Abstract
An increasing body of evidence suggests that the volatile pheromones of cerambycid beetles are much more diverse in structure than previously hypothesized. Here, we describe the identification, synthesis, and field testing of (2E,6Z,9Z)-2,6,9-pentadecatrienal as a male-produced aggregation-sex pheromone of the cerambycid Elaphidion mucronatum (Say) (subfamily Cerambycinae, tribe Elaphidiini). This novel structure is unlike any previously described cerambycid pheromone, and in field bioassays attracted only this species. Males produced about 9 μg of pheromone per 24 h period, and, in field trials, lures loaded with 10, 25, and 100 mg of synthetic pheromone attracted beetles of both sexes, whereas lures loaded with 1 mg of pheromone or less were not significantly attractive. Other typical cerambycine pheromones such as 3-hydroxy-2-hexanone, syn-2,3-hexanediol, and anti-2,3-hexanediol were not attractive to E. mucronatum, and when combined with (2E,6Z,9Z)-2,6,9-pentadecatrienal, the former two compounds appeared to inhibit attraction. Unexpectedly, adults of the cerambycine Xylotrechus colonus (F.) were attracted in significant numbers to a blend of 3-hydroxyhexan-2-one and (2E,6Z,9Z)-2,6,9-pentadecatrienal, even though there is no evidence that this species produces the latter compound. From timed pheromone trap catches, adults of E. mucronatum were determined to be active from dusk until shortly after midnight.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cerambycid beetle Elaphidion mucronatum (Say) (subfamily Cerambycinae, tribe Elaphidiini) is among the most widespread and common cerambycid species throughout eastern North America (Hanks et al. 2014; Lingafelter 2007). The larvae feed in dead twigs and branches of hardwoods, and the species is considered one of the most polyphagous cerambycids in North America (MacRae and Rice 2007). The adults are active from April – October throughout its range (Lingafelter 2007), but populations peak from mid-June to late July in east-central Illinois, where our studies were conducted (Hanks et al. 2014). The species can be destructive, as it has been reported to bore into rustic furniture and other finished wooden products (Duffy 1953; Eyre and Haack 2017; Linsley 1963). In addition, E. mucronatum has the potential to be invasive because the larvae are readily exported accidentally to other continents (Duffy 1953; Eyre and Haack 2017).
Adults of E. mucronatum were known to be attracted to fermenting baits (Champlain and Kirk 1926; Frost and Dietrich 1929), ethanol (Dunn and Potter 1991; Millar et al. 2017; Miller et al. 2015), or odors from freshly cut host trees (Coyle et al. 2015). In addition, we have often caught this species in good numbers during field bioassays testing various cerambycid pheromones, but catches usually appeared to be spread randomly among the traps, with no significant treatment effect (e.g., Hanks and Millar 2013). However, in several instances, both sexes of this species had been caught in significant numbers while screening known cerambycid pheromones such as 3-hydroxy-2-hexanone and anti-2,3-hexanediols (Handley et al. 2015; Millar et al. 2017; Wong et al. 2017b), suggesting that its pheromone might consist of one or more of the compounds known from other species in its subfamily.
We report here that this is not the case. Instead, we present conclusive evidence that the male-produced aggregation-sex pheromone of E. mucronatum consists of (2E,6Z,9Z)-2,6,9-pentadecatrienal, a novel compound completely unrelated in structure to the hydroxyketone or 2,3-alkanediol type pheromones typical of other cerambycine species. Furthermore, we found that if anything, attraction to (2E,6Z,9Z)-2,6,9-pentadecatrienal is inhibited by the latter types of compounds. We also conducted dose-response trials, and used pheromone-baited timer traps to determine the diel activity patterns of the adults.
Methods and Materials
General Trapping Methods
Adults of E. mucronatum were caught with cross-vane panel traps (black corrugated plastic, Alpha Scents, Portland, OR, USA). Capture efficiency of traps was improved by coating the panels and interior surfaces of the bottom funnel with the fluoropolymer dispersion Fluon® (10% aqueous dilution; Northern Specialty Chemicals, Dudley, MA, USA; Graham et al. 2010). For bioassays, trap basins were partially filled with saturated aqueous NaCl solution to kill and preserve captured specimens. Beetles for pheromone collection were captured alive by replacing the trap basins with 2-l plastic jars with the bottoms replaced with aluminum screen to allow rainwater to drain. Traps were hung with bottoms ~0.5 m above the ground from inverted L-shaped frames of polyvinyl chloride pipe, and during bioassays were deployed 10 m apart in linear transects. Lures for bioassays consisted of polyethylene sachets (5.1 × 7.6 cm, Bagettes® model 14770, Cousin Corp., Largo, FL, USA) charged with solutions of test compounds in 1 ml of isopropanol (see below for doses and blends). Control lures were charged with 1 ml of isopropanol alone. Traps were serviced every 2–3 d during bioassays, at which time treatments were moved one position along transects to control for positional effects. Unless otherwise stated, field bioassays were conducted according to these procedures at Robert Allerton Park (Piatt Co., IL, USA; 39.996 lat., −88.651 long.).
Voucher specimens of adults of E. mucronatum that were captured during the studies are available from the laboratory collection of LMH.
Collection and Analysis of Beetle-Produced Compounds
The first adults of E. mucronatum that were used for collection of beetle-produced odors were obtained as bycatch in traps baited with synthesized pheromones of other cerambycid species during 2008–2016 (e.g., Hanks et al. 2014), totaling 54 males and 13 females. In the laboratory, the beetles were caged separately by sex under ambient environmental conditions (~12:12 h L:D, ~20 °C) and provided 10% aqueous sucrose solution as a source of water and nutrition. Beetle-produced volatiles were collected from individual males and females, or from groups of up to four beetles of the same sex. Beetles were held in glass Mason-style canning jars placed adjacent to closed exterior windows (natural photoperiod, ~14:10 h L:D, ~20 °C). Purified air was passed through the jars at a rate of ~1 l/min for 24–48 h. Each jar outlet was fitted with a glass tube cartridge containing the adsorbent polymer HayeSep® Q (150 mg; Sigma-Aldrich, St. Louis, MO, USA) sandwiched between plugs of glass wool, to trap beetle-produced odors. Aerations of jars without beetles were run simultaneously as controls for system contaminants. The insect-produced chemicals were recovered by extracting the adsorbent cartridges with 1.5 ml of dichloromethane containing eicosane (38 μg) as internal standard for analyses. Aerations from an additional eight males of E. mucronatum were used to estimate the rate at which they released the pheromone. These beetles were captured with panel traps baited with synthesized (2E,6Z,9Z)-2,6,9-pentadecatrienal in the yards of three residences in Urbana, IL (Champaign Co.) during 2016 and 2017.
