Abstract
Pseudomonas putida 1290 is a model organism for the study of bacterial degradation of the plant hormone indole-3-acetic acid (IAA). This property is encoded by the iac gene cluster. Insertional inactivation and/or deletion of individual iac genes and heterologous expression of the gene cluster in Escherichia coli were combined with mass spectrometry to demonstrate that iac-based degradation of IAA is likely to involve 2-hydroxy-IAA, 3-hydroxy-2-oxo-IAA, and catechol as intermediates. The first gene of the cluster, iacA encodes for the first step in the pathway, and also can convert indole to indoxyl to produce the blue pigment indigo. Transcriptional profiling of iac genes in P. putida 1290 revealed that they were induced in the presence of IAA. Based on results with an iacR knockout, we propose that this gene codes for a repressor of iacA expression and that exposure to IAA relieves this repression. Transformation of P. putida KT2440 (which cannot degrade IAA) with the iac gene cluster conferred the ability to grow on IAA as a sole source of carbon and energy, but not the ability to chemotaxi towards IAA. We could show such tactic response for P. putida 1290, thus representing the first demonstration of bacterial chemotaxis towards IAA. We discuss the ecological significance of our findings, and specifically the following question: under what circumstances do bacteria with the ability to degrade, recognize, and move towards IAA have a selective advantage?
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of bacteria to destroy chemicals with hormonal activity in higher organisms has been well documented (Dodd et al. 2010; Garcia-Gomez et al. 2013). In plants, indole-3-acetic acid (IAA) is an auxin hormone that plays a key role in plant growth and development (Gray 2004). Bacteria from various environments can inactivate or even mineralize IAA (Faure et al. 2009; Leveau and Gerards 2008; Leveau and Lindow 2005). The ecological function of this activity remains unknown. Does it provide bacteria with a selective advantage in habitats that feature IAA in quantities sufficiently large to serve as an alternative source of carbon, nitrogen, and/or energy? Or does it allow bacteria to alter plant IAA homeostasis and indirectly manipulate plant physiology in a way that confers some as-of-yet-unknown gain in bacterial survival or reproduction? Knowing that plants are not the only source of IAA (Patten and Glick 1996), and that IAA may act as a signal molecule between microorganisms (Spaepen et al. 2007) evokes even more questions about the possible role and ecological significance of bacterial IAA degradation. Answering such questions will require a more complete understanding of the genes and gene products that are involved in this activity.
Our lab has used the bacterial isolate Pseudomonas putida 1290 as a model strain for the identification and characterization of IAA-degradative genes. This bacterium originally was isolated from pear tree foliage and it efficiently exploits IAA as a nutrient source (Leveau and Lindow 2005). Taking a forward genetics approach (Leveau and Gerards 2008), it was discovered that IAA mineralization by P. putida 1290 involved an 8,994-bp genomic DNA fragment that when transferred to P. putida KT2440 rendered this strain able to grow on IAA (Leveau and Gerards 2008). Homologs of the ten so-called iac genes on this DNA fragment were identified in clusters on the genomes of various representatives from the α-, β-, and γ-Proteobacteria, as well as high G+C Gram-positive bacteria (Leveau and Gerards 2008), and the ability to grow on IAA was confirmed for several of these bacterial strains (Leveau and Gerards 2008), including most recently the strain Acinetobacter baumannii ATCC 19606 (Lin et al. 2012).
Little is known still about the contribution of individual iac genes to the IAA degradation pathway. The iacA gene product is believed to carry out one of the first steps, based on its ability to convert indole to indoxyl, which can dimerize to form the blue pigment indigo (Alemayehu et al. 2004). This activity has been reported for the iacA ortholog idoA from Pseudomonas alcaligenes PA-10 (Alemayehu et al. 2004), and iacA from A. baumannii ATCC 19606 (Lin et al. 2012). The involvement of other iac genes in the IAA degradation pathway remains more speculative. A transposon insertion into the iacH gene abolished IAA degradation by P. putida 1290 (Leveau and Gerards 2008), and this defect could be restored only by complementation by both iacH and the downstream iacI gene (Leveau and Gerards 2008). Collectively, the iac genes are thought to code for the conversion of IAA to catechol or a precursor, given that insertional inactivation of the cat and pca genes abolished the ability of P. putida 1290 to grow on IAA (Leveau and Gerards 2008). These cat and pca genes encode the enzymatic, multi-step catalysis of catechol into ß-ketoadipate. There is some evidence that iac gene expression is inducible. In A. baumannii ATCC 19606, the IacA protein was detected by Western blotting in cells cultured on IAA but not in cells on pyruvate (Lin et al. 2012). Extracts of IAA-grown cells of P. putida 1290 showed elevated levels of catechol 1,2-dioxygenase activity (Leveau and Lindow 2005), suggesting a coordinated regulation of iac genes and downstream cat and pca genes. The involvement of the iacR gene in the inducibility of the IAA degradation pathway remains unknown. IacR is one of the genes on the iac gene cluster and predicted to encode a MarR-type transcriptional regulator (Leveau and Gerards 2008).
