Abstract
The compound 1-(1H–pyrrol-2-yl)-1,2-propanedione (“pyrrole”) is an important pheromone component of several Asian and South American species of longhorned beetles in the subfamily Cerambycinae. Here, we report the first confirmed identification of this compound as a pheromone component of a cerambycine species native to North America, the rare beetle Dryobius sexnotatus Linsley. Headspace volatiles from males contained (R)-3-hydroxyhexan-2-one and pyrrole (ratio 1:0.13), neither of which were detected in samples from a female. A field bioassay confirmed that adults of both sexes were attracted only to the binary blend of racemic 3-hydroxyhexan-2-one plus pyrrole, and not by either compound alone. Adults of another cerambycine, Xylotrechus colonus (F.), were attracted by 3-hydroxyhexan-2-one, consistent with this compound being the primary component of the pheromone of this species; attraction was not influenced by the presence of pyrrole. This study attests to the effectiveness of pheromone-baited traps in capturing rarely encountered species of cerambycids. It also provides further evidence that pyrrole represents another conserved pheromone motif within the Cerambycinae, now having been found in representatives of five cerambycid tribes from three continents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Research over the last decade has provided abundant evidence that adults of woodboring beetles of the family Cerambycidae produce volatile pheromones, and that traps baited with synthetic pheromones are effective tools for monitoring populations and detecting endangered and/or rare species (reviewed by Millar and Hanks 2017). Many species in the two largest subfamilies, the Cerambycinae and Lamiinae, are known to use aggregation-sex pheromones (sensu Cardé 2014), which are produced by males but attract both sexes. The chemical structures of some of these pheromones are highly conserved, with closely related species (e.g., congeners) and more distantly related species having pheromones of identical composition, or at least sharing the same structural motif (Hanks and Millar 2016). Structural motifs that are common among cerambycines include 3-hydroxyalkan-2-ones and the related 2,3-alkanediols, whereas motifs that are common among lamiines include hydroxyethers and structures based on the sesquiterpene degradation product geranylacetone (Millar and Hanks 2017). These chemical motifs are also conserved across continents, being shared among species of cerambycids that are native to North and South America, Europe, Asia, and Australia (e.g., Fonseca et al. 2010; Hayes et al. 2016; Imrei et al. 2013; Meier et al. 2016; Pajares et al. 2010, 2013; Sweeney et al. 2014; Wickham et al. 2014).
Because cerambycid larvae are long-lived and develop in wood, they are easily moved to new areas of the world through international commerce. As such, the US Department of Agriculture has developed a list of cerambycids deemed as high risk of being invasive, with potential to damage native forests, ornamental trees, and orchard crops in regions of the world into which they may be accidentally introduced. This list includes some Asian species in the genus Semanotus. As part of an ongoing project to identify pheromones for potentially invasive species, we attempted to obtain, from collaborators, pheromone extracts of Asian Semanotus species. These attempts were unsuccessful and, so, using an alternate, “pheromone identification by proxy” approach, we reasoned that we might be able to exploit biosynthetic parsimony in cerambycid pheromones by identifying pheromones from surrogate North American Semanotus species, in the hope that they might be the same or very similar to those of their Asian congeners. Thus, we obtained samples of headspace odors from several Semanotus species, and several species in the related genus Callidium from northern California (J. G. Millar and A. Richards, unpub. Data). These samples contained a novel compound, which we isolated and identified as 1-(1H–pyrrol-2-yl)-1,2-propanedione (henceforth “pyrrole”). In line with our original intent, we then enlisted Asian collaborators to field test pyrrole, alone and in combination with other known cerambycid pheromone components, as possible pheromones for Asian Semanotus species. Although field trials in Japan did not catch large numbers of Semanotus species, a blend of pyrrole and 3-hydroxyhexan-2-one caught both sexes of Callidiellum rufipenne (Motschulsky), an Asian species that infests cupressaceous plants (Zou et al. 2016). This species invaded the northeastern United States in the 1990s, and has since spread to several states (Maier 2007). Further field tests in China subsequently revealed that the same blend attracted adults of the congener C. villosulum (Fairemaire), as well as adults of Xylotrechus buqueti (Castelnau & Gory), whereas pyrrole alone attracted adults of Allotreus asiaticus (Schwarzer) and Semanotus bifasciatus Motschulsky (Wickham et al. 2016). In total, these species represent three tribes of the Cerambycinae, the Callidiini (Callidium, Callidiellum, Semanotus), Clytini (Xylotrechus), and Phoracanthini (Allotreus). Recent work in Brazil has shown that pyrrole also forms part of the pheromone blend of two species in the genus Ambonus, in a fourth tribe, the Elaphidiini (Silva et al. submitted).