Aeration extracts were analyzed on an Agilent 7890B GC coupled to a 5977A mass selective detector (Agilent Technologies, Santa Clara, CA, USA), in splitless mode with helium carrier gas. The GC was fitted with an HP-5 column (30 m × 0.25 mm ID, Agilent Technologies), and the oven temperature was programmed from 30 °C for 1 min, then at 10 °C/min to 250 °C and held for 5 min. The total amount of pheromone in each of these extracts was estimated by comparison to an external standard curve constructed using the synthetic standard (1.0–100 ng/μl, linear regression r 2 = 0.99).
At UC Riverside, aeration extracts were analyzed on an Agilent 7820A GC coupled to a 5977E mass selective detector, in splitless mode with helium carrier gas. The GC was fitted with an HP-5 column (30 m × 0.25 mm ID, Agilent), and the oven temperature was programmed from 40 °C for 1 min, then at 10 °C/min to 280 °C and held for 10 min. Mass spectra were recorded with electron impact ionization (EI) at 70 eV.
An aliquot of a headspace collection from a male beetle was diluted in 200 μl hexane in a 2 ml septum-capped vial, ~2 mg of 5% palladium on carbon was added, and the vial was briefly flushed with hydrogen, then sealed and stirred for 30 min under hydrogen atmosphere. The mixture was then filtered through a plug of celite filtering aid, and the filtrate was analyzed by GC-MS as described above.
Four headspace extracts were combined and concentrated to ~10 μl in a conical-bottomed vial under a gentle stream of nitrogen. The entire sample was then injected splitless over ~30 s onto a Hewlett-Packard 5890 GC fitted with a DB-5 Megabore column (25 m × 0.53 mm ID, 5 μm film thickness; J&W Scientific, Folsom, CA, USA), with an injector temperature of 250 °C, and an oven temperature program of 50 °C for 1 min, then 10 °C per min to 250 °C and held for 20 min. The column effluent was split between the flame ionization detector and a heated outlet port (200 °C), using a Y-splitter with a 0.1 mm ID fused silica capillary going to the detector and a 0.53 mm ID capillary going to the outlet port. Fractions were collected in dry ice-cooled glass capillaries. After warming to room temperature, the fraction containing the insect-produced compound was rinsed into a conical-bottomed vial with deuterated methylene chloride, then transferred to a 1 mm ID NMR tube. The sample was analyzed on a Bruker Avance 600 spectrometer (Bruker Biospin, Fremont, CA, USA) at 600 MHz, collecting a standard 1H spectrum, followed by a 1H-1H gCOSY spectrum. 1H NMR (600 MHz, CD2Cl2): δ 9.48 (d, 1H, J = 7.9 Hz), 6.85 (dt, 1H, J = 15.6, 6.8 Hz), 6.10 (ddt, 1H, J = 15.6, 7.8, 1.5 Hz), 5.46–5.29 (m, 4H), 2.79 (br t, 2H, J~7.1 Hz), 2.41 (br quart, 2H, J~ 7.3 Hz), 2.29 (br quart, 2H, J~ 7.2 Hz), 2.05 (br quart, J~7.2 Hz), 1.38–1.26 (m, 6H), 0.89 (t, 3H, J = 7.0 Hz).
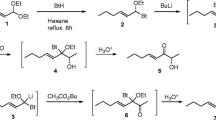
Synthesis of (2E,6Z,9Z)-2,6,9-Pentadecatrienal (Fig. 1)
A mixture of 80 g of a commercial oil containing arachidonic acid as triglycerides (~40% arachidonic acid, catalog #FA76490, Carbosynth LLC, San Diego, CA, USA), 240 ml ethanol, 35.2 ml water, and 18.4 g KOH was heated at 65 °C for 3 h, then cooled to room temperature, diluted with 320 ml water, and extracted twice with 200 ml hexane. The aqueous phase was then acidified with 160 ml 4 M aqueous HCl, and extracted twice with 250 ml hexane. The combined hexane extracts were washed with water and brine, then dried and concentrated, giving 72 g of the crude acid 2 as a dark yellow oil. The oil was taken up in 320 ml THF, and the solution was added in portions to a solution of NaHCO3 (98 g) in 320 ml water. A solution of NaI (30 g, 200 mmol) and iodine (50.8 g, 200 mmol) in 270 ml water was added in portions over 20 min to the crude acid solution (foams!). The resulting dark brown slurry was protected from light and stirred overnight at room temperature. Hexane (300 ml) was then added to the mixture, stirring was continued for 30 min, and the layers were separated. The aqueous layer was extracted again with hexane (200 ml), and the combined hexane layers were washed with 2 M aqueous sodium thiosulphate. After separation of the layers, the resulting cloudy pale yellow organic layer was washed with brine, dried, and concentrated, yielding 36.1 g of the unstable iodide 3 as a viscous yellow oil which began to solidify on standing. EIMS, m/z (relative abundance): 332 (2), 303 (66, M+-I), 285 (10), 267 (4), 257 (5), 237 (4), 219 (6), 201 (11), 175 (17), 161 (18), 145 (20), 131 (26), 119 (32), 105 (50), 91 (89), 79 (90), 67 (100), 55 (73), 41 (50).