Here, we report on our continued efforts to link the iac gene cluster to the IAA degradative phenotype, with the long-term goal to render a more complete understanding of the function of iac genes and their ecological role. Using the model strain P. putida 1290, we demonstrate the inducibility of iac gene expression in response to IAA, explore the contribution of individual iac genes to the IAA degradative phenotype, and identify several intermediates in the IAA degradation pathway. Furthermore, taking advantage of heterologous expression of iac genes in the non-IAA degrader P. putida KT2440, we present evidence for the chemotactic behavior of P. putida 1290 towards IAA.
Methods and Materials
Bacterial Strains and Growth Conditions
Table 1 lists all the bacterial strains used in this study. Strains of Pseudomonas putida were grown at 30 °C on either Lysogeny Broth (LB), King’s B (King et al. 1954), or M9 minimal medium (Sambrook et al. 1989) that was supplemented with 6.58 μM FeSO4 and 8.33 mM glucose, 5 mM indole-3-acetic acid (IAA) (Sigma Aldrich, St. Louis, MO, USA), 8.3 mM benzoate, or 8.33 mM sodium citrate. Escherichia coli strains were grown at 37 °C on LB or M9 minimal medium supplemented with FeSO4, glucose, thiamine, and leucine at concentrations of 6.58 μM, 8.33 mM, 10 μg ml-1, and 100 μg ml-1, respectively. Strains grown in liquid media were routinely incubated in the dark on an orbital shaker at 275 rpm to provide sufficient aeration. Growth media were supplemented with antibiotics as appropriate at the following final concentrations: kanamycin, 50 μg ml-1 (Km50); tetracycline, 15 μg ml-1 (Tc15); ampicillin, 50 μg ml-1 (Amp50); or rifampicin 40 μg ml-1 (Rif40).
Plasmid DNA Isolation, Manipulation, and Transformation
Plasmids used in this study are listed in Table 1. Plasmid DNA was isolated from overnight bacterial cultures using a GeneJet Plasmid Miniprep Kit (Fermentas Thermo Scientific, Waltham, MA, USA), quantified with a NanoDrop (Thermo Scientific, Waltham, MA, USA), and checked for quality by agarose gel electrophoresis and GelRed staining (Biotium, Hayward, CA, USA). Plasmid DNA was digested with restriction enzymes as recommended by the manufacturer, and DNA fragments were recovered from excised agarose gel slices by using the QIAquick Gel Extraction Kit (QIAGEN, Germantown, MD, USA). Ligation reactions were incubated with T4 DNA ligase (Fermentas) at room temperature for 1 h, then heat-inactivated for 10 min at 65 °C. Non-compatible ends were blunted using a Quick Blunting Kit (New England BioLabs, Ipswich, MA, USA) prior to ligation. Chemically competent E. coli TOP10 cells (Life Technologies, Carlsbad, CA, USA) were transformed with 5 μl of the ligation reaction. For the transformation of P. putida or E. coli with purified plasmid DNA, we prepared electrocompetent cells and used 50 ng of DNA, as described previously (Leveau and Gerards 2008). Electroporation was performed with a Gene Pulser Xcell Microbial System (Bio-Rad, Hercules, CA, USA), using the manufacturer’s settings for E. coli or Pseudomonas aeruginosa as needed. A recovery period of 1 hr for E. coli or 2 hr for P. putida occurred in 1 ml of SOC (Sambrook et al. 1989) on an orbital shaker. Transformants were selected on the appropriate agar medium.
Construction of pIAC115 and its Derivatives
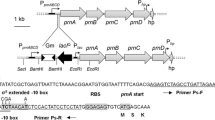
A cluster of ten iac genes involved in the bacterial mineralization of IAA was cut from the vector pCC1FOS11EcoAscPLUS (Leveau and Gerards 2008) as a 9.4-kb HindIII/SacI fragment. This region of DNA was ligated into the 8.3-kb HindIII/SacI-digested backbone of pME6031 (Heeb et al. 2000) to produce pIAC113. This plasmid was used as a template in a PCR with primers pIAC115-F and pIAC115-R (Table 1) to amplify a 1.5-kb fragment that was cut with HindIII and MauBI and ligated into HindIII/MauBI-digested pIAC113 to generate pIAC115. This plasmid then was used in an in vitro EZ-Tn5<KAN-2>Tnp Transposome kit (Epicentre, Madison, WI, USA) as per the manufacturer’s instructions, and transformed into E. coli TOP10. From 48 transformants, plasmid DNA was isolated and sent to the CBS UC DNA Sequencing Facility (UC Davis, CA, USA) for sequencing with the primer KAN-2-FP, which is designed to read from the transposon into the flanking plasmid DNA. DNA sequences were analyzed using the MegAlign and Seqbuilder modules of the Lasergene software (DNASTAR, Madison, WI, USA) to determine the transposon insertion site for each sequenced clone. Ten pIAC115::Tn5 plasmids were selected for further study: nine carrying the transposon inserted in a unique iac gene (pIAC115-iacX::Tn5, where X represents A, B, C, D, E, F, G, R, or H; we never recovered a plasmid carrying Tn5 in iacI) and one carrying the transposon in the vector backbone of pIAC115 (pIAC115-vector::Tn5). The latter plasmid was also used to construct the three deletion derivatives pIAC115-vector::Tn5-ΔiacABCDEFGRHI and -ΔiacCDEFGRHI, by deletion of a 9.0-kb HindIII-EcoRI or 7.0-kb BsrGI-EcoRI internal fragment, respectively.
