (1990) Aggregation pheromone of dried fruit beetle, Carpophilus hemipterus: wind-tunnel bioassay and identification of two novel tetraene hydrocarbons. J Chem Ecol 16:1015–1039
Bauer LS, Liu HP, Miller DL, Gould J (2008) Developing a biological control program for emerald ash borer (Agrilus planipennis), an invasive ash pest in North America. Newsl Michigan Entomol Soc 53:38–39
Bauer LS, Liu H, Miller D (2009) Emerald ash borer biological control: rearing, releasing, establishment, and efficacy of parasitoids, pp. 7–8, in McManus KA, Gottschalk KW (eds.). Proceedings. 20th U.S. Department of Agriculture interagency research forum on invasive species; 2009 January 13–16; Annapolis, MD. Gen. Tech. Rep. NRS-P-51. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station. Available at: https://www.nrs.fs.fed.us/pubs/34330
Bauer LS, Gould J, Duan J, Hansen J, Cossé A, Miller D, Abell K, Van Driesche R, Lelito J, Poland T (2012) Sampling methods for recovery of exotic emerald ash borer parasitoids after environmental release, pp. 2–5, in McManus KA, Gottschalk KW (eds.). Proceedings. 22nd U.S. Department of Agriculture interagency research forum on invasive species; 2011 January 10–13; Annapolis, MD. Gen. Tech. Rep. NRS-P-92. Newtown Square, PA: U.S. Department of Agriculture, Forest Service, Northern Research Station. Available at: http://nrs.fs.fed.us/pubs/39810
Bauer LS, Duan JJ, Gould J (2014) Emerald ash borer (Agrilus planipennis Fairmaire) (Coleoptera: Buprestidae), pp. 189–209, in Van Driesche R, Reardon R. (eds.). The Use of Classical Biological Control to Preserve Forests in North America. U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team, Morgantown, WV. FHTET-2013-02. Available at: http://www.fs.fed.us/foresthealth/technology/pdfs/FHTET-2013-2.pdf
Bauer LS, Duan JJ, Gould JR, Van Driesche R (2015) Progress in the classical biological control of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in North America. Can Entomol 147:300–317
Bauer L, Hansen J, Gould J (2016) Yellow Pan Traps: A Simple Method for Trapping Parasitoids Released for Biological Control of the Emerald Ash Borer. Available at: https://www.nrs.fs.fed.us/disturbance/invasive_species/eab/local-resources/downloads/YPT_Method20160304.pdf
Bray AM, Bauer LS, Poland TM, Haack RA, Cognato AI, Smith JJ (2011) Genetic analysis of emerald ash borer (Agrilus planipennis Fairmaire) populations in Asia and North America. Biol Invasions 13:2869–2887
Cooperband MF, Hartness A, Lelito JP, Cossé AA (2013) Landing surface color preferences of Spathius agrili (Hymenoptera: Braconidae), a parasitoid of emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae). J Insect Behav 26(5):721–729
Cossé AA, Petroski RJ, Zilkowski BW, Vermillion K, Lelito JP, Cooperband MF, Gould JR (2012) Male-produced pheromone of Spathius agrili, a parasitoid introduced for the biological control of the invasive emerald ash borer, Agrilus planipennis. J Chem Ecol 38(4):389–399
Duan JJ, Bauer LS, Abell KJ, Lelito JP, Van Driesche R (2013) Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J Econ Entomol 106:1145–1154
Duan JJ, Bauer LS, Van Driesche RG (2017) Emerald ash borer biocontrol in ash saplings: the potential for early stage recovery of north American ash trees. Forest Ecol Manag 394:64–72
Duan JJ, Bauer LS, Van Driesche RG, Gould JR (2018) Progress and challenges of protecting north American ash trees from the emerald ash borer using biological control. Forests 9:1–17
Duan JJ, Van Driesche RG, Crandall RS, Schmude JM, Rutledge CE, Slager BH, Gould JR, Elkinton JS (2019) Establishment and early impact of Spathius galinae (Hymenoptera: Braconidae) on emerald ash borer (Coleoptera: Buprestidae) in the northeastern United States. J Econ Entomol 112(5):2121–2130
El-Sayed AM, Godde J, Arn H (1999) Sprayer for quantitative application of odor stimuli. Environ Entomol 28:947–953
Emeraldashborer Info (2020) (http://emeraldashborer.info)
Evans DA, Ennis MD, Mathre DJ (1982) Asymmetric alkylation reactions of chiral imide enolates. A practical approach to the enantioselective synthesis of α-substituted carboxylic acid derivatives. J Am Chem Soc 104:1737–1739
Federal Register (2007) Availability of an environmental assessment for the proposed release of three parasitoids for the biological control of the emerald ash borer (Agrilus planipennis) in the continental United States. Fed Regist 72:28947–28948 (http://www.regulations.gov/#!docketDetail;D=APHIS-2007-0060)
Federal Register (2015) Availability of an environmental assessment for field release of the parasitoid Spathius galinae for the biological control of the emerald ash borer (Agrilus planipennis) in the contiguous United States. Fed. Regist. 2015, 80: 7827–7828. (https://www.regulations.gov/ docket?D=APHIS-2014-0094)
Geerdink D, Buter J, van Beek TA, Minnaard AJ (2014) Asymmetric total synthesis of a putative sex pheromone component from the parasitoid wasp Trichogramma turkestanica. Beilstein J Org Chem 10:761–766