The oil was taken up in 200 ml dry MeOH, and 20 g K2CO3 was added. The resulting slurry was stirred overnight at room temp, protected from light. The creamy slurry was then gently extracted (to minimize emulsion formation) twice with 200 ml hexane, and the combined hexane layers were washed with water and brine, then dried and concentrated to yield 15.3 g of crude epoxide 4 as a yellow oil. The oil was purified by vacuum flash chromatography in a 150 ml sintered glass funnel, eluting with 10% EtOAc in hexane, yielding 14.2 g of epoxide 4. EIMS, m/z (relative abundance): 334 (M+, trace), 190 (4), 176 (8), 143 (100), 131 (6), 111 (17), 105 (11), 91 (18), 79 (22), 67 (16), 55 (19), 43 (5). 1H NMR (500 MHz, CDCl3): δ 5.55–5.41 (m, 2H), 5.41–5.29 (m, 4H), 3.67 (s, 3H), 2.97–2.90 (m, 2H), 2.86–2.77 (m, 4H), 2.46–2.34 (m, 3H), 2.26–2.18 (m, 1H), 2.07–2.01 (m, 2H), 1.90–1.75 (m, 2H), 1.67–1.49 (m, 2H), 1.39–1.23 (M, 6H), 0.88 (t, 3H, J = 6.7 Hz). 13C NMR: δ 173.60, 130.62, 130.53, 128.81, 127.43, 127.38, 124.20, 56.53, 56.20, 51.54, 34.64, 33.59, 31.48, 29.28, 27.19, 26.21, 25.78, 25.62, 22.54, 22.02, 14.03 ppm. The 1H NMR data were a reasonable match with literature data (Moustakis et al. 1985).
Epoxide 4 (14.2 g, 42.5 mmol) was added over 15 min to a solution of HIO4.2H2O (11.72 g, 51 mmol) in 150 ml MeOH, and the mixture was stirred for 3 h at room temp. Water was then added (300 ml) and the mixture was extracted twice with hexane (1 × 150 ml, 1 × 50 ml). The combined hexane extracts were washed with saturated aqueous NaHCO3, then dried and concentrated. Upon attempted Kugelrohr distillation, taking the oven temperature up to 100 °C at 0.2 mmHg, the material in the still pot became dark brown. Thus, the remaining crude product was purified by vacuum flash chromatography, eluting with 5% EtOAc in hexane, yielding impure dimethylacetal 5 (5.4 g). EIMS, m/z (relative abundance): 234 (M+, 1), 203 (2), 176 (1), 154 (5), 150 (8), 131 (3), 123 (5), 105 (3), 97 (7), 91 (12), 84 (13), 80 (11), 79 (14), 75 (100), 67 (7), 55 (4), 47 (5), 45 (5). 1H NMR (500 MHz, CDCl3): δ 5.53–5.30 (m, 6H), 4.39 (t, 1H, J = 5.8 Hz), 3.34 (s, 6H), 2.86–2.78 (m, 4H), 2.42 (br t, 2H, J ~ 6.2 Hz), 2.05 (br quart, J ~ 7.1 Hz), 1.39–1.24 (m, 6H), 0.88 (t, 3H, J = 6.4 Hz). 13C NMR: δ 130.45, 130.36, 128.63, 127.61, 127.46, 123.79, 104.05, 52.88, 31.47, 30.95, 29.27, 27.17, 25.79, 25.59, 22.52, 14.04 ppm.
Acetal 5 was taken up in 150 ml acetone, 2 ml of conc. HCl was added, and the mixture was stirred for 2 h. The solution was then cooled in an ice-bath and ice-cold saturated aqueous NaHCO3 (50 ml) was added slowly, giving a cloudy, yellow-orange slurry. Most of the acetone was removed by rotary evaporation, and the residue was diluted with water and extracted twice with hexane. The combined hexane layers were washed with brine, dried, and concentrated, and the residue was purified by vacuum flash chromatography, eluting with 5% EtOAc in hexane. The purest fractions were concentrated (2.9 g) and then Kugelrohr-distilled (bp ~95 °C at 0.05 mmHg), yielding 2.37 g of (2E,6Z,9Z)-2,6,9-pentadecatrienal 1 as a very pale yellow oil. EIMS, m/z (relative abundance): 220 (M+, trace), 202 (trace), 191 (1), 177 (2), 163 (3), 150 (60), 135 (5), 121 (9), 109 (16), 95 (30), 93 (28), 91 (26), 81 (51), 80 (54), 79 (84), 67 (100), 55 (37), 41 (50). 1H NMR (500 MHz, CD2Cl2): δ 9.52 (d, 1H, J = 9.5 Hz), 6.89 (dt, 2H, J = 15.6, 6.9 Hz), 6.14 (ddt, 15.6, 7.9, 1.5 Hz), 5.52–5.32 (m, 4H), 2.83 (br t, 2H, J ~ 7.1 Hz), 2.44 (dtt, 2H, J = ~7, ~7, 1.5 Hz), 2.33 (br quart, 2H, J ~ 7 Hz), 2.09 (br. quart, 2H, J ~ 7 Hz), 1.44–1.28 (m, 6H), 0.93 (t, 3H, J = 6.9 Hz). 13C NMR: δ 193.7, 157.8, 133.1, 130.5, 129.6, 127.7, 127.3, 32.6, 31.5, 29.3, 27.2, 25.6, 25.5, 22.6, 13.8 ppm. The full proton and carbon NMR spectra are shown in the supplementary online information (Figs. S1-S5).
Field Bioassay of Synthesized Pheromone
Attraction of adults of E. mucronatum to the synthesized pheromone was tested with a field bioassay conducted during 14–21 June 2017 (N = 12 replicates over time), using eight traps in a single linear transect, four of which were baited with (2E,6Z,9Z)-2,6,9-pentadecatrienal (25 mg in 1 ml isopropanol) alternating with four traps baited with solvent control lures (1 ml isopropanol). Treatments were moved one position along the transect at each count to minimize positional effects. At this dose, lures released the chemical at an average rate (± SD) of 100.8 ± 4.0 μg/d, estimated from collection of headspace volatiles from five lures, using the methods described above.
Dose-Response Experiment
A bioassay was conducted to determine the optimal dose of synthesized pheromone for attracting E. mucronatum. A transect of six traps was deployed to which were randomly assigned the following dosages of (2E,6Z,9Z)-2,6,9-pentadecatrienal (diluted in 1 ml of isopropanol): 0, 0.01, 0.1, 1, 10, and 100 mg. The experiment was conducted during 17 July – 21 August 2017 (N = 14 replicates over time).