Characterization of E. coli and P. putida Strains Carrying pIAC115::Tn5 Plasmids
To assess the contribution of iac genes to IAA degradation, cells of E. coli TOP10 or P. putida KT2440 carrying pIAC115::Tn5 plasmids were grown in LB Tc15 supplemented with 5 mM IAA for 18 h on an orbital shaker. One-ml aliquots were centrifuged at 10,000 × g for 30 sec, and IAA concentrations were determined using Salkowski reagent as described previously (Leveau and Gerards 2008). To assess the contribution of the iac genes in the utilization of IAA as a source of carbon and energy, cells of P. putida KT2440 carrying pIAC115::Tn5 plasmids were inoculated in liquid M9 Tc15 medium supplemented with 5 mM IAA. To assess indigo production of E. coli BW25113, E. coli BW25113ΔtnaA, and E. coli BW25113ΔtrpL (Lee et al. 2007) carrying selected pIAC115::Tn5 plasmids, strains were streaked onto LB Km50 agar, with or without a few crystals of indole (Sigma Aldrich) in the plastic lid of the inverted petri plates, at 30 °C for 3 days.
Transcriptional Profiling of iac Genes
Relative levels of iac gene expression in P. putida 1290R were quantified by quantitative reverse-transcriptase (RT) real-time PCR. The PrimerSelect module of Lasergene (DNASTAR) was used to design primers that would amplify unique fragments, between 70 and 121 bp in size and internal to different iac genes, the 16S rRNA reference gene, or benC and cbrA control genes (Table 1). Cells were grown as overnight cultures in KB Rif40 or KB Rif40 supplemented with 5 mM IAA, harvested by centrifugation at 8,000 × g for 2 min, washed in 1× M9 minimal salts, and transferred to a sterile 125 ml Erlenmeyer flask as a 1/100 dilution into 20 ml of M9 minimal medium supplemented with 6.58 μM FeSO4 and one or more carbon sources. At mid-exponential phase, approximately 2 × 108 cells were harvested, mixed with two volumes of RNAprotect (QIAGEN), and RNA was extracted using a RNeasy Mini Kit (QIAGEN) with the optional on-column DNAse step. RNA was eluted in 50 μl water twice, quantified by NanoDrop, and quality-checked on a 1 % agarose gel in 1X TAE. One μg of RNA was used as the template for synthesis of cDNA using the Super Script III First-Strand Synthesis Super Mix for qRT-PCR (Invitrogen Life Technologies, Carlsbad, CA, USA) with the following conditions: 10 min at 25 °C, 30 min at 50 °C, 5 min at 85 °C, followed by chilling on ice. To remove RNA, 2U E. coli RNase H was added to each preparation and incubated at 37 °C for 20 min. Fifty pg of template cDNA were mixed with 12.5 μM each of forward and reverse primer in 1x Fast SYBR Green Master Mix (Invitrogen Life Technologies), and run on an Applied Biosystems 7500 Fast Real-Time PCR System (Life Technologies) using the following settings: 10 min at 95 °C, followed by 40 cycles of 15 sec at 95 °C and 1 min at 60 °C. For the standard curves, we generated amplicons for each target gene in a standard PCR using 20 ng of P. putida 1290 genomic DNA that was isolated using a Blood and Tissue DNeasy kit (QIAGEN). PCR primers and unused nucleotides were removed with an UltraClean PCR Clean-Up Kit (Mo Bio Laboratories, Carlsbad, CA, USA). Standard curves were derived from real-time PCRs on ten-fold dilution series (0.002–2 pg range) of each purified amplicon. The relative expression ratio R of individual target genes was calculated as Etarget ΔCPtarget(control − sample)/Eref ΔCPref(control − sample) (Pfaffl 2001) in which Etarget is the real-time efficiency of the target gene, Eref the real-time efficiency of the 16S rRNA reference gene, ΔCP is the difference in crossing point (or Ct), and ‘control’ refers to growth on M9 with citrate as the carbon source.
Gas Chromatography and Mass Spectrometry (GC-MS)
Selected E. coli TOP10 (pIAC115::Tn5) strains were precultured in LB Km50, pelleted by centrifugation, inoculated as a 1/100 dilution into M9 Km50 minimal medium containing glucose, thiamine, and leucine, in addition to 200 μM IAA, and grown for 24 h at 37 °C with shaking at 275 rpm. As a control, we used medium that was not inoculated with cells. Aliquots (2 ml) were centrifuged and 1.5 ml of supernatant was filtered through a sterile 0.2-μm Puradisc 25 AS disposable filter (Whatman, Florham Park, NJ, USA), and frozen at −20 °C until sent to the UC Davis Metabolomics core facility for trimethylsilyl (TMS)-derivatization and GC-MS analysis as described elsewhere (Fiehn et al. 2008). In total, 218 compounds were identified, of which 106 could be annotated using BinBase (Fiehn et al. 2005). Fragmentation profiles for each of the candidate compounds were examined using AMDIS software (Stein 1999) to infer putative structures.
