Abstract
Specialized metabolites in plants influence their interactions with other species, including herbivorous insects, which may adapt to tolerate defensive phytochemicals. The chemical arsenal of Alliaria petiolata (garlic mustard, Brassicaceae) includes the glucosinolate sinigrin and alliarinoside, a hydroxynitrile glucoside with defensive properties to glucosinolate-adapted specialists. To further our understanding of the chemical ecology of A. petiolata, which is spreading invasively in North America, we investigated the metabolite profile and here report a novel natural product, petiolatamide, which is structurally related to sinigrin. In an extensive study of North American populations of A. petiolata, we demonstrate that genetic population differences as well as developmental regulation contribute to variation in the leaf content of petiolatamide, alliarinoside, sinigrin, and a related glycoside. We furthermore demonstrate widely different metabolic fates of these metabolites after ingestion in the glucosinolate-adapted herbivore Pieris rapae, ranging from simple passage over metabolic conversion to sequestration. The differences in metabolic fate were influenced by plant β-glucosidases, insect-mediated degradation, and the specificity of the larval gut transport system mediating sequestration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants produce a range of bioactive natural products that promote their fitness. These specialized metabolites may have defensive properties against herbivores and pathogens (Bednarek 2012; Hopkins et al. 2008; Møller 2010; Morant et al. 2008b) and may function in allelopathic inhibition of competing species directly through toxic effects on plants or indirectly by affecting soil microbes (Cipollini et al. 2012). Alliaria petiolata (garlic mustard, Brassicaceae) is a biennial herb native to Eurasia, which is spreading invasively in eastern North America (Nuzzo 2000). This invasion may be promoted by observed allelopathic effects of A. petiolata, and several studies have demonstrated the inhibition of mycorrhiza by A. petiolata, which similarly to most Brassicaceous species is assumed not to form associations with mycorrhizal fungi (Cipollini et al. 2012). Among other specialized metabolites, Alliaria petiolata produces alliarinoside, a hydroxynitrile glucoside unknown from other species, and glucosinolates, predominantly sinigrin (allylglucosinolate) (Fig. 1) (Agerbirk et al. 2010; Haribal et al. 2001; Huang et al. 1994; Vaughn and Berhow 1999). Glucosinolates and their hydrolytic enzymes, the myrosinases, play important roles in plant-insect interactions. Tissue damage, e.g., by chewing insects, induce myrosinase-catalyzed breakdown of glucosinolates into isothiocyanates and other compounds that can have toxic effects on non-adapted organisms, but can serve as oviposition attractants or feeding stimulants for adapted specialists (Hopkins et al. 2008; Winde and Wittstock 2011). In addition to the established roles as defense compounds, evidence of glucosinolate breakdown in living tissue without herbivore or pathogen challenge has fostered suggestions that glucosinolates serve additional roles as nutrient storage (Brown et al. 2003; Falk et al. 2007; Janowitz et al. 2009; Petersen et al. 2002; Piotrowski 2008; Svanem et al. 1997). Nitrogen and sulfur availability often are limiting factors in plant nutrition, and glucosinolates contain both these elements. The operation of glucosinolate turnover pathways enabling remobilization of the bound sulfur and/or nitrogen into primary metabolism would promote metabolic plasticity and increase fitness. Glucosinolate turnover has been suggested to involve nitrilase activity (EC 3.5.5.1) catalyzing hydrolysis of the CN triple bond of glucosinolate-derived simple nitriles into the corresponding carboxylic acid (Janowitz et al. 2009; Piotrowski 2008). In the Brassicaceae, a subgroup of nitrilases homologous to NIT1 in Arabidopsis thaliana accepts glucosinolate-derived simple nitriles as substrates in in vitro assays (Janowitz et al. 2009). However, conclusive evidence of the involvement of NIT1 homologs in in vivo glucosinolate turnover remains to be obtained.
The structural similarity of the aglucons of sinigrin and alliarinoside, and the fact that sinigrin is a known precursor of a simple nitrile and epithionitrile with 4 carbons and a CN triple bond as alliarinoside, suggests a biosynthetic relationship between sinigrin and alliarinoside. This would imply considerable glucosinolate turnover in A. petiolata.
Alliarinoside has been shown to function as a feeding deterrent to young larval instars of Pieris oleracea (formerly P. napi oleracea, Lepidoptera), a glucosinolate-adapted butterfly native to North America (Haribal et al. 2001; Renwick et al. 2001). In the field, P. oleracea and another North American Pierid, Pieris virginiensis, oviposit on A. petiolata and other glucosinolate-containing Brassicaceae species, but the larvae develop poorly or are unable to survive on A. petiolata (Davis and Cipollini 2014; Keeler and Chew 2008). In contrast, another specialist on glucosinolate-containing plants, Pieris rapae, will feed on A. petiolata in the field in North America and can reach pupation on it in the laboratory (unpublished results reported in Huang et al. 1994; Davis and Cipollini 2014). This butterfly is native to Eurasia and was accidentally introduced to North America. As a result of its shared evolutionary history with A. petiolata, P. rapae may possess effective mechanisms permitting tolerance of toxic specialized metabolites of A. petiolata.