Testing for Synergism by 3-Hydroxy-2-Hexanone and 2,3-Hexanediols
As mentioned above, earlier studies had suggested that adults of E. mucronatum were attracted by 3-hydroxy-2-hexanone (henceforth 3-ketol) and anti-2,3-hexanediol, although these compounds had not been found in extracts of headspace volatiles from males (see Results). An experiment was conducted to test for attraction to 3-ketol and both diastereomers of 2,3-hexanediol, as well as for synergistic effects of these compounds on attraction to the pheromone. Thus, a transect of eight traps was deployed that were baited with solutions of test compounds in 1 ml isopropanol, with 25 mg of (2E,6Z,9Z)-2,6,9-pentadecatrienal alone or combined with 50 mg of the racemic ketol or diols. The eight treatments were as follows: (2E,6Z,9Z)-2,6,9-pentadecatrienal alone, racemic 3-ketol alone, syn-2,3-hexanediol alone, anti-2,3-hexanediol alone, (2E,6Z,9Z)-2,6,9-pentadecatrienal +3-ketol, (2E,6Z,9Z)-2,6,9-pentadecatrienal + syn-2,3-hexanediol, (2E,6Z,9Z)-2,6,9-pentadecatrienal + anti-2,3-hexanediol, and solvent control. The experiment was conducted during 21 June – 17 July 2017 (N = 9 replicates over time). The racemic 3-ketol was purchased from Bedoukian Research (Danbury CT, USA), and the syn- and anti-2,3-hexanediols were synthesized as described in Lacey et al. (2004).
Diel Phenology of Adult Beetles
Diel flight phenology of adult E. mucronatum was characterized by capturing beetles with a panel trap as described above, but with the collection basin replaced with a mechanism which changed eight collection bottles at programmable intervals (collection bottle rotator model #2850, BioQuip Products, Rancho Dominguez, CA, USA). A Fluon-coated plastic funnel attached to the bottom funnel of the trap guided the captured beetles into the bottles. The trap was deployed in the yard of a private residence in Urbana, IL, and baited with (2E,6Z,9Z)-2,6,9-pentadecatrienal during 20 June – 13 August 2017. The mechanism was set to rotate the bottles at 1-h intervals from 1900 h to 0300 h the following morning, which was intended to encompass the expected flight period of this species.
Statistical Analyses
Differences among treatment means in numbers of adult beetles captured were tested with the nonparametric Friedman’s test (PROC FREQ, option CMH; SAS Institute 2011), with replicates defined by number of traps per treatment within transects and collection date. Replicates that contained no specimens in any treatment of the beetle species in question (e.g., due to inclement weather) were dropped from analyses. Pairs of treatments were compared using the REGWQ test (controlling experiment-wise error rates; SAS Institute 2011), and were protected (i.e., assuming a significant overall Friedman’s test).
Results
Collection and Analysis of Beetle-Produced Compounds
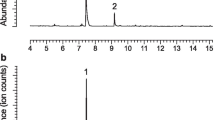
Aeration extracts from adult males of E. mucronatum contained a single dominant compound which was not present in aerations of females, nor in control aerations (Fig. 2a). The compound was detected in 14 of the 54 aeration extracts from males, with emission rates of 8.8 ± 5.1 μg/d (mean ± SD; N = 8). The EI mass spectrum of the insect-produced compound (Fig. 2b) had a trace of a possible molecular ion at m/z 220, a small m/z 202 from loss of water, a small m/z 191 (loss of 29 Da), and a prominent even-mass ion at m/z 150 (loss of 70 Da, possibly loss of C5H10). Catalytic reduction with hydrogen over 5% palladium on carbon gave a mixture of pentadecanol (m/z 228) and pentadecanal (m/z 226), which were confirmed with authentic standards. This provided several useful pieces of information. First, it proved that the basic carbon skeleton was a straight chain, with four double bond equivalents. Second, because pentadecanal was one of the products of reduction, this suggested that one of the unsaturations corresponded to the aldehyde function, leaving three C = C double bonds or the equivalent still to be accounted for. The production of the fully reduced pentadecanol may have been an artefact of the relatively large amount of palladium catalyst that was used in the microscale reduction.
The insect-produced compound was isolated by preparative gas chromatography, and the NMR spectra allowed unequivocal identification of the purified compound. Thus, a one-proton doublet at 9.48 ppm (J = 7.9 Hz) was coupled to a vinylic proton at 6.10 ppm (ddt, J = 15.6, 7.9, 1.5 Hz) which in turn was coupled to another vinylic proton at 6.85 ppm (dt, J = 15.6, 6.8 Hz), indicating the presence of a conjugated, trans-1,2-disubstituted aldehyde with a methylene at carbon 4 (2.41 ppm, broadened quartet, J ~7.3 Hz). This methylene was in turn coupled to another allylic methylene at 2.29 ppm (broadened quartet, J ~7.2 Hz), which was also coupled to a vinylic proton in a 4-proton multiplet at 5.46–5.29 ppm. A broadened 2-proton triplet at 2.79 ppm (J ~7.1 Hz) was assigned to a bisallylic methylene between two 1,2-disubstituted double bonds, accounting for all the unsaturations in the molecule. A 2-proton broadened quartet at 2.05 ppm (J ~7.2 Hz) was assigned to an allylic methylene on the distal side of the double bond system. The remaining protons consisted of a 6-proton multiplet from 1.38–1.26 ppm and a terminal methyl at 0.89 ppm (J = 7.0 Hz), corresponding to a 4-carbon, straight-chain saturated alkane fragment. In total, these data unequivocally proved the structure to be a (2E,6,9)-pentadecatrienal. The protons from the two 1,2-disubstituted double bonds at carbons 6–7 and 9–10 all had similar chemical shifts, suggesting that they were probably both cis or both trans, rather than one of each. Furthermore, biosynthetic considerations suggested that both were likely to be cis, based on the similarity of the structure to a polyunsaturated fatty acid such as arachidonic acid in terms of the positions of the double bonds relative to the end of the chain, and the 1,4-diene motif with the double bonds separated by a methylene. Thus, the insect-produced compound was tentatively identified as (2E,6Z,9Z)-2,6,9-pentadecatrienal 1, and this was confirmed by synthesis of the authentic material.