Chemotaxis Experiments
A soft agar plate assay (Harwood et al. 1994) was used to evaluate the ability of P. putida 1290R-carrying plasmid pPROBE’-gfp[tagless] (Miller et al. 2000) and P. putida KT2440R carrying plasmid pIAC115-vector::Tn5 to chemotaxi towards IAA. Soft agar plates (94-mm diam) contained 0.3 % agar, M9 Km50 medium supplemented with 6.58 μM FeSO4, and either 5 mM IAA, 8.3 mM sodium benzoate, or no carbon source. Single colonies of each strain were grown overnight in 3 ml LB Km50 with shaking at 30 °C, and a 5-μl aliquot was placed in the center of each soft agar plate. After incubation at 30 °C for 16 h, the diameters of bacterial spread were measured and compared.
Results
IAA-Induced Expression of iac Genes
Quantitative RT real-time PCR analysis of RNA extracted from P. putida 1290 cultures revealed that on mineral medium, with IAA as the sole source of carbon and energy, the expression of iacA and iacC was >2 orders of magnitude greater than with glucose (Fig. 1). In the presence of both glucose and IAA, iacA and iacC expression levels were lower than with IAA only, but still well above those with glucose alone, suggesting that iac genes are not subject to catabolite repression. For iacH and iacR, we also observed elevated expression on IAA and on glucose plus IAA, albeit to a lower level. We did not observe IAA-induced expression of cbrA, which is predicted to be involved in IAA sensing (Leveau and Gerards 2008), nor did we see induction of iac gene expression when the cells were grown on benzoate (Fig. 1). Conversely, the benC gene, which contributes to growth on benzoate (Leveau and Gerards 2008), was induced by benzoate but not IAA (Fig. 1).
Indole-3-acetic acid (IAA)-induced expression of iac genes. Shown is the relative expression ratio (R) of 6 target genes (iacA, iacC, iacH, iacR, cbrA, and benC) for cultures of Pseudomonas putida 1290R that were grown on mineral medium containing different carbon sources (glucose, glucose plus IAA, IAA, or benzoate). R was calculated using the 16S rRNA gene as a reference gene and citrate as the control carbon source. Error bars indicate standard deviations of the means of technical replicates within this representative biological experiment
Characterization of P. putida and E. coli Strains Carrying pIAC115::Tn5 Plasmids
The plasmid pIAC115 carries the complete iac gene cluster in the broad-host vector pME6031 (Fig. 2a). In vitro transposon mutagenesis of this plasmid and flank-sequencing of insertion sites revealed 29 derivatives of pIAC115 that carried the transposon in one of the iac genes. Nine of these were selected for further study, representing insertions in iacA, iacB, iacC, iacD, iacE, iacF, iacG, iacR, and iacH, so as to assess the individual role of these genes in IAA degradation. Also included was derivative pIAC115-vector::Tn5, which carries a transposon insertion outside of the iac gene cluster. In addition, we constructed two deletion derivatives of pIAC115-vector::Tn5: one by removal of the entire iac gene cluster to give pIAC115-Δiac, and the other by removal of iacCDEFGRHI to generate pIAC115-iacAB. P. putida KT2440 or E. coli TOP10 were transformed with derivatives of pIAC115 and tested for various phenotypes. The results are summarized in Table 2 and described in the next paragraphs.
a Diagram showing part of plasmid pIAC115 carrying the iac gene cluster and the location of 30 EZ-Tn5<KAN-2>transposon insertion events. Each insertion event is represented by a triangle: down- or upward direction of the triangles correspond to right or left orientations, respectively, of the transposed KAN-2 gene. The ten insertions labeled with a solid black triangle were selected for subsequent experiments. Of these, nine are in one of the iac genes and one in the vector backbone. The stippled line represents a DNA fragment that shows homology to the Tn5<KAN-2>sequence and was removed from pIAC113 to create pIAC115 using primers pIAC115-F and pIAC115-R. b Relative size and overlap of DNA inserts in four pCR4Blunt-TOPO plasmids from the original shotgun clone library of Pseudomonas putida 1290 genomic DNA (Leveau and Gerards 2008). All four conferred a blue color to colonies of host E. coli DH5α. They share a 1,568-bp fragment harboring the iacA gene
Introduction of pIAC115-vector::Tn5 (complete iac gene cluster) into P. putida KT2440 conferred the ability to grow on IAA as the sole source of carbon and energy. Insertional inactivation of iacA, iacC, iacD, iacE or iacH, but not iacF, iacG, or iacR, completely abolished this phenotype. E. coli TOP10 carrying pIAC115-vector::Tn5 did not gain the ability to grow at the expense of IAA. However, this strain was able to make IAA disappear during growth on glucose plus IAA. This ability was lost with insertional inactivation of iacA or with removal of the entire iac gene cluster. E. coli TOP10 carrying an insertion in any one of the other iac genes or carrying pIAC115-iacAB showed the same capability of IAA destruction as E. coli (pIAC115-vector::Tn5). When grown in LB plus IAA, cultures of E. coli (pIAC115-vector::Tn5) turned brown after overnight incubation. A similar observation was made for E. coli strains carrying pIAC115-iacF::Tn5, -iacG::Tn5, and -iacR::Tn5. We suspect that the brown color results from the polymerization of catechol that accumulates in the supernatant (see GC-MS analysis below) and which E. coli is unable to metabolize further. We note that these three plasmids and pIAC115-vector::Tn5 were the only ones to confer growth on IAA to P. putida KT2440, suggesting that iac-encoded growth on IAA is linked to the ability to metabolize catechol.