Gould JR, Bauer LS, Duan JJ, Williams D, Liu H (2015) History of emerald ash borer biological control. pp. 83–95. In: Van Driesche RG, Reardon RC (eds.), Biology and Control of Emerald Ash Borer. U.S. Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team, Morgantown, WV. FHTET-2014-09. Available at: https://www.nrs.fs.fed.us/pubs/49321
Haack RA, Jendek E, Liu H, Marchant KR, Petrice TR, Poland TM, Ye H (2002) The emerald ash borer: a new exotic pest in North America. Newsletter Michigan Entomol Soc 47:1–5 Available at: https://www.nrs.fs.fed.us/pubs/1858
Haack RA, Baranchikov Y, Bauer LS, Poland TM (2015) Emerald ash borer biology and invasion history, pp. 1–13, in Van Driesche RG, Reardon R (eds.). The Biology and Control of Emerald Ash Borer. USDA FS FHTET 2014–09
Heath RR, Sonnet PE (1980) Technique for in situ coating of Ag+ onto silica gel in HPLC columns for the separation of geometrical isomers. J Liq Chromatogr 3:1129–1135
Hooie HA, Wiggins GJ, Lambdin PL, Grant JF, Powell SD, Lelito JP (2015) Native parasitoids and recovery of Spathius agrili from areas of release against emerald ash borer in eastern Tennessee, USA. Biocontrol Sci Tech 25:345–351
Jin Y, Roberts FG, Coates RM (2007) Stereoselective isoprenoid chain extension with acetoacetate dianion: (E,E,E)-geranylgeraniol from (E,E)-farnesol. Org Synth 84:43–57
Kasun ZA, Gao X, Lipinski RM, Krische MJ (2015) Direct generation of triketide stereopolyads via merged redox-construction events: Total synthesis of (+)-zincophorin methyl ester. J Am Chem Soc 137:8900–8903
Keeling C, Plettner E, Slessor KN (2004) Hymenopteran Semiochemicals. Top Curr Chem 239:133–177
Lister T, Perkins MV (2004) Total synthesis of a hemiacetal polypropionate from Siphonaria australis. Aus J Chem 57:787–797
Liu HP, Bauer LS, Gao RT, Zhao TH, Petrice TR, Haack RA (2003) Exploratory survey for the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), and its natural enemies in China. Great Lakes Entomol 36:191–204
Liu HP, Bauer LS, Miller DL, Zhao TH, Gao RT, Song L, Luan Q, Jin R, Gao C (2007) Seasonal abundance of Agrilus planipennis (Coleoptera: Buprestidae) and its natural enemies Oobius agrili (Hymenoptera: Encyrtidae) and Tetrastichus planipennisi (Hymenoptera: Eulophidae) in China. Biol Control 42:61–71
Mapbiocontrol (2020) Available at: http://www.mapbiocontrol.org/2020
NIST (2017) NIST Standard Reference Database Number 173, National Institute of Standards and Technology, Gaithersburg MD, 20899
Tröger A, Van Beek TA, Huigens ME, Silva IMMS, Posthumus MA, Francke W (2014) Structure elucidation of female-specific volatiles released by the parasitoid wasp Trichogramma turkestanica (Hymenoptera: Trichogrammatidae). J Organomet Chem 10:767–773
Ulyshen MD, Duan JJ, Bauer LS, Fraser I (2010) Suitability and accessibility of immature Agrilus planipennis (Coleoptera: Buprestidae) stages to Tetrastichus planipennisi (Hymenoptera: Eulophidae). J Econ Entomol 103:1080–1085
USDA–APHIS/ARS/FS (2019) 2020. Emerald ash borer biological control release and recovery guidelines. USDA–APHIS–ARS-FS, Riverdale, Maryland. Available at: https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/EAB-FieldRelease-Guidelines.pdf
Wang Q, Deredas D, Huynh C, Schlosser M (2003) Sequestered alkyllithiums: why phenyllithium alone is suitable for betaine-ylide generation. Chem Eur J 9:570–574
Wiley (2007) Wiley. Spectral library, [CD-ROM], 7th edn. Wiley, New York
Yang Z, Strazanac JS, Yanxia Y, Xaioyi W (2006) A new species of emerald ash borer parasitoid from China belonging to the genus Tetrastichus Haliday (Hymenoptera: Eulophidae). Proc Entomol Soc Wash 108:550–558
Zilkowski BW, Bartelt RJ, Cossé AA, Petroski RJ (2006) Male-produced aggregation pheromone compounds from the eggplant flea beetle (Epitrix fuscula): identification, synthesis, and field biossays. J Chem Ecol 32:2543–2558
Acknowledgements
We thank Karl Vermillion for the NMR analysis (USDA/ARS/NCAUR, Peoria, IL). We thank Robert Bartelt (USDA/ARS/NCAUR, Peoria, IL, retired) for very helpful suggestions towards the structure determination. We thank Miriam Cooperband (USDA/APHIS/PPQ, Otis Laboratory, MA) for the statistical analysis. We are grateful for financial support from the U.S. Department of Agriculture, Forest Service, and Animal & Plant Health Inspective Service (APHIS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclaimer
This article reports the results of research only. Mention of a proprietary product does not constitute an endorsement or a recommendation by the U.S. Department of Agriculture (USDA) for its use. This research was supported [in part] by the intramural research program of the USDA Agricultural Research Service and the USDA Animal and Plant Health Inspection Service, Plant Protection and Quarantine. USDA is an equal opportunity provider and employer.
Electronic Supplementary Material
ESM 1
(DOCX 131 kb)
Rights and permissions
About this article
Cite this article
Cossé, A.A., Zilkowski, B.W., Zou, Y. et al. Female-Produced Sex Pheromone of Tetrastichus planipennisi, a Parasitoid Introduced for Biological Control of the Invasive Emerald Ash Borer, Agrilus planipennis. J Chem Ecol 46, 508–519 (2020). https://doi.org/10.1007/s10886-020-01188-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-020-01188-0