Abstract
Sequestration of plant secondary metabolites is a widespread phenomenon among aposematic insects. Sarmentosin is an unsaturated γ-hydroxynitrile glucoside known from plants and some Lepidoptera. It is structurally and biosynthetically closely related to cyanogenic glucosides, which are commonly sequestered from food plants and/or de novo synthesized by lepidopteran species. Sarmentosin was found previously in Parnassius (Papilionidae) butterflies, but it was not known how the occurrence was related to food plants or whether Parnassius species could biosynthesize the compound. Here, we report on the occurrence of sarmentosin and related compounds in four different Parnassius species belonging to two different clades, as well as their known and suspected food plants. There were dramatic differences between the two clades, with P. apollo and P. smintheus from the Apollo group containing high amounts of sarmentosin, and P. clodius and P. mnemosyne from the Mnemosyne group containing low or no detectable amounts. This was reflected in the larval food plants; P. apollo and P. smintheus larvae feed on Sedum species (Crassulaceae), which all contained considerable amounts of sarmentosin, while the known food plants of the two other species, Dicentra and Corydalis (Fumariaceae), had no detectable levels of sarmentosin. All insects and plants containing sarmentosin also contained other biosynthetically related hydroxynitrile glucosides in patterns previously reported for plants, but not for insects. Not all findings could be explained by sequestration alone and we therefore hypothesize that Parnassius species are able to de novo synthesize sarmentosin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
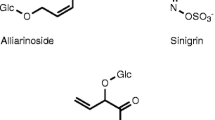
Almost all plants and insects contain characteristic allelochemicals with defensive properties. The chemical interactions of insects and their food plants are variable and hard to predict, and are dependent on types of chemicals, and the insects’ ability to sense and avoid, sequester, degrade/transform, or excrete chemicals as well as cost-benefit involved for the plants and the insects. Sarmentosin is an unsaturated γ-hydroxynitrile glucoside (γ-HNG) (Fig. 1) known from plants as well as butterflies and moths (Nishida, 1994; Nishida et al., 1994; Nishida and Rothschild, 1995; Bjarnholt et al., 2008). The importance and biological function of the compound is not understood, although it is found in significant amounts in both types of organisms, i.e., 500–650 μg/insect (Nishida et al., 1994; Nishida and Rothschild, 1995) and up to 75 % of total HNG content in a given plant (Bjarnholt et al., 2008). In plants, sarmentosin is derived from the amino acid isoleucine (Ile), and most often co-occurs with other Ile-derived HNGs (Nishida, 1994; Bjarnholt et al., 2008) (Fig. 1). Commonly known are the α-HNGs, also denoted cyanogenic glucosides (CNglcs) because they release hydrogen cyanide (HCN) upon hydrolysis of the glucosidic bond. The HNGs are mainly β-glucosides, and most plants that harbor CNglcs also contain a β-glucosidase (BGD) committed to hydrolyzing the compounds upon herbivore attack causing HCN release. The CNglcs are thus considered to be part of the plants’ chemical defense (Morant et al., 2008). Many CNglc-containing insects have been reported to be cyanogenic, and some have been shown to contain a corresponding β-glucosidase (Franzl et al., 1989), which may be released along with the CNglcs as a defense mechanism. Insects that contain either sarmentosin or the leucine (Leu)-derived analog sutherlandin are generally known to be deterrent to many predators, and sutherlandin itself has been reported to be deterrent to predating ants (Braekman et al., 1982; Aldrich et al., 1990; Nishida et al., 1994).
In Lotus japonicus, the six co-occurring Ile-derived α-, β-, and γ-HNGs (Fig. 1) share the first step of the biosynthetic pathway where Ile is converted to its corresponding oxime by a cytochrome P450, CYP79D3 (Takos et al., 2011) (Fig. 2). Furthermore, the presence or absence of the five non-cyanogenic HNGs in this plant is determined by a single recessive genetic trait, demonstrating a tight biosynthetic connection between these compounds further downstream in the pathway as well (Bjarnholt et al., 2008). Subsequent investigation of the pathway in L. japonicus has led to the proposed pathway seen in Fig. 2 (Saito et al., 2012), however, some key enzymes remain unknown. Sarmentosin is not present in L. japonicus, but the typical co-occurrence with the remaining Ile-derived HNGs in other plants renders it a likely product of rhodiocyanoside A hydroxylation. Sarmentosin is, therefore, presumably also biosynthetically linked to CNglcs and other HNGs in plants (Fig. 2). The CNglcs are widespread in the plant kingdom (Bak et al., 2006) and are also present in some insects, especially butterflies and moths (Zagrobelny et al., 2008). Insects either sequester the compounds from their food plants or biosynthesize the compounds themselves, and some are even able to do both (Zagrobelny et al., 2008). It is possible that the situation is the same for sarmentosin (Nishida, 1994). It has recently been established that the individual steps in CNglc biosynthesis are the same in plants and insects, although catalyzed by members of different subfamilies of the involved cytochrome P450s and glucosyl transferases (Jensen et al., 2011). Furthermore, the pathway of sequestration from plant to insect appears to be the same for cyanogenic and non-cyanogenic HNGs, as demonstrated for the larvae of the burnet moth Zygaena filipendulae. These larvae naturally sequester linamarin and lotaustralin from the food plant Lotus corniculatus, which does not contain non-cyanogenic HNGs. When reared instead on L. japonicus, Z. filipendulae also sequestered the remaining HNGs found in this plant (Zagrobelny et al., 2007a). In addition, the zygaenid moth Pryeria sinica was reported to contain sarmentosin as well as linamarin and lotaustralin, all of which also were found in its larval food plant Euonymus japonicus (Nishida, 1994). This indicates that it sequesters all HNGs from the food plant. On the other hand, the Magpie moth Abraxas grossulariata (Geometridae) appears to be able to de novo synthesize sarmentosin. The compound was found in pupae as well as adults regardless of whether the food plant was a Prunus species (only known to contain phenylalanine-derived HNGs) or E. japonicus (Nishida et al., 1994; Nishida and Rothschild, 1995). Several other Abraxas moths have also been found to contain sarmentosin, most of them after being reared on E. japonicus, but interestingly none were reported to contain other HNGs (Nishida, 1994).