On LB agar plates, colonies of E. coli TOP10 harboring plasmid pIAC115-iacAB or pIAC115-iacR::Tn5 developed a blue color. None of the other pIAC115 derivatives conferred this property (Table 2). The pIAC115-iacR::Tn5-induced blue colony phenotype also was observed with the host strain E. coli BW25113 (Fig. 3). When introduced into E. coli BW25113 ΔtnaA, which has reduced indole production (Lee et al. 2007), pIAC115-iacR::Tn5 failed to produce the blue color, but pigment production could be restored to wildtype levels by exogenously providing indole (Fig. 2). A ΔtrpL derivative of BW25113 that overproduces indole (Lee et al. 2007) showed blue color formation similar to wildtype. These results suggest that the blue color is produced from indole by a iacAB-encoded activity that is repressed by iacR. During the shotgun sequencing of the iac gene cluster of P. putida 1290 (Leveau and Gerards 2008), four library clones were observed to produce a similar blue color. Sequence of the inserts revealed that they shared the iacA gene (Fig. 2b), suggesting the iacA gene product is the catalyst for pigment formation and that iacB is not needed.
Production of indigo by Escherichia coli BW25113 strains carrying different pIAC115 derivatives. E. coli BW25113 ΔtnaA has been described as a strain with reduced indole production (compared to wildtype E. coli BW25113), whereas E. coli BW25113 ΔtrpL is an overproducer of indole. Plasmid pIAC115 harbors the entire iac gene cluster, whereas pIAC115-iacR::Tn5 carries a transposon insertion in the iacR gene
Intermediates of the iac-Directed IAA Degradation Pathway
GC-MS analyses were performed on the supernatants of E. coli strains carrying different pIAC115 derivatives (full iac gene cluster, pIAC115-iacC::Tn5, -iacE::Tn5, -Δiac, or -iacAB), after overnight growth on mineral medium containing glucose supplemented with 0.2 mM IAA. Authentic IAA was identified based on a BinBase-annotated parent ion with m/z 319 (IAA-2TMS), as well as a strong daughter peak at m/z 202, which likely resulted from loss of the trimethylsilylated carboxylic acid group on the IAA side chain. IAA was near the detection limit in the supernatants of E. coli carrying pIAC115-vector::Tn5 (the full iac gene cluster) and pIAC115-iacE::Tn5 (transposon insertion in iacE), while the level of IAA in the supernatants of cells carrying the empty vector (pIAC115-Δiac) was similar to that of the no-cells-added control. Supernatants of E. coli (pIAC115-iacC::Tn5) and E. coli (pIAC115-iacAB) showed residual concentrations of IAA.
We identified three compounds that accumulated differently in the supernatants of the tested E. coli strains by GC-MS analysis (Table 2). Compound #3 was unambiguously identified as catechol, with an m/z 254 parent ion and daughter ions with m/z 136, 151, 166, and 239. Catechol only appeared in supernatants of cells carrying the complete iac cluster. Compound #1 was putatively annotated as 5-hydroxyindoleacetic acid (5-OH-IAA), using the ion peak at m/z 290 and lower intensity peaks at m/z 407 (5-OH-IAA-3TMS) and 202 for identification. This compound was found in the supernatants of E. coli (pIAC115-iacE::Tn5) and E. coli (pIAC115-iacAB). Compound #2 could not be annotated. It had a complex peak profile with signature ion peaks at m/z 202, 292, 306, 364, and 423. This compound appeared in the supernatants of cells carrying the full iac gene cluster as well as the plasmid pIAC115-iacC::Tn5.
Chemotaxis of P. putida 1290 towards IAA
On soft agar plates containing IAA, P. putida 1290 exhibited chemotactic behavior, as demonstrated by the radial growth of the bacteria (Fig. 4). P. putida KT2440 carrying plasmid pIAC115-vector::Tn5 (complete iac gene cluster) did not show such behavior (Fig. 4), although it could utilize IAA as a sole source of carbon and energy (Table 2). This suggests that IAA catabolism alone is not sufficient for chemotaxis towards IAA. Figure 4 also shows a positive control (benzoate) and negative control (no carbon source) for chemotaxis.