Synthesis of (2E,6Z,9Z)-2,6,9-Pentadecatrienal (Fig. 1)
Our synthesis was designed to exploit the preexisting placement and stereochemistry of the double bonds in a common fatty acid, arachidonic acid, i.e., (5Z,8Z,11Z,14Z)-5,8,11,14-eicosatetraenoic acid 2 in Fig. 1. Thus, a sample of crude arachidonic acid was prepared from a commercial triglyceride sample enriched in the acid by hydrolysis in alkaline aqueous ethanol (Vali et al. 2003). Then, adopting a strategy pioneered by E. J. Corey, the crude acid fraction, containing about 40% arachidonic acid, was submitted to iodolactonization (Corey and Wright 1984). This resulted in selective iodolactonization of the arachidonic acid to a favored six-membered ring lactone, whereas the other fatty acids present in the mixture did not cyclize. Thus, simple extraction of the mildly basic reaction mixture with hexane allowed the separation in reasonably high purity of the unstable iodolactone 3, leaving the remaining acids in the aqueous layer as their salts. The iodolactone 3 was immediately treated with K2CO3 in dry methanol (Flock et al. 1999), resulting in transesterification of the lactone to the methyl ester, with the resulting secondary alcohol subsequently displacing the iodide on the adjacent carbon to give epoxy-ester 4. Cleavage of the epoxide with periodic acid in MeOH then gave acetal 5 (Flock et al. 1999). The synthesis was then completed by trans-acetalization of 5 with HCl in acetone (Mustafa et al. 2014), which both cleaved the acetal to the aldehyde, and induced isomerization of the C = C double bond at carbon 3 into conjugation with the aldehyde, giving the desired (2E,6Z,9Z)-2,6,9-pentadecatrienal 1.
Field Bioassay of Synthesized Pheromone
A total of 44 adult males and females of E. mucronatum was trapped during the initial field bioassay of the candidate pheromone, all of which were caught in traps baited with the synthesized pheromone (mean ± SE: 5.5 ± 2 beetles per replicate, significantly different from the zero mean catch in unbaited traps, Friedman’s Q 1,16 = 12.9, P < 0.001). The pheromone appeared to be species-specific because only four beetles of another three cerambycid species were caught in total, apparently by random encounters with traps.
Dose-Response Experiment
In the experiment to determine the optimal dose for trapping E. mucronatum, treatment means differed significantly in numbers of beetle trapped (Fig. 3a; Q 5,36 = 21.0, P < 0.001). The two highest doses (10 and 100 mg) were significantly more attractive than all other treatments, which were not significantly different than the solvent control. No other species were caught in significant numbers in any of the treatments.
Captures of adults of Elaphidion mucronatum (both sexes combined) by traps baited with: a varying dosages of the candidate pheromone (2E,6Z,9Z)-2,6,9-pentadecatrienal (“PentaDec” or “PD”), and b traps baited with the pheromone alone, 3-hydroxy-2-hexanone (“Ketol”), syn-(2,3)-hexanediol (“syn-diol”), anti-(2,3)-hexanediol (“anti-diol”), the same three compounds individually blended with (2E,6Z,9Z)-2,6,9-pentadecatrienal, and a solvent control. For each figure, means with different letters are significantly different (REGWQ test, P < 0.05)
Testing for Synergism by 3-Hydroxy-2-Hexanone and 2,3-Hexanediols
In the field trial testing attraction to 3-hydroxy-2-hexanone, syn-2,3-hexanediol, anti-2,3-hexanediol, and (2E,6Z,9Z)-2,6,9-pentadecatrienal as individual components, and blends of each of the former three compounds with (2E,6Z,9Z)-2,6,9-pentadecatrienal, adults of E. mucronatum were significantly attracted only to (2E,6Z,9Z)-2,6,9-pentadecatrienal alone, and not to 3-hydroxy-2-hexanone or to either of the 2,3-hexanediols (Fig. 3b; means significantly different, Q 7,56 = 40.4, P < 0.001). Furthermore, addition of 3-hydroxy-2-hexanone and at least the syn-2,3-hexanediol to (2E,6Z,9Z)-2,6,9-pentadecatrienal resulted in significant depression of attraction.
Another 165 adult males and females of other cerambycid species were captured during this bioassay, in most cases responding to their known 3-ketol or 2,3-hexanediol pheromones (see Hanks and Millar 2016), notably the cerambycines Neoclytus acuminatus acuminatus (F.) (N = 76 beetles), Neoclytus mucronatus mucronatus (F.) (15 beetles), and Xylotrechus colonus (F.) (32 beetles). The pheromone of N. a. acuminatus is composed solely of (2S,3S)-hexanediol, and adults indeed were attracted in significant numbers only to traps baited with syn-2,3-hexanediol (mean 5.3 ± 2.6 beetles), with the mean for the blend of the diol and (2E,6Z,9Z)-2,6,9-pentadecatrienal being intermediate (2.8 ± 1.3 beetles) compared to the means of 0.2 beetles or lower for the remaining treatments (Q 7,72 = 41.1, P < 0.001). The (R)-enantiomer of 3-hydroxy-2-hexanone is the sole pheromone component for N. m. mucronatus and the primary component for X. colonus. It therefore was no surprise that adults of N. m. mucronatus were significantly attracted only by racemic 3-ketol (1.8 ± 0.5 beetles), attraction to the ketol apparently being antagonized by (2E,6Z,9Z)-2,6,9-pentadecatrienal (mean for the blend: 0.67 ± 0.3 beetle), which did not differ significantly from the zero means of the remaining treatments (Q 7,48 = 31.1, P < 0.001). However, unexpectedly, adults of X. colonus were significantly attracted only by the blend of the racemic 3-ketol and (2E,6Z,9Z)-2,6,9-pentadecatrienal (2.6 ± 0.75 beetles), with the mean for the ketol alone (1.0 ± 0.46 beetles) being not significantly different from the means of 0.25 beetles or lower for the remaining treatments (Q 7,64 = 37.6, P < 0.001).