However, until now, despite having originally identified pyrrole from several North American cerambycids, it has not been shown that it is indeed a pheromone component for any North American species. Here, we present the first evidence of this, by showing that pyrrole is a key component of the pheromone of a representative of a fifth tribe (Dryobini), Dryobius sexnotatus Linsley. Both pyrrole and (R)-3-hydroxyhexan-2-one were identified in headspace volatiles of live males and, in field trials, a blend of pyrrole with 3-hydroxyhexan-2-one attracted both sexes. These results, in combination with the results summarized above, suggest that pyrrole represents another “generic” pheromone component for cerambycine species, which is broadly shared among a number of tribes across several continents. We expect that this compound will be found in numerous additional species, worldwide.
Methods and Materials
Basic Biology of Study Species
Dryobius sexnotatus Linsley is the only member of its tribe, the Dryobiini, and is native to the eastern United States, but rarely collected (for biology, see Lingafelter 2007; Linsley 1964; Perry et al. 1974). The larvae develop in dead and dying elms (Ulmus species), ash (Fraxinus species), beech (Fagus species), and American basswood (Tilia americana L.), although the primary host is thought to be sugar maple, Acer saccharum Marshall. The brightly colored adults are diurnal and fly primarily in June–July.
Sources of Synthetic Pheromones
Racemic 3-hydroxyhexan-2-one (henceforth “3-ketol”) was purchased from Bedoukian Research (Danbury, CT, USA), while pyrrole was synthesized as described in Zou et al. (2016).
Collection and Analysis of Beetle-Produced Compounds
The pheromone of D. sexnotatus was identified by collecting headspace volatiles from adult beetles on an adsorbent. The first beetle to be aerated was an adult male collected on 17 June 2015 in southern Illinois (Pope County; 37.415, −88.667, lat., long.). Subsequent aerations were conducted on one male and one female that were collected during July 2016 (after the field bioassay of synthetic pheromone; see below) at a privately owned woodlot in southwestern Illinois (Randolph County; 37.955, −89.834, lat., long.; 32 ha). The latter site was forested primarily with oaks (Quercus species) and hickories (Carya species), but also included sugar maples.
Beetles were captured with black cross-vane panel traps (corrugated plastic; Alpha Scents, Inc., West Linn, OR) coated with Fluon® PTFE dispersion (10% aqueous dilution; Northern Products, Woonsocket, RI, USA) to improve capture efficiency (see Graham et al. 2010). Traps were modified to capture beetles alive by replacing trap basins with 2 l plastic jars with bottoms cut out and replaced by aluminum screen. Traps were suspended (at ~1.5 m from the ground) from inverted L-shaped frames of polyvinylchloride irrigation pipe that were mounted on 1-m long sections of steel reinforcing bar driven partway into the ground. Pheromone emitters were polyethylene sachets (clear press-seal bag, Bagette model 14770, 5.1 × 7.6 cm, 0.05 mm thick, Cousin Corp., Largo, FL, USA) with a cotton roll (1 × 4 cm) to stabilize release rates. Lures were formulated to contain 50 mg of 3-ketol (i.e., 25 mg of each enantiomer), 25 mg of the achiral pyrrole, or a blend of the two, dissolved in 1 ml of isopropanol. Emitters released 3-ketol and pyrrole at rates of ~25 and 5.4 μg/h, as estimated by sampling headspace volatiles using the same apparatus as used to collect volatiles from beetles (see below). Control lures contained 1 ml of isopropanol.