Biosynthesis of Ile-derived HNGs and linamarin as known from plants and insects. Lj = Lotus japonicus, Me = Manihot esculenta, Zf = Zygaena filipendulae, CYP = cytochrome P450, FMO = flavin monooxygenase, OGDD = 2-oxoglutarate-dependent dioxygenase. CNglc pathway in plants and insects: The first cytochrome P450 catalyzes the conversion of amino acids to oximes, and the second converts oximes to α-hydroxynitriles, most likely via a nitrile intermediate. The α-hydroxynitriles are glucosylated to CNglcs by family 1 UDP-glycosyl transferases (Takos et al., 2011). In L. japonicus, the pathway for non-cyanogenic HNGs share the first step with the CNglc pathway (Bjarnholt et al., 2008), the second step is catalyzed by an unknown cytochrome P450 converting the Ile-derived oxime to the corresponding nitrile (Saito et al., 2012). The following steps towards the β- and γ-hydroxynitriles are unknown but hypothesized to be catalyzed by any of the mentioned oxygenases or combinations of them (Saito et al., 2012), and the putative UGT(s) finally producing the β- and γ-HNGs has not been identified yet either. In sarmentosin producing plants, the extra hydroxylation can take place before or after glucosylation and may be catalyzed by CYPs or FMOs
Sarmentosin was additionally found in two species of Parnassius (Papilionidae) butterflies, P. apollo and P. smintheus Footnote 1 (Nishida, 1994; Shepard and Manley, 1998). These species are reported to feed on Sedum plants, which are likely to contain sarmentosin, as the compound was first isolated from S. sarmentosum (Lechtenberg and Nahrstedt, 1999) and subsequently found in S. stenopetalum (Nishida, 1994). There are no reports on other HNGs occurring in the butterflies or the plants. In order to shed some light on the possible co-occurrence and sequestration or biosynthesis of various cyanogenic and non-cyanogenic HNGs in these insects, we here report on the content of HNGs in four different species of Parnassius butterflies and larvae, and in their known and suspected food plants. Around 50 species of Parnassius butterflies are known, mostly occurring in areas in Central Asia, the Himalayas, and western China at high altitudes. Based on phylogenetic analyses, they are divided into eight clades (Michel et al., 2008; Omoto et al., 2009). The species examined here belong to two clades (Fig. 5): European P. apollo and North American P. smintheus represent the Apollo group (subgenus Parnassius s.str.) feeding on Crassulaceae, while European P. mnemosyne and North American P. clodius represent the Mnemosyne group (subgenus Driopa) feeding on Fumariaceae.
Methods and Materials
Samples and Sampling Procedure
To elucidate the presence of sarmentosin and related compounds in Parnassius butterflies and their respective food plants, four butterfly species (P. smintheus, P. mnemosyne, P. apollo, P. clodius) and four plant species representing Crassulaceae (S. telephium ssp. maximum, S. lanceolatum) and Fumariaceae (Dicentra uniflora, Corydalis solida) were sampled as summarized in Table 1. All samples were collected alive in the field, where they were frozen quickly and subsequently transported on dry ice to Copenhagen University. Here, the samples were kept at −80°C until they were extracted and analyzed according to the procedures below.
Extraction Procedure
Metabolites were extracted from frozen samples with 55 % MeOH containing 0.1 % formic acid and 0.044 mM amygdalin (phenylalanine derived CNglc) as internal standard. Butterfly samples were crushed with a mortar and pestle (in some cases with wings and body as separate samples), while plant samples were crushed in an eppendorf tube with a plastic pestle. Both sample types were subsequently passed through an anopore 0.45 mm filter (Whatman).
LC-MS Analyses
The extracts were analyzed by LC-MS in their year of collection and subsequently stored at −20°C. All extracts were re-analyzed together in 2011 to be able to compare samples from different years, and the resulting chromatograms had not changed during storage of extracts (data not shown). All samples were diluted four times with water before analysis by LC-MS. Analytical LC-MS was carried out using an Agilent 1100 Series LC (Agilent Technologies, Germany) coupled to a Bruker HCT-Ultra ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany). A Zorbax SB-C18 column (Agilent; 1.8 μM, 2.1 × 50 mm) was used at a flow rate of 0.2 ml/min. The oven temperature was maintained at 35°C. The mobile phases were: A, water with 0.1 % (v/v) HCOOH and 50μM NaCl; B, acetonitrile with 0.1 % (v/v) HCOOH. The gradient program was: 0 to 0.5 min, isocratic 2 % B; 0.5 to 7.5 min, linear gradient 2 to 40 % B; 7.5 to 8.5 min, linear gradient 40 % to 90 % B; 8.5 to 11.5 isocratic 90 % B; 11.60 to 17 min, isocratic 2 % B. The flow rate was increased to 0.3 ml/min in the interval 11.2 to 13.5 min. The mass spectrometer was run in positive electrospray mode. Mass spectral data were analysed with the native DataAnalysis software. Sodium adducts of sarmentosin (m/z 298, retention time (rt) 1.8 min), linamarin (m/z 270, rt 2.6 min), rhodiocyanoside A (m/z 282, rt 4.6 min), lotaustralin (m/z 284, rt 5.5 min), β-HNGs (ribesuvanins and rhodiocyanosides D + E)(m/z 282 + 284, rt 4.0-4.5 min) and amygdalin (m/z 480, rt 6.6 min) (Fig. 3) were detected and compared to standards of linamarin, lotaustralin, and sarmentosin. Total amounts of HNGs were estimated based on peak areas and quantified by injection of known amounts of linamarin, lotaustralin, and sarmentosin. As the ionization efficiency of linamarin, lotaustralin, and rhodiocyanoside A under these analytical conditions is the same (Bjarnholt et al., 2008), all rhodiocyanosides and ribesuvanins were quantified using the standard curve for lotaustralin.