Diel Phenology of Adult Beetles
The trap with the timer mechanism captured 15 males and 12 females of E. mucronatus, with average (± SD) hour of capture being 22:14 ± 1:4 h. The sexes did not differ significantly in the mean hour of capture (ANOVA F 1,23 = 1.7, P = 0.21). Numbers of beetles trapped per 1-h period were skewed towards the beginning of the scotophase, with 8, 5, 4, 5, and 3 beetles caught during the intervals 20:00–21:00, 21:01–22:00, 22:01–23:00, 23:01–24:00, and 24:01–01:00 h, respectively. Thus, beetles began to fly shortly before the onset of complete darkness (solar radiation fell to zero during 21:00–22:00 h throughout the seasonal flight period of E. mucronatum; Illinois Climate Network 2014, Illinois State Water Survey, Champaign, IL: http://dx.doi.org/10.13012/J8MW2F2Q), indicating that adults of E. mucronatum are crepuscular to nocturnal.
Discussion
Over the past decade, research on the chemical ecology of longhorned beetles (Coleoptera: Cerambycidae) has undergone a rapid expansion, driven in large part by the realization that, contrary to earlier hypotheses (Hanks 1999; Allison et al. 2004), volatile male-produced aggregation-sex pheromones (subfamilies Cerambycinae, Lamiinae, and Spondylidinae) or female-produced sex pheromones (subfamilies Prioninae and Lepturinae) are widely used within the family as crucial mediators of reproduction (reviewed in Hanks and Millar 2016; Millar and Hanks 2017). Initially, probable pheromones of many species were identified by field testing the few known cerambycid pheromones or analogs thereof, such as 3-hydroxy-2-alkanones and the corresponding 2,3-alkanediols. Follow-up studies then showed that many of these species did indeed produce the compounds to which they had been attracted, confirming that the attractants were pheromones. Thus, over the space of a few years, pheromones or likely pheromones were identified for more than 100 species (reviewed in Hanks and Millar 2016), but the number of unique compounds that constituted these pheromones was much lower because of the extensive sharing of compounds among related taxa. Although this strategy was highly successful for rapid identification of pheromones, it may have unintentionally biased researchers towards thinking that pheromone structures in general were likely to be highly conserved among related cerambycid species. However, recent research has identified an increasing number of structures which are entirely different from the previously known, “typical” cerambycid pheromones. Furthermore, at least some of these structures may be species-specific, or restricted to a very limited number of species (e.g., Ray et al. 2009; Silva et al. 2016a, b; Zou et al. 2015). These findings suggest that a more nuanced hypothesis would be more realistic, possibly spanning a continuum from widely shared structures such as 2,3-alkanediols, 3-hydroxy-2-alkanones, and hydroxyethers, which are shared among tribes or even subfamilies, through to pheromones which are unique to one species. It is also becoming clear that the chemistry of the cerambycid pheromones is diverse, with pheromone components likely arising from a variety of different biosynthetic pathways, and with some containing heteroatoms such as nitrogen (e.g., Diesel et al. 2017; Zou et al. 2016) or sulfur (Silva et al. 2017).
(2E,6Z,9Z)-2,6,9-Pentadecatrienal, the pheromone of E. mucronatum, continues this trend towards increasing diversity of cerambycid pheromone structures. Although three aldehydes have been reported as cerambycid pheromones, one has a saturated methyl-branched structure (10-methyldodecanal in Eburodachrys vittata [Blanchard]; Silva et al. 2016a), the second consists of a 15-carbon straight chain with a conjugated diene in the middle of the chain (the congeners Chlorida festiva [L.] and C. costata Audinet-Serville; Silva et al. 2016b), and the third has a straight-chain alkyl ether skeleton (Anoplophora glabripennis [Motschulsky]; Zhang et al. 2002). None of these is very closely related structurally to the pheromone of E. mucronatum, which, judging by the placement and stereochemistry of its double bonds, may arise from a polyunsaturated fatty acid precursor, such as the arachidonic acid starting material used in our synthesis of the pheromone. Males of E. mucronatum do not have the prothoracic gland pores that have been associated with the production of hydroxyketone and 2,3-alkanediol pheromones in many other cerambycine species (Hoshino et al. 2015; Ray et al. 2006), and the site of production and release of the pheromone remain unknown. It should be noted that the association between pheromone gland pores and hydroxyhexanone and/or 2,3-hexanediol pheromones holds true for other species in the same tribe as E. mucronatum, the Elaphidiini, including species in the genera Ambonus, Anelaphus, Enaphalodes, Orwellion, and Parelaphidion (Mitchell et al. 2013, 2015; Ray et al. 2006; Silva et al. 2017). The fact that no other cerambycid species were caught in significant numbers during field bioassays of the E. mucronatum pheromone (except in the trial in which 3-hydroxyhexan-2-one and 2,3-hexanediols were included as test components) suggests that the pheromone may be species-specific.
As has been noted with other cerambycids which produce male-produced aggregation-sex pheromones (e.g., Álvarez et al. 2016; Lacey et al. 2004, 2007; Wong et al. 2017a), relatively high doses/release rates of synthesized pheromone were required to achieve significant levels of attraction to traps. Our data suggested that, with the dispensers used in this study, there is a minimum threshold dose somewhere between 1 and 10 mg/lure, below which no significant attraction occurs. Under laboratory conditions, males of E. mucronatum released about 9 μg of pheromone per 24 h period, but given that the timer trap data showed that the beetles were only active for 4–5 h per day, the actual release rate was probably several μg/h for a period of several hours. The release rate measured from our pheromone dispensers loaded with 25 mg of pheromone was about 4 μg/h, similar to the natural release rate from individual males. In sum, these data suggest that a release rate approximating that released by a single male is sufficient to achieve significant attraction.
The fact that attraction was virtually the same for the 10 and 100 mg lure doses also indicates that above a certain dose/release rate, attraction reaches a plateau rather than continuing to increase with dose. The significant attraction to the largest dose of 100 mg also suggests that beetle responses are not inhibited by what may be unnaturally high release rates. Thus, for practical purposes, with the dispensers used in this study, doses of about 25 mg may represent an optimal compromise between field effectiveness and cost-effective use of the synthesized pheromone.