Adults of D. sexnotatus were sexed, based on antennal length (males have longer antennae; Linsley 1964). The two males and one female that were captured for volatiles collection were housed separately in aluminum screen cages under laboratory conditions (~12:12 h L:D, ~20 °C) and provided with 10% sucrose solution (glass vial with a cotton wick) for nourishment. Beetles were allowed to acclimate to laboratory conditions for at least 24 h prior to collection, and allowed at least 24 h to recuperate between collections. Beetles were aerated individually in glass Mason-style canning jars (~0.5 l) that were sealed with a Teflon® gasket, for a total of four aerations of males and one of the female. Aeration chambers were adjacent to closed exterior windows (natural photoperiod, ~14:10 h L:D). Charcoal-purified air was drawn through the apparatus at ~1 l/min−1 ~ 24 h, with headspace volatiles trapped in a glass tube containing the adsorbent HayeSep® Q (150 mg, Sigma-Aldrich, St. Louis, MO, USA) between plugs of glass wool. Aerations of empty chambers were run simultaneously to check for system contaminants. Volatile compounds were recovered from tubes by elution with 1.5 ml of dichloromethane.
Aeration extracts were analyzed by coupled gas chromatography/mass spectrometry (GC/MS) using an Agilent 7890B gas chromatograph (Agilent Technologies, Santa Clara, CA, USA), fitted with a HP-5 ms column (30 m × 0.25 mm i.d., 0.25 μm film; Agilent J&W Columns, Agilent Technologies), coupled to an Agilent 5977A mass selective detector. Injections were made in splitless mode with an injector temperature of 250 °C, and an oven temperature program of 30 °C for 1 min, increased by 10 °C.min−1 to 250 °C, and held for 5 min. Sex-specific peaks were identified by comparing spectra and retention times with those of authentic standards.
The absolute configuration of the insect-produced 3-ketol was determined using an HP 5890 GC fitted with a Cyclodex-B column (30 m × 0.25 mm i.d., 0.25 μm film; Agilent Technologies). The injector temperature was set at 110 °C, to minimize isomerization of the 3-ketol (Millar et al. 2009), while an oven temperature program of 50 °C for 1 min, increased by 2.5 °C.min−1 to 150 °C, and held for 5 min (retention time of 3R–ketol: 18.55 min) was used.
Field Bioassay of Synthesized Pheromones
A field experiment was conducted to test for attraction of adult beetles to racemic 3-ketol, pyrrole, or the binary blend (see Results). The experiment was conducted at the study site in Randolph County, Illinois. Traps and lures described above were used, with the exception that trap funnels were fitted with plastic buckets that were partially filled with saturated aqueous NaCl solution to kill and preserve captured beetles. A linear transect of four traps, separated by ~10 m, was deployed on 27 May 2016 with four treatments: 1) 3-ketol, 2) pyrrole, 4) 3-ketol + pyrrole, and 4) solvent control. Traps were checked for beetles every ~3 d, at which time treatments were shifted one position along the transect to control for positional effects. The experiment finished on 16 June 2016, for a total of 18 d (for 7 replicates defined by collection date).
Representative specimens of species caught are available from the laboratory collection of LMH, and voucher specimens have been deposited at the Illinois Natural History Survey, Champaign, IL.
Statistics
The nonparametric Friedman’s Test (PROC FREQ, option CMH; SAS Institute 2011) was used to test differences among treatment means, because data violated homoscedasticity assumptions of ANOVA (Sokal and Rohlf 1995). Two replicates that contained no specimens of D. sexnotatus were dropped from the analysis. The nonparametric Dunn-Nemenyi multiple comparison test, which limits Type I errors to acceptable levels (Elliot and Hynan 2011; Zar 2010), was used to compare pairs of treatment means.
Results
Collection and Analysis of Beetle-Produced Compounds
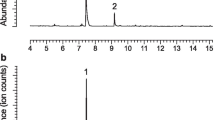
Analyses of all four extracts of headspace volatiles from males of D. sexnotatus revealed two peaks that were not detected in the sample obtained from the female, nor from control aerations (Fig. 1a). The first peak was identified as 3-hydroxyhexan-2-one (retention time 6.42 min) and the second peak as pyrrole (11.33 min; ratio 1:0.13) by comparison of retention times and mass spectra with those of authentic standards (Fig. 1b). Analysis of the extracts on the chiral Cyclodex-B column revealed that the absolute configuration of the ketol was R. During aerations, males released 3-ketol and pyrrole at average rates (± SD) of 0.7 ± 0.35 and 0.17 ± 26 μg/h (N = 4), respectively, or about 3% of the rate of the synthetic pheromone lures.