Test of chemotactic behavior by Pseudomonas putida 1290 and P. putida KT2440 (pIAC115) towards indole-3-acetic acid (IAA). A drop of overnight culture was placed in the center of a M9 0.3 % agar plate containing benzoate, IAA, or no carbon source, incubated for 6 hr at 30 °C, and analyzed for outward spread of bacterial growth
Discussion
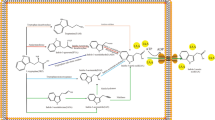
The experimental results we present here are consistent with the following model for iac-encoded catabolism of IAA. A knockout of iacA but none of the other genes in the iac gene cluster abolished the ability of E. coli to make IAA disappear (as measured by Salkowski staining), suggesting that iacA codes for the first step in the IAA degradation pathway. The iacA gene product also catalyzed blue pigment production in E. coli, which we showed to be dependent on the presence of indole. This suggests that iacA codes for the hydroxylation of position 3 of the indole ring to produce indoxyl (tautomeric with 3-oxindole), which in the presence of oxygen dimerizes to form indigo. We hypothesize that hydroxylation of IAA in the same position would yield either 3-oxindole with release of the acetic acid side chain, or 2-hydroxy-indole-3-acetic acid (2-OH-IAA) by migration of the hydroxyl group from position 3 to position 2 on the indole ring. 2-OH-IAA is a tautomer of OxIAA, which is a major degradation product of IAA in plants (Ljung et al. 2002). Published GC-MS analysis of silylated 2-OH-IAA (Ostin et al. 1998) showed ion peaks at m/z 290, as well as 407 and 202. This profile is very similar to what we found for compound #2, which BinBase annotated as 5-OH-IAA, which is an isomer of 2-OH-IAA/OxIAA. OxIAA is unreactive with Salkowski reagent (Reinecke and Bandurski 1981), whereas 5-OH-IAA produces a beige to brown color (Glickmann and Dessaux 1995). Given the absence of Salkowski-induced color formation in supernatants that harbored compound #1, we propose that compound #1 is in fact 2-OH-IAA. It accumulated in the supernatants of IAA-exposed E. coli cells carrying pIAC115-iacAB or pIAC115-iacE::Tn5 (Table 2). The iacB gene is not needed for blue pigment production (Fig. 2b) or for making IAA disappear, as determined by Salkowski reagent (Table 2), suggesting that the iacA gene product is sufficient to catalyze the conversion of IAA into 2-OH-IAA. The data also indicate that 2-OH-IAA might be the substrate for iacE in the IAA degradation pathway. We note that hydroxylation of 2-OH-IAA at position 3 of the indole ring would yield 3-hydroxy-2-oxindole-3-acetic acid (dioxindole-3-acetic acid, or diOxIAA), which has been proposed as an intermediate of IAA catabolism in the bacterium Bradyrhizobium japonicum (Jensen et al. 1995). A 3TMS-derivative of diOxIAA is predicted to have a parent ion with an m/z of 423, and loss of the COO-TMS would yield a 306 peak. These values are part of the spectrum for compound #2, and so we tentatively propose that compound #2 is diOxIAA. It accumulated to the highest levels in the supernatants of E. coli cells carrying plasmid pIAC115-iacC::Tn5, but we also found it in cells carrying the full iac gene cluster. Possibly, this indicates that diOxIAA is the substrate for the iacC gene product, and that this reaction is the rate limiting step in the iac-encoded pathway of IAA degradation. Catechol (compound #3) was identified only in the supernatants of E. coli cells carrying the full iac cluster, indicating that it is the end product of the iac-encoded pathway. This is consistent with the observation that P. putida KT2440, which carries the cat/pca genes for catechol utilization, can grow on IAA when transformed with the iac gene cluster, while E. coli, which does not possess such genes, cannot. This also explains the accumulation in E. coli supernatants of catechol, which is known to polymerize to produce a brown color (Leveau and Gerards 2008). A tentative pathway for IAA degradation in P. putida 1290 is presented in Fig. 5. The roles of other iac genes in this pathway remain unknown. Based on gene synteny and sequence homology, it has been suggested (Leveau and Gerards 2008) that IacD is a subunit of the IacC protein. We suspect that the conversion of diOxIAA to catechol involves multiple steps that require the products of iacF and iacG. Our data show that a functional copy of these genes is not required to bestow upon P. putida KT2440 the ability to grow on IAA. On the genome of P. putida KT2440 (Nelson et al. 2002), we identified orthologs of iacF and iacG (NCBI accession numbers AAN69333 and AAN69243, respectively) that may be similar enough in function (based on sequence identity of 51 % and 28 %, respectively) to rescue the absence of iacF and iacG and allow IAA degradation.