Extracted ion chromatograms from LC-MS analyses of Sedum lanceolatum from 2009 USA illustrating the peaks of HNGs. The peak with a retention time of approximately 4 min and the same m/z value as sarmentosin is an unidentified glucoside (as determined by MS/MS-analysis, data not shown) which was not found in the insects
Estimation of P. apollo Larval Leaf Consumption
The food and energy budget of P. apollo reared in the semi-natural colony was experimentally estimated in 1996, 1997, and 2003 as described in detail for 1996 (Kędziorski et al., 1997). These numbers were used to calculate the estimated average amount of sarmentosin ingested by larvae (Table 2). Only the two last instars were considered, as the amount of food consumed at earlier instars is relatively small.
Results
Four different species of Parnassius butterflies were analyzed for their content of linamarin, lotaustralin, and the suite of known Ile-derived HNGs, including sarmentosin. The specimens of P. apollo had been reared in a semi-natural open-air colony and fed with food plants freshly collected in their natural biotopes, whereas the remaining three species originated from three different native habitats. In the cases of P. apollo and P. clodius, it was possible to analyze both larvae and adults, while only adults were available from the other species. Samples of plants known to or suspected to have served as the food plants of the larvae were also analyzed. The results on the overall occurrence of the compounds are shown in Table 3 (for details on number of replicates etc., see Table 1). Corydalis solida did not contain any of our compounds of interest, and in P. clodius from 2009 and 2010 we found only linamarin and lotaustralin. In the remaining plant and insect species, we found lotaustralin co-occurring with sarmentosin and/or the other Ile-derived HNGs, and/or sometimes with linamarin. Although it is not surprising that these compounds co-occur, this is the first report of rhodiocyanosides and ribesuvanins found in insects reared on a natural diet. Some of these compounds were found previously in Z. filipendulae, but only when larvae were reared on a species of Lotus, which was not their natural food plant (Zagrobelny et al., 2007a). To our knowledge, this is also the first report of CNglcs in the Fumariaceae (D. uniflora). The analyzed Sedum plants (Crassulaceae) had similar amounts of sarmentosin, lotaustralin, rhodiocyanoside D, and ribesuvanins across years and continents, but at least in the USA S. lanceolatum has the ability to produce linamarin and rhodiocyanoside A as well. Also, to the best of our knowledge, presence of linamarin has not been reported previously in the Crassulaceae, although several members of this order contain Ile-derived HNGs (Bjarnholt and Møller, 2008).
Sarmentosin was found in three of the four analyzed Parnassius species (Table 3). In P. smintheus and P. apollo, the levels were respectively 602 ± 74 and 845 ± 151 μg per individual adult. It is noteworthy that the P. smintheus imagines were on average approximately one third the size of the P. apollo imagines (130 vs. 420 mg). This means that the concentrations found in P. smintheus were almost three times higher than those found in P. apollo, as can be seen from Fig. 4. The total amounts are in accordance with the previously reported levels of 460 μg per individual for P. smintheus [Clade I (Omoto et al., 2009)], whereas the amount of sarmentosin found in P. apollo has to our knowledge not been published (Nishida, 1994; Nishida and Rothschild, 1995). Furthermore, the reported levels in various Abraxas moths are 270–670 μg per individual, and in one species of Yponomeuta moth 120 μg per individual (Nishida, 1994; Nishida et al., 1994). On the other hand, we found no sarmentosin in any individuals of P. mnemosyne from 2011, and in P. clodius the occurrence varied among the 3 years they were collected; furthermore, when sarmentosin was present, the level was very low (~0.5 μg/individual).
The larvae that gave rise to the examined P. apollo adults were from a batch of early hatched larvae, and their average leaf consumption can be estimated to be approximately 25 g for males and 38 g for females (Table 2). The difference is mainly due to sex differences in duration of the last instar (Kędziorski et al., 1997). The sarmentosin content of 25 g of S. telephium is approximately 1000 μg [40 μg/g FW (Fig. 4)], which is within the confidence interval (P < 0.05) for the amount found in P. apollo adults (roughly 850 μg/individual ± 150 μg). The “concentration factor” from larval food plant to imago was 55 (data from Fig. 4: C P. apollo : C S. telephium = 2.2 μg/g : 0.04 μg/g). Interestingly, this factor for the other species containing high sarmentosin amounts was essentially the same (C P. smintheus : C S. lanceolatum = 5.9 μg/g : 0.1 μg/g = 59). As seen from Fig. 4, the remaining HNGs were not concentrated from food plant to butterflies.
For S. telephium and C. solida, intact leaves as well as leaves that had been damaged by larval feeding were analyzed. There were no differences in the amount of HNGs due to grazing stress (data not shown), and this had also previously been observed in S. lanceolatum in Canada for sarmentosin only (our unpublished results). This is consistent with the traditional perception that CNglcs – and possibly also the other HNGs – are phytoanticipins, and that the plants’ inducible defense involves different compounds. There were no differences in the type or amounts of compounds between males and females in P. apollo and P. clodius (data not shown), although differences between the sexes were previously shown for linamarin and lotaustralin in Z. filipendulae adults (Zagrobelny et al., 2007b). Adult specimens of P. apollo and P. clodius from 2010 were divided into wings and body, which were extracted separately, and it was evident that 30–40 % of the sarmentosin content of the entire P. apollo butterflies was present in the wings (Table 4). Since the body is much heavier than the wings, wings actually contain up to 10 times as much sarmentosin per mg fresh weight. This was shown previously to also be the case for P. phoebus from the US (Nishida and Rothschild, 1995).