a Total ion chromatogram of a representative collection of volatiles produced by a male Dryobius sexnotatus. The peak at 6.42 min is 3-hydroxyhexan-2-one, that at 11.33 min is 1-[1H–pyrrol-2-yl]-1,2-propanedione, and that at 20.73 min is the internal standard eicosane. b Electron ionization mass spectrum (70 eV) of insect-produced 1-[1H–pyrrol-2-yl]-1,2-propanedione
Field Bioassay of Synthesized Pheromones
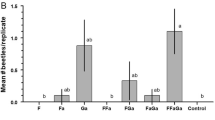
Two species of cerambycids were captured during the field bioassay in numbers sufficient for statistical analysis: the target species D. sexnotatus and another cerambycine native to Illinois, Xylotrechus colonus (F.). Thirty adults of D. sexnotatus (~2:1 M:F) were caught, of which 29 (97%) were in traps baited with the blend of 3-ketol + pyrrole, and the other in a trap baited with 3-ketol. Only the mean for the blend was greater than that of the control (Fig. 2a; Friedman’s Q 3,20 = 15.9, P = 0.0012). Adults of X. colonus were caught in significant numbers only in traps baited with 3-ketol alone or in the blend with pyrrole (Fig. 2b; total of 57 beetles of both sexes; means different; Q 3,28 = 14.2, P = 0.0026).
Mean (± SE) number of adults of a Dryobius sexnotatus and b Xylotrechus colonus (sexes combined) caught per replicate of a trap during the field bioassay in southwestern Illinois. Chemical abbreviations: 3-ketol = racemic 3-hydroxyhexan-2-one, pyrrole = 1-[1H–pyrrol-2-yl]-1,2-propanedione. Means within species with different letters are different (Dunn-Nemenyi multiple comparison test, P < 0.05)
Discussion
Attraction of adult D. sexnotatus only to the blend of 3-ketol plus pyrrole confirmed that both components were essential for attraction. The blend of these two compounds also proved to be critical for attraction of the cerambycines C. rufipenne, C. villosulum, and X. buqueti during field trials in China and Japan (Wickham et al. 2016; Zou et al. 2016). However, those field trials also showed that for two other species, A. asiaticus and S. bifasciatus, pyrrole was attractive as a single component, suggesting that males of those species do not produce the ketol as a pheromone component. 3-Ketol had no effect on attraction of A. asiaticus to pyrrole, but apparently inhibited attraction of S. bifasciatus. Such differences among sympatric species of cerambycids in the synergistic and inhibitory effects of the same pheromone components may have evolved to limit interspecific attraction (Millar and Hanks 2017). These cumulative results suggest that pyrrole represents yet another conserved pheromone structural motif within the Cerambycinae.
(R)-3-Hydroxyhexan-2-one is the most common cerambycid pheromone component identified to date, being the primary or sole component of pheromones for 13 species of cerambycines, and a likely component for an additional ~50 species of cerambycines worldwide (Hanks and Millar 2016). Whereas this overlap in pheromone chemistry might seem to present opportunities for cross-attraction among species, there are several possible mechanisms by which they may remain segregated, including differences in seasonal phenology, diel activity period, or the presence of minor pheromone components that synergize attraction of conspecifics and/or antagonize attraction of heterospecifics (Millar and Hanks 2017). Mitchell et al. (2015) showed how such mechanisms serve to minimize cross attraction among eleven species of cerambycids native to the eastern United States, which probably overlap in distribution with D. sexnotatus. Adults of D. sexnotatus would not be attracted by the pheromones of these other species because they lack the critical pyrrole, but the possibility remains that the 3-ketol produced by male D. sexnotatus could attract other species. Xylotrechus colonus is among the small number of these species that flies at the same time of the year as D. sexnotatus (midsummer; see Hanks et al. 2014, Mitchell et al. 2015), and its pheromone is composed of (R)- and (S)-3-hydroxyhexan-2-one and 2,3-hexanediols (Lacey et al. 2009). In the present study, adults of X. colonus were attracted to 3-ketol but were not influenced by pyrrole, as was observed in an earlier study (Zou et al. 2016), suggesting that this species could be attracted by the pheromone blend of D. sexnotatus. However, the two species are segregated by diel activity period, with X. colonus being crepuscular (adults flying between 8:00–10:00 pm; LMH unpub. Data.), whereas adults of D. sexnotatus fly during early afternoon (Perry et al. 1974).