Proposed pathway for iac-encoded degradation of indole-3-actic acid (IAA) in Pseudomonas putida 1290. Shown are three intermediates, one confirmed (catechol) and two suspected (2-hydroxy-IAA, or 2-OH-IAA; 3-hydroxy-2-oxo-IAA, or diOxIAA) based on GC-MS analysis. Insertional inactivation experiments suggest that iacA is involved in step 1, iacE in step2 and iacC in step 3. The role of other iac genes (iacB, iacD, iacE, iacF, iacG, iacH, and iacI) in these steps remains unknown
We demonstrated that the iac-encoded degradation of IAA in P. putida 1290 involves IAA-inducible expression of the iac genes (Fig. 1). The experiments with plasmids pIAC115-iacR::Tn5 and pIAC115-iacAB in E. coli show that in the absence of iacR, expression of iacA is induced, as deduced from iacA-catalyzed indigo production. This suggests that iacR is a repressor of iacA expression. IacR shows high amino acid sequence identity to members of the Multiple Antibiotic Resistance Regulator (MarR) family, many of which regulate the expression of genes involved in antibiotic resistance, stress responses, virulence, or catabolism of aromatic compounds (Perera and Grove 2010). In Comamonas testosteroni, the MarR homolog CbaR represses the cbaABC operon, which codes for the degradation of 3-chlorobenzoate (Providenti and Wyndham 2001). Repression is relieved when CbaR binds the ligand 3-chlorobenzoate, which causes a reduction in DNA binding affinity of CbaR to its operator sites in the cbaA promoter region. Interestingly, the DNA binding affinity of CbaR is enhanced upon binding with 3-hydroxy- or 3-carboxyl-benzoate. It was suggested that this represents a mechanism to prevent gratuitous induction of cbaABC (Providenti and Wyndham 2001). The ligand of iacR remains to be identified, but IAA is a likely candidate. Based on results with E. coli (pIAC115-iacB::Tn5), we tentatively conclude that indole is not an inducer of iacA gene expression, otherwise we would have expected this strain to produce indigo, assuming that iacA is solely responsible for the conversion of indole to indoxyl.
To the best of our knowledge, this study offers the first demonstration of bacterial chemotaxis towards IAA. The mechanism underlying this property in P. putida 1290 will be a subject of future investigations. Most likely, it involves one or more methyl-accepting proteins (MCPs), which are chemoreceptors that recognize a specific attractant and transmit a signal to the flagellar machinery (Krell et al. 2011). We can assume that an MCP-based chemotaxis towards IAA would involve a certain degree of specificity in P. putida 1290, since none of the 27 MCPs or MCP-like proteins identified on the genome of P. putida KT2440 (Parales et al. 2013) appeared to be able to surrogate and to induce chemotactic behavior toward IAA in P. putida KT2440 carrying the full iac gene cluster (Fig. 4). The knowledge that bacteria have the capability to chemotaxi towards IAA implies that this property is of use to these bacteria and offers a selective advantage to their survival. Many chemotactic behaviors are linked to metabolism of the attractant (Krell et al. 2011), and so bacteria like P. putida 1290 would have a competitive advantage over IAA-mineralizing bacteria that lack chemotactic capability. Another potential benefit is that bacteria can actively move towards biological sources of IAA. These might be plants (Cho et al. 2007), but also microbial producers of IAA, such as bacteria or fungi (Spaepen et al. 2007). Certain environments such as the plant leaf surface (Enya et al. 2007) are ubiquitously colonized by IAA-producing bacteria, and possibly, bacterial chemotaxis towards bacterially-produced IAA could contribute to the aggregative colonization patterns that are observed in such environments (Monier and Lindow 2004).
References
Alemayehu D, Gordon LM, O’Mahony MM, O’Leary ND, Dobson AD (2004) Cloning and functional analysis by gene disruption of a novel gene involved in indigo production and fluoranthene metabolism in Pseudomonas alcaligenes PA-10. FEMS Microbiol Lett 239:285–293
Cho M, Lee OR, Ganguly A, Cho HT (2007) Auxin-signaling: short and long. J Plant Biol 50:79–89
Dodd IC, Zinovkina NY, Safronova VI, Belimov AA (2010) Rhizobacterial mediation of plant hormone status. Annal Appl Biol 157:361–379
Enya J, Shinohara H, Yoshida S, Negishi TTH, Suyama K, Tsushima S (2007) Culturable leaf-associated bacteria on tomato plants and their potential as biological control agents. Microb Ecol 53:524–536
Faure D, Vereecke D, Leveau JHJ (2009) Molecular communication in the rhizosphere. Plant Soil 321:279–303
Fiehn O, Wohlgemuth G, Scholz M (2005) Setup and annotation of metabolomic experiments by integrating biological and mass spectrometric metadata. Data Integr Life Sci Proc 3615:224–239
Fiehn O, Wohlgemuth G, Scholz M, Kind T, Lee DY, Lu Y, Moon S, Nikolau B (2008) Quality control for plant metabolomics: reporting MSI-compliant studies. Plant J 53:691–704
Garcia-Gomez E, Gonzalez-Pedrajo B, Camacho-Arroyo I (2013) Role of sex steroid hormones in bacterial-host interactions. Biomed Res Int 2013:928290
Glickmann E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Gray WM (2004) Hormonal regulation of plant growth and development. PLoS Biol 2:E311
Harwood CS, Nichols NN, Kim MK, Ditty JL, Parales RE (1994) Identification of the pcaRKF gene cluster from Pseudomonas putida: involvement in chemotaxis, biodegradation, and transport of 4-hydroxybenzoate. J Bacteriol 176:6479–6488