Discussion
Many Lepidoptera are strongly associated with poisonous plants and sequester toxic compounds instead of or in addition to manufacturing their own (Nishida, 1994, 2002; Opitz and Muller, 2009). The potential of insects to take up, transport, and sequester plant derived defense compounds to their own benefit probably derives from their general nutrient uptake system, which has a broad selectivity for transport of plant glucosides. If food plants provide compounds that match the substrate profile of the larval transport systems, the larvae have a high probability of adopting compounds into their defense system (Kunert et al., 2008; Zagrobelny and Møller, 2011). At first sight, the results of our study present a case of sequestration, in that the patterns of compounds found in larvae and butterflies roughly reflect those found in the food plants. However, some specimens contained “extra compounds” (Table 3). Larvae of the P. apollo ssp. frankenbergeri feed almost exclusively on S. telephium spp. maximum, so this was the only food plant made available to the specimens in the semi-natural colony (Nakonieczny and Kedziorski, 2005). The pattern of compounds found in the insects and the food plant was the same, except for the presence of rhodiocyanoside A in larvae and butterflies. As this compound is likely to be the biosynthetic precursor of sarmentosin it could have been present in the food plant at other time points or in different tissues from the ones sampled. The same applies for the rhodiocyanoside A found in the sampled P. smintheus; the Canadian samples of its food plant S. lanceolatum (Matter et al., 2003) did not contain this compound, although S. lanceolatum from USA did. Furthermore, larvae of P. clodius are known to feed on D. uniflora (Scott, 1986), which did not contain sarmentosin, whereas P. clodius butterflies from 2011 did. It is also possible that these plants contain sarmentosin in different tissues from those sampled here, but other plants are known which produce rhodiocyanoside A without producing sarmentosin (Bjarnholt et al., 2008). Because of the limited total biomass available from the food plant D. uniflora and the co-occurence of S. lanceolatum at the population site, we wondered whether P. clodius specimens collected at this location may have supplemented their diet with S. lanceolatum. The presence of sarmentosin in S. lanceolatum supports this hypothesis, but it is also possible that the P. clodius specimens from 2011 have synthesized the compound, either de novo or by hydroxylation of rhodiocyanoside A sequestered from D. uniflora. The P. mnemosyne butterflies contained little or none of our compounds of interest, and in the food plant C. solida there were no detectable levels at all. This Parnassius species feeds exclusively on Corydalis ssp. (Turlin and Malin, 2005), and to our knowledge there are no other reports of HNGs in Corydalis. The present results, therefore, indicate that P. mnemosyne can biosynthesize HNGs, unless the compounds found in the butterflies originate from tissues or Corydalis species not analyzed. In all cases, it is theoretically possible that the compounds found in insects, but not in food plants, were bio-concentrated from undetectable levels in plants to detectable levels in insects. On the other hand, the remaining results show that sarmentosin is the only compound that is concentrated from plant to insect.
Interestingly, in larvae and butterflies of P. apollo and P. smintheus where the sarmentosin content was high, the content of the remaining HNGs were several orders of magnitude lower (Fig. 4). This is in stark contrast to the pattern found in the food plants where the levels of the different HNGs were of comparable magnitude. Similar HNG levels were previously also observed in plants from the genera Rhodiola, Ribes, and Lotus (Bjarnholt et al., 2008). From the report of co-occurring sarmentosin, lotaustralin, and linamarin in Pryeria sinica, it seems that the levels of the compounds were more equal in this species, but there are no exact measurements stated (Nishida, 1994). The dramatic increase in the ratio of sarmentosin to the remaining HNGs in P. apollo and P. smintheus demonstrates clearly that there is preferential accumulation in these species. This could be achieved either by preferential uptake of sarmentosin, preferential degradation of the remaining HNGs, or by de novo synthesis of sarmentosin. As previously mentioned, the uptake system in Z. filipendulae larvae does not appear to discriminate between CNglcs and other HNGs found in the food plant, and the ratio between the different CNglcs linamarin and lotaustralin is influenced by the ratio in the food plant at the larval stage (Zagrobelny et al., 2007a). During pupation, this changes to almost identical ratios of compounds in the imagines, irrespective of food plant composition (Zagrobelny et al., 2007a). Simultaneously, the total amount of CNglcs decreases (Zagrobelny et al., 2007a), indicating that the changed ratio is obtained by preferential degradation, but in P. apollo the preferential accumulation of sarmentosin appears already at the larval stage (Fig. 4). De novo synthesis of CNglcs is a widespread phenomenon in Papilionoidea, where imagines of butterflies from the Heliconiinae, Acraeinae, Nymphalinae, and Polyommatinae groups have been shown to accumulate linamarin and often lotaustralin, which is probably achieved by biosynthesis of the compounds (Engler et al., 2000; Turlin and Malin 2005; Rebourg et al., 2006). The ability to produce CNglcs has also been proposed to be present in all species of Zygaenidae (Zagrobelny et al., 2008). When the biosynthetic pathway was recently resolved for Z. filipendulae (Jensen et al. 2011), it was found to be highly similar to the pathways known from plants (Takos et al., 2011), although clearly convergent. In L. japonicus, it appears likely that only two more enzymes are necessary to produce all the non-cyanogenic HNGs, and they could very well be closely related to the two cytochromes P450 already known to be involved in the pathway (Bjarnholt et al., 2008; Takos et al., 2011; Saito et al., 2012). As previously mentioned, biosynthesis of sarmentosin presumably only requires an additional hydroxylation of rhodiocyanoside A, a reaction frequently used by insects to handle sequestered toxins (Langel and Ober, 2011; Pinto et al., 2011) and possibly present in P. clodius if it does not feed on S. lanceolatum. Given the similar structure of the CNglc biosynthetic pathway in insects and plants, it is likely that some insects could also possess the few additional enzymes needed to produce the remaining compounds as suspected for the Abraxas species (Nishida, 1994; Nishida et al., 1994).