To further the understanding of the chemical ecology of A. petiolata and its interaction with insect herbivores, we investigated sources of variations in the content of alliarinoside and sinigrin in an extensive study of American and European populations of A. petiolata. We found that genetic population differences as well as developmental regulation contributed to variation in leaf metabolite contents. Furthermore, in the course of these studies of A. petiolata, we identified a novel specialized metabolite, petiolatamide (Fig. 1), with structural resemblance to the simple nitrile product of sinigrin. Additionally, we found a hexoside of a hydroxybutenoic acid (HHBA) present in A. petiolata. The metabolic fate of these two metabolites, alliarinoside and sinigrin following ingestion by the adapted specialist P. rapae was measured in relation to the presence of plant β-glucosidases able to hydrolyze the ingested glycosides. This demonstrated widely different metabolic fates of the phytochemicals ranging from simple passage through the digestive system over metabolic conversion to sequestration. This was influenced by differential susceptibility to plant β-glucosidases, degradation in the insect gut, and the specificity of the larval gut transport system mediating sequestration.
Methods and Materials
Plant Material and Growth Conditions
Alliaria petiolata first year plants were collected in August and September 2010–2012 at three Danish locations: Køge (Decimal degrees: 55.4328; 12.2087), Frederiksberg (55.6814; 12.5378), and Valby (55.6648; 12.5072). The plants were transplanted to 1.6 L pots with Pindstrup Substrate no. 2 potting medium (www.pindstrup.com) and grown in a greenhouse with a 15:9 h L:D cycle and minimum day/night temperatures of 18 °C/15 °C. Plants were fertilized monthly with a general purpose fertilizer, “Min Have” Næring (5–1-4) plus micronutrient, at the concentration recommended by the supplier.
Seeds from North American populations of A. petiolata were collected in Illinois (IL6, IL19, IL21, IL28), Indiana (IN1, IN2), New Jersey (NJ1), New York (NY7, NY8), Ohio (OH7, OH8, OH17, UK2, UK3), and Vermont (VT1) (Table 1). Seeds were cold stratified at 4 °C on moist filter paper in a petri dish and germinated after approximately 3 months. Seedlings were planted in 300 ml pots in ProMix BX (Grace-Sierra) potting medium and grown under fluorescent lights (approximately 75 μmol PAR m−2 s−1) in a growth room on a 16:8 h L:D cycle. Plants were watered with distilled water as necessary and fertilized biweekly with Jack’s Complete Fertilizer (20—20-20) plus micronutrients at the rate recommended for indoor plants.
Brassica juncea seeds (Sand Mountain Herbs) were planted in 300 ml pots in ProMix BX potting medium and grown under the same conditions as the North American A. petiolata seeds above.
Purification and Spectroscopy of Petiolatamide
Rosette leaves (40 g FW) from Danish A. petiolata plants were extracted by boiling for 5 min in 100 ml 70 % aq. MeOH. After evaporation of MeOH, the extract was frozen, freeze dried, and the dark brown residue obtained re-dissolved in 6 ml H2O. The aqueous extract was repeatedly passed through LC18 SPE cartridges (0.5 g, Supelco, activated in 3 ml of MeOH and equilibrated in 4 ml H2O) with intermittent passage through an anion exchange mini column (Agerbirk and Olsen 2012) or a freeze drying step followed by solubilization in a few ml of H2O and centrifugation (4,000 × g, 10 min, pellet discarded). The resulting 5 ml aqueous extract with highly polar compounds was subjected to fractionation on an analytical HPLC (Shimadzu LC-10AT) fitted with a Luna C18(2) column (250 × 4.6 mm, particle size: 4.6 μm, Phenomenex, Torrance, CA, USA; flow rate: 1 ml min−1). The injection volume was 100 μL and the mobile phases were: A, water; B, MeOH. The gradient program was: 0 to 10 min, isocratic A; 10 to 15 min, linear gradient to 5 % B; 15 to 18 min, linear gradient to 80 % B; 18 to 21 min, isocratic 100 % B; 21 to 24 min, linear gradient to 0 % B; 24 to 31 min, isocratic 100 % A. The elution of components was monitored by their UV absorbance (200 nm). A prominent component eluted at 6 min, and LC-MS and NMR analysis of collected fractions (0.5 ml) demonstrated this peak to be alliarinoside. A second component eluted at 5 min along with a group of compounds giving rise to minute absorbance peaks. Individually collected 0.2 or 0.5 ml fractions from 30 runs were combined, freeze dried and subjected to 1H NMR for structural identification of the components present. Further attempts to purify combined NMR preparations were performed by preparative HPLC employing a different column with a cyanopropyl stationary phase (Luna 5u CN, 250 × 4.6 mm, particle size: 5 μm, 100 A, Phenomenex, Torrance, CA, USA).