Heeb S, Itoh Y, Nishijyo T, Schnider U, Keel C, Wade J, Walsh U, O’Gara F, Haas D (2000) Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Mol Plant Microbe Interact 13:232–237
Jensen JB, Egsgaard H, Vanonckelen H, Jochimsen BU (1995) Catabolism of indole-3-acetic acid and 4-chloroindole-3-acetic and 5-chloroindole-3-acetic acid in Bradyrhizobium japonicum. J Bacteriol 177:5762–5766
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Krell T, Lacal J, Munoz-Martinez F, Reyes-Darias JA, Cadirci BH, Garcia-Fontana C, Ramos JL (2011) Diversity at its best: bacterial taxis. Environ Microbiol 13:1115–1124
Lee JT, Jayaraman A, Wood TK (2007) Indole is an inter-species biofilm signal mediated by SdiA. BMC Microbiol 7:15
Leveau JHJ, Gerards S (2008) Discovery of a bacterial gene cluster for catabolism of the plant hormone indole 3-acetic acid. FEMS Microbiol Ecol 65:238–250
Leveau JHJ, Lindow SE (2005) Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol 71:2365–2371
Lin GH, Chen HP, Huang JH, Liu TT, Lin TK, Wang SJ, Tseng CH, Shu HY (2012) Identification and characterization of an indigo-producing oxygenase involved in indole 3-acetic acid utilization by Acinetobacter baumannii. A Van Leeuw J Microb 101:881–890
Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G (2002) Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Mol Biol 50:309–332
Miller WG, Leveau JHJ, Lindow SE (2000) Improved gfp and inaZ broad-host-range promoter-probe vectors. Mol Plant Microbe Interact 13:1243–1250
Monier JM, Lindow SE (2004) Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl Environ Microbiol 70:346–355
Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, dos Santos VAPM, Fouts DE, Gill SR, Pop M, Holmes M et al (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4:799–808
Ostin A, Kowalyczk M, Bhalerao RP, Sandberg G (1998) Metabolism of indole-3-acetic acid in Arabidopsis. Plant Physiol 118:285–296
Parales RE, Luu RA, Chen GY, Liu X, Wu V, Lin P, Hughes JG, Nesteryuk V, Parales JV, Ditty JL (2013) Pseudomonas putida F1 has multiple chemoreceptors with overlapping specificity for organic acids. Microbiology Published online ahead of print(doi: 10.1099/mic.0.065698-0)
Patten CL, Glick BR (1996) Bacterial biosynthesis on indole-3-acetic acid. Can J Microbiol 42:207–220
Perera IC, Grove A (2010) Molecular mechanisms of ligand-mediated attenuation of DNA binding by MarR family transcriptional regulators. J Mol Cell Biol 2:243–254
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. NAR 29:e45
Providenti MA, Wyndham RC (2001) Identification and functional characterization of CbaR, a MarR-Like modulator of the cbaABC-encoded chlorobenzoate catabolism pathway. Appl Environ Microbiol 67:3530–3541
Reinecke DM, Bandurski RS (1981) Metabolic Conversion of C-14-Labeled Indole-3-Acetic-Acid to C-14-Labeled Oxindole-3-Acetic Acid. Biochem Bioph Res Co 103:429–433
Sambrook J, Maniatis T, Fritsch EF (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory. 3 v. p
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Stein SE (1999) An integrated method for spectrum extraction and compound identification from gas chromatography/mass spectrometry data. J Am Soc Mass Spectr 10:770–781
Acknowledgments
We thank Dr. Oliver Fiehn from the UC Davis Metabolomics Facility for his help with interpretation of some of the GC-MS data. We thank Seok Hoon Hong from the laboratory of Thomas Wood at Texas A&M, College Station, TX, US, for sharing the E. coli BW15113 strains, and Becky Parales at UC Davis for helpful advice regarding chemotaxis assays. We also acknowledge the constructive criticism of three anonymous reviewers. This study was funded in part by USDA-NIFA grant 2011-67017-30024 from the United States Department of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure 1
GC-MS ion counts of indole-3-acetic acid (IAA) (blue), compound #1 (red), compound #2 (green), and catechol (purple, compound #3) in the supernatants of Escherichia coli TOP10 cultures grown overnight on M9 glucose medium supplemented with 0.2 mM IAA and carrying plasmid pIAC115-iacE::Tn5, -iacC::Tn5, -iacAB, -vector::Tn5, or -Δiac. ‘IAA Alone’ is M9 glucose medium supplemented with 0.2 mM IAA but no cells (control). Data were averaged over three biological replicates, with error bars representing standard deviations. (PPTX 87 kb)
Supplemental Figure 2
(A) Deconvoluted mass spectrum of compound #1 (top) and Binbase library spectrum for 5-hydroxyindole-3-acetic acid (bottom); ion peaks at m/z 290, 407 and 202 were used for putative annotation. (B) Deconvoluted mass spectrum of compound #2, putatively dioxindole-3-acetic acid, could not be annotated or identified using BinBase library; signature ion peaks at m/z 202, 292, 306, 364 and 423. (C) Deconvoluted mass spectrum of compound # 3 (top) and library spectrum for catechol (bottom); identified by m/z 254 parent ion and daughter ions with m/z 136, 151, 166, and 239. (PPTX 320 kb)
Rights and permissions
About this article
Cite this article
Scott, J.C., Greenhut, I.V. & Leveau, J.H.J. Functional Characterization of the Bacterial iac Genes for Degradation of the Plant Hormone Indole-3-Acetic Acid. J Chem Ecol 39, 942–951 (2013). https://doi.org/10.1007/s10886-013-0324-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-013-0324-x