The calculations of larval sarmentosin intake for the two Parnassius species from the Apollo group are based on estimates of larval leaf intake and variable sarmentosin concentrations in plants. However, they show that sequestration efficiency must be quite high if all the sarmentosin found in the male insects originate from sequestration during the larval stage. The literature contains few attempts to quantify sequestration of plant secondary metabolites by insects, but in general significantly less than 100 % of ingested compounds can be recovered in the insects. Furthermore, the concentration factors from larval food plant to imagines are much lower than the values of 55–60 found for P. apollo and P. smintheus in the present study. Larvae of Heliconius butterflies sequester CNglcs derived from the non-protein amino acid cyclopentenyl glycine from their Passifloraceae food plants, and in some cases possibly also minor amounts of linamarin and lotaustralin. Some or all of them are also able to biosynthesize linamarin and lotaustralin. In an extensive study of different Heliconius species reared on different Passiflora plants, the concentrations of CNglcs were measured in the butterflies and the food plants (Engler-Chaouat and Gilbert, 2007). No attempts were made to quantify sequestration, but the concentrations found in butterflies were generally equal to or much less than concentrations in the food plants, with few examples of a 6–10 fold increase.Footnote 2 Our unpublished results indicate that Z. filipendulae larvae sequester around 80–85 % of CNglcs present in the leaves they eat, with the rest being lost due to degradation and HCN release during chewing. Before reaching maturity, Zygeana species lose additional CNglcs by at least two routes: degradation during pupation and degradation during the larval stage, where they have been shown to be continuously surrounded by a HCN “cloud” (Witthohn and Naumann, 1987; Zagrobelny et al., 2007a, b). In the case of Z. filipendulae, the concentration factor from larval food plant to adult moth was around 3 when the larvae were reared on natural food plants (Zagrobelny et al., 2007a). Larvae of Thessalia leanira fulvia (Nymphalidae) sequester iridoid glycosides (IGs) from Castilleja integra. Cultured larvae reared on this food plant contained IGs to the same order of magnitude as the plant tissue, and so did the larval frass (Mead et al., 1993), clearly demonstrating less than 100 % sequestration. Among Papilionidae sequestering non-glucosidic compounds such as aristolochic acids, quinolizidine alkaloids, or pyrrolizidine alkaloids, the recovery of compounds in larvae can be estimated to correspond to either 0.5-50 % of ingested compounds, or up to 1 g of plant material (Montllor et al., 1990; Vonnickischrosenegk and Wink, 1993; Pinto et al., 2011).
All in all, it appears somewhat unlikely that the P. apollo and P. smintheus larvae should have sequestered and retained all the sarmentosin ingested with their food plant. At the same time, not all HNGs found in P. clodius and P. mnemosyne can be unequivocally accounted for by sequestration, and there is also the question of the presence of rhodiocyanoside A in P. apollo and P. smintheus. Based on this, we hypothesize that Parnassius species are capable of biosynthesizing HNGs. We propose that a pathway for production of all HNGs evolved in the common ancestor of Zygaenoidea and Papilionoidea (Fig. 5) or even earlier. This made that ancestor able to produce all of the different Ile-derived HNGs and also valine-derived linamarin, which is biosynthesized by the same enzyme system as lotaustralin in insects and plants (Jensen et al. 2011; Takos et al., 2011). If the Abraxas moths (Geometridae) are indeed capable of biosynthesizing sarmentosin, the pathway must have evolved even before this branch was split from Zygaenoidea and Papilionoidea (Fig. 5). During evolution, some branches must have specialized to produce only one or two compounds, and some branches lost the ability altogether. However, most species in all branches probably retained the ability to handle and detoxify the compounds, since HNGs are widespread in plants (Bjarnholt and Møller, 2008), making it easy for species to sequester the compounds from newly colonized food plants during evolution.
Parnassius butterflies are divided into eight clades (Omoto et al., 2009) (Fig. 5). Clade I which is the most basal split on the Parnassius species tree, comprise species feeding on Crassulaceae, mainly Sedum spp., and in one instance Saxifragaceae (Michel et al., 2008), with several species from both plant families having been shown to contain sarmentosin (Nishida and Rothschild, 1995; Bjarnholt et al., 2008). The other seven Parnassius clades feed on Fumariaceae, mostly on Corydalis species, and in one instance on Scrophulariaceae (Michel et al., 2008), so far not shown to contain sarmentosin. According to molecular data Parnassius underwent a major speciation event some 24.3 ± 4.1 MY BP (Omoto et al., 2009) which probably resulted in a shift from ancestral food-plants (possibly Aristolochiaceae) onto Crassulaceae and Fumariaceae (Rebourg et al., 2006; Michel et al., 2008). We hypothesize that Parnassius butterflies were able to biosynthesize HNGs even before they colonized Crassulaceae since the ability came from a proposed Ditrysian ancestor (Fig. 5). They were then immediately able to sequester the encountered compounds, when colonizing Crassulaceae, and this would have enabled them to conserve energy from the production of defense compounds. Their own biosynthetic activity may then have become lower over time, because it was not necessary to maintain a high activity anymore. The later shift to Fumariaceae as food plant probably entailed a shift in defense compounds as well, as D. uniflora contains poisonous alkaloids that P. clodius is suspected of sequestering, and C. solida apparently does not contain HNGs at all, rendering it unfavorable for P. mnemosyne to base its chemical defense on these compounds. This could be the reason why we found only trace amounts of HNGs in the Mnemosyne clade and why P. mnemosyne has apparently lost the ability to perform the last step of sarmentosin biosynthesis and accumulates rhodiocyanoside A instead.
Whether the sarmentosin found in P. apollo, P. smintheus, and P. clodius was a result of sequestering and/or biosynthesis, the high amounts and the comparatively low amounts of the remaining HNGs found in the two first species clearly show that sarmentosin is of importance to these larvae and butterflies. The observation that the sarmentosin concentrations were much higher in wings than in the bodies of all analyzed sarmentosin-containing specimens is consistent with sarmentosin being a defense compound, since wings and their scales are the first thing a predator comes in contact with when attacking a butterfly. In addition, P. apollo is avoided by naive chicks, either initially or after a few attacks (Bohlin et al., 2008). Likewise, Abraxas moths are known to be deterrent to a long list of predators (Nishida et al., 1994). The young black Apollo larvae are hard to detect by predators, while the colored spots appearing later significantly increase larval conspicuousness (Bohlin et al., 2008). This could be linked to the lower amounts of sarmentosin presumably present during the first few instars where the larvae have not yet had the opportunity to sequester or biosynthesize as much sarmentosin as compared to later instars. Larvae of P. smintheus show a similar pattern with conspicuous yellow markings generally appearing after the second instar, whereas P. clodius larvae are more cryptic in coloration patterns, brown in background color with small dark brown and cream colored spots. Likewise, P. apollo and P. smintheus butterflies both have large and distinct red spots on their wings, suggesting aposematism, whereas the spots on adult P. clodius are smaller and more variable in color, and adult P. mnemosyne have no spots at all.