In contrast to the results from several previous studies in which significant numbers of E. mucronatum had been attracted to traps baited with 3-hydroxy-2-hexanone and 2,3-hexanediols, either as single components or as components of blends (Handley et al. 2015; Millar et al. 2017; Wong et al. 2017b), we found no evidence of attraction to such compounds in a field trial that directly compared attraction to these compounds versus (2E,6Z,9Z)-2,6,9-pentadecatrienal. In fact, when these compounds were released as blends with (2E,6Z,9Z)-2,6,9-pentadecatrienal, they appeared to inhibit attraction to the pheromone. One possible explanation for these results, which appear to contradict previous findings (Handley et al. 2015; Millar et al. 2017; Wong et al. 2017b), may be that in the absence of their own pheromone, beetles may be weakly attracted to the pheromones of other members of their guild, as a way of indirectly locating suitable hosts for their larvae. Such cross-attraction to the pheromones of heterospecifics previously has been documented for several cerambycid species (reviewed in Hanks and Millar 2016). Conversely, when the appropriate pheromone is present, that attraction may completely override any weak attraction to the pheromones of other species.
The unexpected attraction of X. colonus to the blend of 3-ketol and (2E,6Z,9Z)-2,6,9-pentadecatrienal may represent a further example of exploitation of the pheromone of a heterospecific. In particular, no trace of (2E,6Z,9Z)-2,6,9-pentadecatrienal has been found in analyses of >40 collections of headspace volatiles from male X. colonus (LMH, unpublished data), confirming that (2E,6Z,9Z)-2,6,9-pentadecatrienal is not produced by this species. However, E. mucronatum and X. colonus are both highly polyphagous, adults of both species are attracted to felled or girdled hardwoods (Waters 1981), their larvae develop in dead and dying hardwoods, the adults overlap broadly in seasonal flight period (Hanks et al. 2014), and the adults have overlapping diel activity patterns (X. colonus flies from ~19:00–22:00; LMH, unpublished data). Thus, exploitation of the pheromone of a heterospecific guild member with very similar host range and biology seems to be the only reasonable explanation for the attraction.
In summary, we have presented analytical and field bioassay data showing that the aggregation-sex pheromone of E. mucronatum consists of the single male-produced compound (2E,6Z,9Z)-2,6,9-pentadecatrienal. This novel compound extends the chemical diversity of known cerambycid pheromones, providing further evidence that the “chemical space” occupied by such pheromones is probably much larger than originally hypothesized. A minimum threshold dose of several mg, resulting in a release rate of ~1 μg/h or more was required to obtain significant attraction, and attraction appeared to plateau at release rates equivalent to that produced by several males. Fortunately, the pheromone can be synthesized in several straightforward steps from inexpensive and readily available starting materials, and so cost and availability should not represent barriers to its development for detection and monitoring of this species.
References
Allison JD, Borden JH, Seybold SJ (2004) A review of the chemical ecology of the Cerambycidae (Coleoptera). Chemoecology 14:123–150
Álvarez G, Gallego D, Hall DR, Jactel H, Pajares JA (2016) Combining pheromone and kairomones for effective trapping of the pine sawyer beetle Monochamus galloprovincialis. J Appl Entomol 140:58–71
Champlain AB, Kirk HB (1926) Bait pan insects. Entomol News 37:288–291
Corey EJ, Wright SW (1984) A simple process for the purification of arachidonic acid. Tet Lett 26:2729–2730
Coyle DR, Brissey CL, Gandhi KJK (2015) Species characterization and responses of subcortical insects to trap-logs and ethanol in a hardwood biomass plantation. Ag For Entomol 17:258–269
Diesel NM, Zou Y, Johnson TD, Diesel DA, Millar JG, Mongold-Diers JA, Hanks LM (2017) The rare north American cerambycid beetle Dryobius sexnotatus shares a novel pyrrole pheromone component with species in Asia and South America. J Chem Ecol 43:739–744
Duffy EAJ (1953) A monograph of the immature stages of British and imported timber beetles (Cerambycidae), Brit Mus (Nat. Hist.), London
Dunn JP, Potter DA (1991) Synergistic effects of oak volatiles with ethanol in the capture of saprophagous wood borers. J Entomol Sci 26:425–429
Eyre D, Haack RA (2017) Invasive cerambycid pests and biosecurity measures. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC Press/Taylor & Francis, Boca Raton, pp 563–607
Flock S, Lundquist M, Skattebǿl L (1999) Syntheses of some polyunsaturated sulfur- and oxygen-containing fatty acids related to eicosapentaenoic and docosapentaenoic acids. Acta Chem Scand 53:436–445
Frost SW, Dietrich H (1929) Coleoptera taken from bait-traps. Ann Entomol Soc Am 22:427–436
Graham EE, Mitchell RF, Reagel PF, Barbour JD, Millar JG, Hanks LM (2010) Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. J Econ Entomol 103:641–647
Handley K, Hough-Goldstein J, Hanks LM, Millar JG, D'Amico V (2015) Species richness and phenology of cerambycid beetles in urban forest fragments of northern Delaware. Ann Entomol Soc Am 108:251–262
Hanks LM (1999) Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu Rev Entomol 44:483–505
Hanks LM, Millar JG (2013) Field bioassays of cerambycid pheromones reveal widespread parsimony of pheromone structures, enhancement by host plant volatiles, and antagonism by components from heterospecifics. Chemoecology 23:21–44
Hanks LM, Millar JG (2016) Sex and aggregation pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654
Hanks LM, Reagel PF, Mitchell RF, Wong JCH, Meier LR, Silliman CA, Graham EE, Striman BL, Robinson KP, Mongold-Diers JA, Millar JG (2014) Seasonal phenology of the cerambycid beetles of east-central Illinois. Ann Entomol Soc Am 107:211–226
Hoshino K, Nakaba S, Inoue H, Iwabuchi K (2015) Structure and development of male pheromone gland of longicorn beetles and its phylogenetic relationships within the tribe Clytini. J Exp Zool Part B: Mol Dev Evol 324:68–76