1H NMR (400.1 MHz) and 13C NMR (100.6 MHz) spectra were recorded in D2O using a Bruker Avance 400 instrument following lyophilisation of HPLC fractions. For further characterization, selected fractions were subjected to DEPT-135, COSY, HSQC, HMBC, and J-resolved 1H NMR. For quantification, following NMR analysis, solvents were evaporated and selected samples dried overnight in vacuo and weighed using a 5 decimal balance.
Metabolite Extraction for LC-MS
For targeted metabolite profiling of North American populations, metabolites were extracted from flash frozen leaf discs (Ø 7 mm, 5–15 mg FW) from the youngest fully unfolded leaf from 2–3 –mo- old plants sampled at the leaf base near the petiole. Each leaf disc was boiled in 85 % MeOH (300 μl, 5 min), cooled on ice, and stored at−20 °C in glass HPLC vials.
Developmental variation in the leaf metabolite content was analyzed by sampling and extracting leaf discs as described above from three rosette leaves of different developmental stage (leaf 1 (youngest fully unfolded leaf), leaf 2, and leaf 3) from 4 individuals in 5 North American populations.
Frozen samples of caterpillars, frass, and leaf discs from bioassays were homogenized in 1.5 ml microcentrifuge tubes using plastic pestles and liquid N2. Homogenates of frass and plant material were extracted by boiling in 85 % MeOH (300 μl, 3 min). Extracts were cooled on ice, transferred to glass HPLC vials, and stored at−20 °C. Caterpillar homogenates were extracted in ice cold 85 % MeOH, and the supernatants from centrifugation (2 min, 17.000 × g, 4 °C) were stored at−20 °C in glass HPLC vials.
For preparation of extracts for LC-MS, the samples were randomized within each experiment and diluted five times in H2O. Linamarin (α-hydroxyisobutyronitrile-β-D-glucopyranoside; AG Scientific, San Diego, CA, USA) was added as internal standard (final concentration 40 μM), and samples were filtered through a 0.45 μm MultiScreen HTS HV filterplate (Millipore) before LC-MS analysis.
LC-MS.LC-MS
was carried out using an Agilent 1100 Series LC (Agilent Technologies, Germany) coupled to a Bruker HCT-Ultra ion trap mass spectrometer (Bruker Daltonics, Bremen, Germany) and fitted with a Luna C8(2) column (150 × 2.0 mm; particle size: 3 μm, 100 A, www.phenomenex.com) preceded by a Gemini C18 SecurityGuard (Phenomenex; 4 × 2 mm; flow rate: 0.2 ml min−1). The oven temperature was maintained at 35 °C. Mobile phases were: A, water with 0.1 % (v/v) HCOOH and 50 μM NaCl; B, acetonitrile with 0.1 % (v/v) HCOOH. The gradient program was: 0 to 2 min, isocratic 1 % B; 2 to 8.5 min, linear gradient 1 to 3 % B; 8.6 to 9.6 min, isocratic 99 % B; 9.7 to 17 min, isocratic 1 % B. The mass spectrometer was run in positive electrospray mode.
Accurate masses were determined on a Bruker microTOF-Q spectrometer using LC parameters similar to those described above.
Alliarinoside (m/z 268, [M + Na]+; standard chemically synthesized as described elsewhere (Olsen et al. 2014) and sinigrin (m/z 404, [M + 2Na]+; standard: sinigrin monohydrate from horseradish, www.sigmaaldrich.com) were quantified using dilution series ranging 1–200 μM and 2–120 μM, respectively. Quantification of the components in the large sample series in the population study was based on the mean of three technical replicate standard series analyzed intermittingly in the sample series. Based on the EIC peak area relative to that of the internal standard, the best fitting standard curve equation was determined in GraphPad Prism 5 software. Polynomial curves of first to third order were fitted using the least squares method and compared by an Extra sum-of-squares F-test. For each experiment, the best fitting curve was defined as the simplest (lowest order) and significantly different fit (P < 0.05).