The mechanism by which sarmentosin might work as a defense compound is not known. In plants, the action of the non-cyanogenic HNGs is most likely mediated by BGD-hydrolysis, as L. japonicus contains BGDs committed to rhodiocyanoside hydrolysis (Takos et al., 2010), and our unpublished results for eight cultivars of gooseberry show that sarmentosin is hydrolyzed upon tissue disruption. Insects may also possess such BGDs, but the γ-hydroxynitrile resulting from sarmentosin hydrolysis (Fig. 6) will not release HCN as do the α-hydroxynitriles derived from CNglcs hydrolysis. The hypothetical defensive effect of sarmentosin could simply be related to the reported bitterness of the intact compound (Nishida et al., 1994), which could also be the cause of the repellency towards predating ants reported for the Leu-derived analog, sutherlandin (Braekman et al., 1982). On the other hand, a number of reaction schemes may also be envisioned to transform sarmentosin into more potent compounds as described in Fig. 6 and the following. First, a related compound has been identified in S. cepaea, namely sarmentosin epoxide (Fig. 6) (Nahrstedt et al., 1982). The epoxide group can be hydrolyzed, causing the formation of a free α-hydroxynitrile moiety (Scheme C, Fig. 6). The reaction has been demonstrated to be accelerated in the presence of rat liver microsomes containing epoxide hydrolases (Nahrstedt et al., 1982). We investigated the possibility that the presently analyzed plants or insects also contained sarmentosin epoxide, but did not find clear evidence of the compound or its possible degradation products (scheme C, Fig. 6) being present. However, the compound may represent an activated form of sarmentosin, the production of which could depend on an enzyme released upon cell disruption, as is the case for the CNglc-BGD system, or upon epoxidation by reactive oxygen species. Alternatively, the action of sarmentosin may depend on BGD-mediated hydrolysis, which could lead to defense in several hypothetical ways: 1. HCN release from γ-hydroxynitriles by an unknown mechanism, probably enzyme-mediated (scheme A, Fig. 6); 2. Toxicity or deterrent effects of the γ-hydroxynitrile itself; 3. Nitrilase-mediated formation of a lactone, isosiphonodin (scheme B, Fig. 6) as we have previously suggested (Bjarnholt and Møller, 2008). So far, there is no experimental evidence to support possibilities 1 and 2, but isosiphondin has been found in several Yponomeuta larvae (Yponomeutidae) and in small amounts in E. europaeus on which some of them feed (Fung et al., 1988). As previously mentioned, one Yponomeuta moth and E. japonica contain sarmentosin (Nishida, 1994). Isosophonodin was subsequently also found in roots of S. telephium (Fung et al., 1990), and its glucoside, β-miroside, has been isolated from a gymnosperm, Prymnopitys ferruginea (Lorimer et al., 1995). In addition, analogous lactones and corresponding glucosides suspected to be derived from Leu-derived hydroxynitrile compounds in plants have been found in larvae and adults of true bugs (Braekman et al., 1982; Aldrich et al., 1990), and similar lactones and corresponding glucosides have been identified in various plants (Fung et al., 1988; Lorimer et al., 1995). Antifungal, antibacterial, and/or cytotoxic activities have been reported for β-miroside and several of the related compounds (Fung et al., 1988; Lorimer et al., 1995). We did not find any evidence of lactones in the analyzed specimens in this study either, but the analytical method was not optimized for such compounds. More importantly, if isosophonodin has a biological activity, sarmentosin could also in this case be the storage form that is enzymatically activated upon attack by predators and, therefore, not found in the intact insect.
This study has demonstrated that sarmentosin and related HNGs can be found in Crassulaceae-feeding Parnassius species as well as species feeding on Fumariaceae, albeit in highly variable amounts. Since the members of the remaining clades also feed on Fumariaceae species, it is likely that HNGs are widespread in this genus, just as is the case for sarmentosin in the Abraxas genus (Nishida, 1994) and for CNglcs in the Zygeana and Heliconius genera (Engler-Chaouat and Gilbert, 2007; Zagrobelny et al., 2008). Based on the observed discrepancies between occurrence of specific compounds in butterflies and known food plants, and on the apparently high accumulation of sarmentosin in P. apollo and P. smintheus, we have hypothesized that Parnassius species possess a biosynthetic pathway for production of HNGs. This has also been suggested for Abraxas, and is known to be the case for Zygeana and Heliconius (Nishida, 1994; Engler-Chaouat and Gilbert, 2007; Zagrobelny et al., 2008). The presence of such a biosynthetic pathway remains to be experimentally verified, e.g., by controlled feeding of the larvae or biochemical investigations of enzymatic activities. If the larvae are not able to de novo synthesize the compounds, the present study demonstrates a highly efficient selection for sarmentosin accumulation in species of the Apollo clade. Lack of such a biosynthetic pathway would also indicate that at least the populations of species from the Mnemosyne clade sampled in this study are or can be polyphagous since they apparently can not sequester all the observed compounds from their known food plants. It is of great interest to researchers from the insect as well as the plant field to uncover the biological role of sarmentosin and other non-cyanogenic HNGs, but this is more complicated. As a first step, it should be determined if any of the schemes in Fig. 6 can be verified, in order to identify compounds that may be tested for effects on predators, mates etc.
Notes
At the time of sampling P. smintheus was considered to be P. phoebus and the results reported at such (Nishida, 1994). Later, the two species were separated. Given the reported host plant and because P. phoebus does not exist in the state of Washington, USA, where the butterflies were sampled, the species investigated must have been P. smintheus (Shepard and Manley, 1998).