Lacey ES, Ginzel MD, Millar JG, Hanks LM (2004) Male-produced aggregation pheromone of the cerambycid beetle Neoclytus acuminatus acuminatus. J Chem Ecol 30:1493–1507
Lacey ES, Moreira JA, Millar JG, Ray AM, Hanks LM (2007) Male-produced aggregation pheromone of the cerambycid beetle Neoclytus mucronatus mucronatus. Entomol Exp Appl 122:171–179
Lingafelter SW (2007) Illustrated key to the longhorned wood-boring beetles of the eastern United States. Special Publication No. 3. Coleopterists Society, North Potomac
Linsley EG (1963) The Cerambycidae of North America, Part IV Taxonomy and classification of the subfamily Cerambycinae, tribes Elaphidionini through Rhinotragini University of California Publications in Entomology 21 University of California Press, Berkeley
MacRae TC, Rice ME (2007) Biological and distributional observations on north American Cerambycidae (Coleoptera). Coleopt Bull 61:227–263
Millar JG, Hanks LM (2017) Chemical ecology of cerambycids. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC press. Taylor & Francis, Boca Raton, pp 167–202
Millar JG, Hanks LM, Mitchell RF, Mongold-Diers JA, Zou Y, Bográn CE, Dodds KJ, Fierke MK, Ginzel MD, Johnson CW, Meeker JR, Poland TM, Ragenovich I (2017) Identifying possible pheromones of cerambycid beetles by field testing known pheromone components in four widely separated regions of the United States. J Econ Entomol (in press)
Miller DR, Crowe CM, Mayo PD, Silk PJ, Sweeney JD (2015) Responses of Cerambycidae and other insects to traps baited with ethanol, 2,3-hexanediol, and 3,2-hydroxyketone lures in north-central Georgia. J Econ Entomol 108:2354–2365
Mitchell RF, Millar JG, Hanks LM (2013) Blends of (R)-3-hydroxyhexan-2-one and alkan-2-ones identified as potential pheromones produced by three species of cerambycid beetles. Chemoecology 23:121–127
Mitchell RF, Reagel PF, Wong JCH, Meier LR, Silva WD, Mongold-Diers J, Millar JG, Hanks LM (2015) Cerambycid beetle species with similar pheromones are segregated by phenology and minor pheromone components. J Chem Ecol 41:431–440
Moustakis CA, Viala J, Capdevila J, Falck JR (1985) Total synthesis of cytochrome P450 epoxygenase metabolites 5(R),6(S)-, 5(S),6(R)-, and 14(R),15(S)-epoxyeicosatrienoic acid (EET) and hydration products 5(R),6(R)- and 14(R),15(R)-dihydroxyeicosatrienoic acid (DHET). J Am Chem Soc 107:5283–5285
Mustafa HH, Baird MS, Juma’a R, Tverezovskiy VV (2014) A nine-carbon homologating system for skip-conjugated polyenes. Chem Phys Lipids 183:34–42
Ray AM, Lacey ES, Hanks LM (2006) Predicted taxonomic patterns in pheromone production by longhorned beetles. Naturwissenschaften 93:543–550
Ray AM, Millar JG, McElfresh JS, Swift IP, Barbour JD, Hanks LM (2009) Male-produced aggregation pheromone of the cerambycid beetle Rosalia funebris. J Chem Ecol 35:96–103
SAS Institute (2011) SAS/STAT 9. User's Guide. SAS Institute Inc., Cary, NC, p 3
Silva WD, Millar JG, Hanks LM, Bento JMS (2016a) 10-Methyldodecanal, a novel attractant pheromone produced by males of the south American cerambycid beetle Eburodacrys vittata. PLoS One 11:e0160727
Silva WD, Millar JG, Hanks LM, Bento JMS (2016b) (6E,8Z)-6,8-Pentadecadienal, a novel attractant pheromone produced by males of the cerambycid beetles Chlorida festiva and Chlorida costata. J Chem Ecol 42:1082–1085
Silva WD, Zou Y, Bento JMS, Hanks LM, Millar JG (2017) Aggregation-sex pheromones and likely pheromones of 11 South American cerambycid beetles, and partitioning of pheromone channels. Frontiers Ecol Evol 5 article 101 https://doi.org/10.3389/fevo.2017.00101
Vali SJ, Sheng HY, Y-H J (2003) An efficient method for the purification of arachidonic acid from fungal single-cell oil (ARASCO). J Am Oil Chem Soc 80:725–730
Waters DJ (1981) Life history of Neoclytus acuminatus with notes on other cerambycids associated with dead or dying deciduous trees. Auburn University, Auburn, AL, M. Sc. thesis
Wong JCH, Meier LR, Zou Y, Mongold-Diers J, Hanks LM (2017a) Evaluation of methods used in testing attraction of cerambycid beetles to pheromone-baited traps. J Econ Entomol 110:2269–2274
Wong JCH, Zou Q, Millar JG, Hanks LM (2017b) Attraction of adult cerambycid beetles to their aggregation-sex pheromones is influenced by volatiles from host plants of their larvae. Environ Entomol 46:649–653
Zhang A, Oliver JE, Aldrich JR, Wang B. Mastro VC (2002) Stimulatory beetle volatiles for the Asian longhorned beetle, Anoplophora glabripennis (Motschulsky). Zeit Naturforsch C 57:553–558
Zou Y, Millar JG, Blackwood JS, Duzor RV, Hanks LM, Mongold-Diers JA, Wong JCH, Ray AM (2015) (2S,4E)-2-hydroxyoct-4-en-3-one, a male-produced attractant pheromone of the cerambycid beetle Tylonotus bimaculatus. J Chem Ecol 41:670–677
Zou Y, Rutledge CE, Nakamuta K, Maier CT, Hanks LM, Richards AB, Lacey ES, Millar JG (2016) Identification of a pheromone component and a critical synergist for the invasive beetle Callidiellum rufipenne (Coleoptera: Cerambycidae). Environ Entomol 45:216–222
Acknowledgements
We thank Kate Johnson for technical assistance in the field, and Steve Buck and James Ellis of the University of Illinois Committee on Natural Areas for access to field sites. JGM thanks Lars Skattebǿl (Dept. of Chemistry, University of Oslo) for the gift of a sample of (2E,6Z,9Z,12Z)-2,6,9,12-pentadecatetraenol. We appreciate funding support from The Alphawood Foundation of Chicago (to LMH) and from the USDA National Institute of Food and Agriculture (award # 2015-67013-23173 to LMH and JGM).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(PDF 592 kb)
Rights and permissions
About this article
Cite this article
Millar, J.G., Mitchell, R.F., Meier, L.R. et al. (2E,6Z,9Z)-2,6,9-Pentadecatrienal as a Male-Produced Aggregation-Sex Pheromone of the Cerambycid Beetle Elaphidion mucronatum . J Chem Ecol 43, 1056–1065 (2017). https://doi.org/10.1007/s10886-017-0905-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0905-1