Capture of 30 adults of D. sexnotatus over an 18-d period, during a trial that included, at any time, only one trap baited with the binary blend, is further evidence of the effectiveness of pheromone-baited traps in detecting rarely-encountered cerambycids, and in delineating their geographical distributions (Millar and Hanks 2017). This species is thought to be distributed broadly across the eastern United States (Linsley 1964; Lingafelter 2007), but our trapping surveys suggest that it is not present in some areas of Illinois. For example, during the last two years, no specimen has been captured by sentinel traps baited with the 3-ketol + pyrrole blend in four wooded areas of east-central Illinois. In fact, only two specimens of D. sexnotatus were trapped during our previous ten years of intensive field trapping in these areas (Hanks et al. 2014), and both were collected at our western-most study site, Funk’s Grove Nature Preserve in McLean County (40.363, −89.115). Dryobius sexnotatus may have been favored at that site by on-going successional replacement of oaks and hickories by sugar maple (McFall and Karnes 1995), which is reported to be the most common host of the larvae (Perry et al. 1974). Our findings, therefore, suggest that D. sexnotatus is patchily distributed within its reported geographical range, but may be more abundant where suitable host plants are abundant.
References
Cardé RT (2014) Defining attraction and aggregation pheromones: teleological versus functional perspectives. J Chem Ecol 40:519–520
Elliot AC, Hynan LS (2011) A SAS® macro implementation of a multiple comparison post hoc test for a Kruskal-Wallis analysis. Computer Meth Prog Biomed 102:75–80
Fonseca MG, Vidal DM, Zarbin PHG (2010) Male-produced sex pheromone of the cerambycid beetle Hedypathes betulinus: chemical identification and biological activity. J Chem Ecol 36:1132–1139
Graham EE, Mitchell RF, Reagel PF, Barbour JD, Millar JG, Hanks LM (2010) Treating panel traps with a fluoropolymer enhances their efficiency in capturing cerambycid beetles. J Econ Entomol 103:641–647
Hanks LM, Millar JG (2016) Sex and aggregation pheromones of cerambycid beetles: basic science and practical applications. J Chem Ecol 42:631–654
Hanks LM, Reagel PF, Mitchell RF, Wong JCH, Meier LR, Silliman CA, Graham EE, Striman BL, Robinson KP, Mongold-Diers JA, Millar JG (2014) Seasonal phenology of the cerambycid beetles of east-central Illinois. Ann Entomol Soc Am 107:211–226
Hayes RA, Griffiths MW, Nahrung HF, Arnold PA, Hanks LM, Millar JG (2016) Optimizing generic cerambycid pheromone lures for Australian biosecurity and biodiversity monitoring. J Econ Entomol 109:1741–1749
Imrei Z, Millar JG, Janik G, Tóth M (2013) Field screening of known pheromone components of longhorned beetles in the subfamily Cerambycinae (Coleoptera: Cerambycidae) in Hungary. Z Naturforsch 68c:236–242
Lacey ES, Millar JG, Moreira JA, Hanks LM (2009) Male-produced aggregation pheromones of the cerambycid beetles Xylotrechus colonus and Sarosesthes fulminans. J Chem Ecol 35:733–740
Lingafelter SW (2007) Illustrated key to the longhorned wood-boring beetles of the eastern United States. Special publication no. 3. Coleopterists Society, North Potomac
Linsley EG (1964) The Cerambycidae of North America: part V. Taxonomy and classification of the subfamily Cerambycinae, tribes Callichromini through Ancylocerini. Univ Calif Publ Entomol 22:1–197
Maier CT (2007) Distribution and hosts of Callidiellum rufipenne (Coleoptera: Cerambycidae), an Asian cedar borer established in the eastern United States. J Econ Entomol 100:1291–1297