The concentrations stated are based on dry weight of insects and plants. Our calculations of concentration factors are based on the assumption that butterfly and plant dry matter constitute approximately 20 % and 10 % respectively.
References
Aldrich, J. R., Carroll, S. P., Lusby, W. R., Thompson, M. J., Kochansky, J. P., and Waters, R. M. 1990. Sapindaceae, cyanolipids, and bugs. J. Chem. Ecol. 16:199–210.
Bak, S., Paquette, S. M., Morant, M., Rasmussen, A. B., Saito, S., Bjarnholt, N., Zagrobelny, M., Jørgensen, K., Hamann, T., Osmani, S., Simonsen, H. T., Perez, R. S., van Hesswijck, T. B., Jørgensen, B., and Møller, B. L. 2006. Cyanogenic glycosides: a case study for evolution and application of cytochromes P450. Phytochem. Rev. 5:309–329.
Bjarnholt, N. and Møller, B. L. 2008. Hydroxynitrile glucosides. Phytochemistry 69:1947–1961.
Bjarnholt, N., Rook, F., Motawia, M. S., Jørgensen, C., Olsen, C. E., Jaroszewski, J. W., Bak, S., and Møller, B. L. 2008. Diversification of an ancient theme: hydroxynitrile glucosides. Phytochemistry 69:1507–1516.
Bohlin, T., Tullberg, B. S., and Merilaita, S. 2008. The effect of signal appearance and distance on detection risk in an aposematic butterfly larva (Parnassius apollo). Anim. behav. 76:577–584.
Braekman, J. C., Daloze, D., and Pasteels, J. M. 1982. Cyanogenic and other glucosides in a neo-guinean bug Leptocoris isolata - possible precursors in its host plant. Biochem. Syst. Ecol. 10:355–364.
Engler-Chaouat, H. S. and Gilbert, L. E. 2007. De novo synthesis vs. sequestration: Negatively correlated metabolic traits and the evolution of host plant specialization in cyanogenic butterflies. J. Chem. Ecol. 33:25–42.
Engler, H. S., Spencer, K. C., and Gilbert, L. E. 2000. Insect metabolism - Preventing cyanide release from leaves. Nature 406:144–145.
Franzl, S., Ackermann, I., and Nahrstedt, A. 1989. Purification and characterization of beta-glucosidase (linamarase) from the hemolymph of Zygaena trifolii esper, 1783 (Insecta, Lepidoptera). Experientia 45:712–718.
Fung, S. Y., Herrebout, W. M., Verpoorte, R., and Fischer, F. C. 1988. Butenolides in small ermine moths, Yponomeuta ssp. (Lepidoptere, Yponomeutidae), and spindle tree, Euonymus europaeus (Celastraceae). J. Chem. Ecol. 14:1099–1111.
Fung, S. Y., Schripsema, J., and Verpoorte, R. 1990. Alpha, beta-unsaturated gamma-lactones from Sedum telephium roots. Phytochemistry 29:517–519.
Jensen, N. B., Zagrobelny, M., Hjerno, K., Olsen, C. E., Houghton-Larsen, J., Borch, J., Møller, B. L., and Bak, S. 2011. Convergent evolution in biosynthesis of cyanogenic defense compounds in plants and insects. Nat. Comm. 2: doi: 10.1038/ncomms1271.
Kędziorski, A., Nakonieczny, M., Pyrak, K., Bembenek, J., and Rosiński, G. 1997. Energy Metabolism in the Apollo Butterfly (Parnassius apollo L., Lepidoptera, Papilionidae). pp 114–120 in: D. Konopińska, G. Goldsworthy, R. J. Nachman, J. Nawrot, I. Orchard, and G. Rosiński, (eds.). Insects-Chemical, Physiological and Environmental Aspects. University of Wroclaw.
Kunert, M., Soe, A., Bartram, S., Discher, S., Tolzin-Banasch, K., Nie, L., David, A., Pasteels, J., and Boland, W. 2008. De novo biosynthesis versus sequestration: A network of transport systems supports in iridoid producing leaf beetle larvae both modes of defense. Insect Biochem. Mol. Biol. 38:895–904.
Langel, D. and Ober, D. 2011. Evolutionary recruitment of a flavin-dependent monooxygenase for stabilization of sequestered pyrrolizidine alkaloids in arctiids. Phytochemistry 72:1576–1584.
Lechtenberg, M. and Nahrstedt, A. 1999. Cyanogenic glycosides, pp. 147–191, in R. Ikan (ed.), Naturally Occurring Glycosides. John Wiley and Sons, Chichester.
Lorimer, S. D., Mawson, S. D., Perry, N. B., and Weavers, R. T. 1995. Isoltaion and synthesis of beta-miroside - an antifungal furanone glucosides from Prumnopitys ferruginea. Tetrahedron 51:7287–7300.
Matter, S. F., Roland, J., Keyghobadi, N., and Sabourin, K. 2003. The effects of isolation, habitat area and resources on the abundance, density and movement of the butterfly Parnassius smintheus. Am. Midl. Nat. 150:26–36.
Mead, E. W., Foderaro, T. A., Gardner, D. R., and Stermitz, F. R. 1993. Iridoid glycosides sequestration by Thessalia leanira (Lepidoptera, Nymphalidae) feeding on Castilleja integra (Scrophulariaceae). J. Chem. Ecol. 19:1155–1166.
Michel, F., Rebourg, C., Cosson, E., and Descimon, H. 2008. Molecular phylogeny of Parnassiinae butterflies (Lepidoptera: Papilionidae) based on the sequences of four mitochondrial DNA segments. Ann. Soc. Entomol. Fr. (n. s.) 44:1–36.
Montllor, C. B., Bernays, E. A., and Barbehenn, R. V. 1990. Importance of quinolizidine alkaloids in the relationship between larvae of Uresiphita reversalis (Lepidoptera, Pyralidae) and a host plant, Genista monspessulana. J. Chem. Ecol. 16:1853–1865.
Morant, A. V., Jørgensen, K., Jørgensen, C., Paquette, S. M., Sánchez-Perez, R., Møller, B. L., and Bak, S. 2008. β-glucosidases as detonators of plant chemical defense. Phytochemistry 69:1795–1813.
Nahrstedt, A., Walther, A., and Wray, V. 1982. Sarmentosin epoxide, a new cyanogenic compound from Sedum cepaea. Phytochemistry 21:107–110.
Nakonieczny, M. and Kedziorski, K. 2005. Feeding preferences of the Apollo butterfly (Parnassius apollo ssp frankenbergeri) larvae inhabiting the Pieniny Mts (southern Poland). C. R. Biol. 328:235–242.
Nishida, R. 1994. Sequestration of plant secondary compounds by butterflies and moths. Chemoecology 5:127–138.
Nishida, R. 2002. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 47:57–92.
Nishida, R. and Rothschild, M. 1995. A cyanoglucoside stored by a Sedum-feeding apollo butterfly, Parnassius phoebus. Experientia 51:267–269.
Nishida, R., Rothschild, M., and Mummery, R. 1994. A cyanoglucoside, sarmentosin, from the magpie moth, Abraxas grossulariata, Geometridae: Lepidoptera. Phytochemistry 36:37–38.
Omoto, K., Yonezawa, T., and Shinkawa, T. 2009. Molecular systematics and evolution of the recently discovered "Parnassian" butterfly (Parnassius davydovi Churkin, 2006) and its allied species (Lepidoptera, Papilionidae). Gene 441:80–88.
Opitz, S. E. W. and Muller, C. 2009. Plant chemistry and insect sequestration. Chemoecology 19:117–154.
Pinto, C. F., Urzua, A., and Niemeyer, H. M. 2011. Sequestration of aristolochic acids from meridic diets by larvae of Battus polydamas archidamas (Papilionidae: Troidini). Eur. J. Entomol. 108:41–45.
Rebourg, C., Petenian, F., Cosson, E., and Faure, E. 2006. Patterns of speciation and adaptive radiation in Parnassius butterflies. J. Entomol. 3:204–215.
SAITO, S., MOTAWIA, M. S., OLSEN, C. E., MØLLER, B. L., and BAK, S. 2012. Biosynthesis of rhodiocyanosides in Lotus japonicus: Rhodiocyanoside A is synthesized from (Z)-2-methylbutanaloxime via 2-methyl-2-butenenitrile. Phytochemistry. doi: dx.doi.org/10.1016/j.phytochem.2012.01.020
Scott, J. A. 1986. The Butterflies of North America: A Natural History and Field Guide. Stanford University Press, Stanford, California.
Shepard, J. H. and Manley, T. R. 1998. A species revision of the Parnassius phoebus complex in North America (Lepidoptera: Papilionidae), pp. 731–736, in T. C. Emmel (ed.), Systematics of Western North American Butterflies. Mariposa Press, Gainsville, FL.
Takos, A. M., Knudsen, C., Lai, D., Kannangara, R., Mikkelsen, L., Motawia, M. S., Olsen, C. E., Saito, S., Tabata, S., Jørgensen, K., Møller, B. L., and Rook, F. 2011. Genomic clustering of cyanogenic glucoside biosynthetic genes aids their identification in Lotus japonicus and suggests the repeated evolution of this chemical defense pathway. Plant J. 68:273–86.
Takos, A., Lai, D., Mikkelsen, L., Abou hachem, M., Shelton, D., Motawia, M. S., Olsen, C. E., Wang, T. L., Martin, C., and Rook, F. 2010. Genetic screening identifies cyanogenesis-deficient mutants of Lotus japonicus and reveals enzymatic specificity in hydroxynitrile glucoside metabolism. Plant Cell 22:1605–1619.
Turlin, B. and Malin, L. 2005. Etude synoptique et Répartition mondiale des Espèces du Genre Parnassius Latreille 1804 (Lepidoptera Papilionidae). L. Manil, (ed.). Paris.
Vonnickischrosenegk, E. and Wink, M. 1993. Sequestration of pyrrolizidine alkaloids in several arctiid moths (Lepidoptera, Arctiidae). J. Chem. Ecol. 19:1889–1903.
Witthohn, K. and Naumann, C. M. 1987. Genus Zygaena F and related taxa (Insecta, Lepidoptera) 53. Active cyanogenesis - in Zygaenids and other Lepidoptera. Z. Naturforsch. C 42c:1319–1322.
Zagrobelny, M., Bak, S., Ekstrom, C. T., Olsen, C. E., and Møller, B. L. 2007a. The cyanogenic glucoside composition of Zygaena filipendulae (Lepidoptera: Zygaenidae) as effected by feeding on wild-type and transgenic lotus populations with variable cyanogenic glucoside profiles. Insect Biochem. Mol. Biol. 37:10–18.
Zagrobelny, M., Bak, S., and Møller, B. L. 2008. Cyanogenesis in plants and arthropods. Phytochemistry 69:1457–1468.
Zagrobelny, M., Bak, S., Olsen, C. E., and Møller, B. L. 2007b. Intimate roles for cyanogenic glucosides in the life cycle of Zygaena filipendulae (Lepidoptera, Zygaenidae). Insect Biochem. Mol. Biol. 37:1189–1197.
Zagrobelny, M. and Møller, B. L. 2011. Cyanogenic glucosides in the biological warfare between plants and insects: the Burnet moth-Birdsfoot trefoil model system. Phytochemistry 72:1585–1592.
Acknowledgements
We express our thanks to Managing Director and Scientific Board of The Pieniny National Park for official support of this project, and to Dr. Paweł Adamski from Polish Academy of Sciences in Cracow and Mr. Tadeusz Oleś, Apollo breeder from PNPark, for their help in collecting insects for this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bjarnholt, N., Nakonieczny, M., Kędziorski, A. et al. Occurrence of Sarmentosin and Other Hydroxynitrile Glucosides in Parnassius (Papilionidae) Butterflies and Their Food Plants. J Chem Ecol 38, 525–537 (2012). https://doi.org/10.1007/s10886-012-0114-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-012-0114-x