McFall D, Karnes J (eds) (1995) A directory of Illinois nature preserves, vol 2. Illinois Department of Natural Resources, Springfield
Meier LR, Zou Y, Millar JG, Mongold-Diers JA, Hanks LM (2016) Synergism between enantiomers creates species-specific pheromone blends and minimizes cross-attraction for two species of cerambycid beetles. J Chem Ecol 42:1181–1192
Millar JG, Hanks LM (2017) Chemical ecology of cerambycids. In: Wang Q (ed) Cerambycidae of the world: biology and pest management. CRC Press/Taylor & Francis, Boca Raton, pp 161–208
Millar JG, Hanks LM, Moreira JA, Barbour JD, Lacey ES (2009) Pheromone chemistry of cerambycid beetles. In: Nakamuta K, Millar JG (eds) Chemical ecology of wood-boring insects. Forestry and Forest Products Research Institute, Ibaraki, pp 52–79
Mitchell RF, Reagel PF, Wong JCH, Meier LR, Silva WD, Mongold-Diers J, Millar JG, Hanks LM (2015) Cerambycid beetle species with similar pheromones are segregated by phenology and minor pheromone components. J Chem Ecol 41:431–440
Pajares JA, Álvarez G, Ibeas F, Gallego D, Hall DR, Farman DI (2010) Identification and field activity of a male-produced aggregation pheromone in the pine sawyer beetle, Monochamus galloprovincialis. J Chem Ecol 36:570–583
Pajares JA, Alvarez G, Hall DR, Douglas P, Centeno F, Ibarra N, Schroeder M, Teale SA, Wang Z, Yan S, Millar JG, Hanks LM (2013) 2-(Undecyloxy)-ethanol is a major component of the male-produced aggregation pheromone of Monochamus sutor. Entomol Exp Appl 149:118–127
Perry RH, Surdick RW, Anderson DM (1974) Observations on the biology, ecology, behavior, and larvae of Dryobius sexnotatus Linsley (Coleoptera: Cerambycidae). Coleopt Bull 28:169–176
SAS Institute (2011) SAS/STAT 9.3 user's guide. SAS Institute Inc., Cary
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W. H. Freeman, New York
Sweeney JD, Silk PJ, Grebennikov V (2014) Efficacy of semiochemical-baited traps for detection of longhorn beetles (Coleoptera: Cerambycidae) in the Russian far east. Eur J Entomol 111:397–406
Wickham JD, Harrison RD, Lu W, Guo Z, Millar JG, Hanks LM, Chen Y (2014) Generic lures attract cerambycid beetles in a tropical montane rain forest in southern China. J Econ Entomol 107:259–267
Wickham JD, Lu W, Zhang L-W, Chen Y, Zou Y, Hanks LM, Millar JG (2016) Likely aggregation-sex pheromones of the invasive beetle Callidiellum villosulum, and the related Asian species Allotraeus asiaticus, Semanotus bifasciatus, and Xylotrechus buqueti (Coleoptera: Cerambycidae). J Econ Entomol 109:2243–2246
Zar J (2010) Biostatistical analysis, 5th edn. Pearson Prentice-Hall, Upper Saddle River
Zou Y, Rutledge CE, Nakamuta K, Maier CT, Hanks LM, Richards AB, Lacey ES, Millar JG (2016) Identification of a pheromone component and a critical synergist for the invasive beetle Callidiellum rufipenne (Coleoptera: Cerambycidae). Environ Entomol 45:216–222
Acknowledgments
We appreciate collection of the initial beetle specimen for the collection of volatiles by Tyler Hedlund, and funding in support of research from the Alphawood Foundation of Chicago (to LMH), and Hatch Act project CA-R*ENT-5181-H (to JGM). This research was in partial fulfillment of a Masters degree for NMD from the University of Illinois at Urbana-Champaign.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Diesel, N.M., Zou, Y., Johnson, T.D. et al. The Rare North American Cerambycid Beetle Dryobius sexnotatus Shares a Novel Pyrrole Pheromone Component with Species in Asia and South America. J Chem Ecol 43, 739–744 (2017). https://doi.org/10.1007/s10886-017-0875-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-017-0875-3