Search by GC/MS for Biosynthetic Precursors of Petiolatamide
Leaf homogenates were prepared by grinding young rosette leaves from minimum three Danish A. petiolata plants in liquid nitrogen using mortar and pestle. Thirty mg homogenate was transferred to 100 μl 50 mM KPi (pH 8.0) containing 2 mM DTT. This mixture was either extracted immediately for GC-MS analysis or incubated during shaking (250 rpm) at room temperature for 2 h followed by extraction. The samples were acidified by 1 M HCl (5 μl), supplemented with benzonitrile as internal standard (final concentration: 100 ng μl-1) and extracted with CH2Cl2 (3 times, 4 volumes). The combined CH2Cl2 phase was dried over MgSO4, filtered through glass wool in a Pasteur pipette, and concentrated to approximately 10 μl under a N2 atm. The sample was diluted with CHCl3 (total volume: 100 μl), derivatized with trimethylsilyl cyanide (TMSCN; www.sigmaaldrich.com) (50 μl sample, 20 μl TMSCN) (Khakimov et al. 2013) and analyzed by direct injection into the GC/MS. To correct for variation in sample preparation and injection volume, the internal standard EIC peak area was used to calculate the relative peak area of other analytes. All experiments were repeated three times with different rosette leaf pools from at least three petiolatamide-containing plants.
The reference compounds 3-butenenitrile (www.sigmaaldrich.com) and 2-hydroxy-3-butenenitrile (Enamine, Kiev, Ukraine) (100 ng μl−1 in CHCl3) were likewise TMSCN-derivatized and analyzed by direct injection in the GC/MS.
GC/MS was performed in a Turbomass spectrometer coupled to an Autosystem XL gas chromatograph (both from PerkinElmer, USA). The transfer line was kept at 200 °C. Split injection (1:10) of 1 μl at 200 °C, solvent delay 3 min, helium as carrier gas (1 ml min−1). An SGE column (SGE 30QC2 / BPX5-0.25, P/N 054142, 30 m × 0.22 mm, 0.25 μm film thickness) was used. The oven temperature program was as follows: 80 °C for 2 min, 80 to 160 °C at 5 °C min−1, 160 to 280 °C at 20 °C min−1, 280 °C for 3 min. The ion source was run in EI mode (70 eV) at 200 °C. The mass range m/z 50–500 was acquired.
Pieris rapae Feeding Experiment
Fully expanded leaves were harvested from 6-weeks-old plants of B. juncea and A. petiolata (population IL19) and painted with chemically synthesized alliarinoside (Olsen et al. 2014) in 1 % Triton X-100 (final level: 20.4 μmol g FW−1; B. juncea), or an equal volume of 1 % Triton X-100 without alliarinoside (B. juncea and A. petiolata). Before feeding to caterpillars, a disc (Ø 7 mm; ca. 16 mg FW) was sampled from each leaf, frozen in liquid N2, and stored at −80 °C until metabolite extraction. Remaining, untreated foliage of the plants was combined, frozen in liquid N2 and stored at −80 °C until subsequent enzyme activity analysis.
Pieris rapae caterpillars were purchased from Carolina Biological Supply Company (NC, USA) and raised on the provided artificial diet. At the third instar, caterpillars were removed from artificial diet, starved for 6 h, and weighed. Frass was discarded, and the caterpillars were placed in petri dishes with one of the three types of leaf material for 24 h. Following final starvation for 6 h, frass produced during and after feeding was collected. Caterpillars and frass were weighed, frozen in liquid N2, and stored at −80 °C.
Consumption was quantified by weighing the leaf material before and after allowing the caterpillars to feed. As the amounts of B. juncea fed to the caterpillars proved to be limited, the consumption of B. juncea was not included in correlation analyses of consumption and plant metabolite content.
Investigations of β-Glucosidase and Myrosinase Activity
Degradation of phytochemicals by endogenous enzymes was investigated at pH 6–10 using the following buffers: 50 mM MES pH 6.0, 50 mM Tris pH 7.0, and Fluka Fixanal buffers pH 8.0, pH 9.0, and pH 10.0 (www.sigmaaldrich.com). Leaf material from A. petiolata and B. juncea plants used in the P. rapae feeding experiments were homogenized in liquid N2 using cold mortars and pestles. In triplicates, 15 mg aliquots of homogenized plant material were combined with 150 μl buffer and, if relevant, alliarinoside (final level: 10 μmol g leaf FW−1), and incubated at 30 °C, 600 rpm, for 2 h. Samples were inactivated and metabolites extracted by addition of 150 μl ice cold MeOH and boiling for 3 min. Control samples were extracted at t =0 min. Addition of internal standard and preparation for LC/MS was performed as described above.
Results
Investigating the Metabolite Profile of
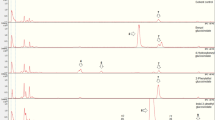
A. petiolata. Studies of specialized metabolites in A. petiolata were initiated by analyses of the metabolite profile of rosette leaves by LC/MS. Separation of the very polar alliarinoside and several unknown highly polar compounds yielding minor chromatographic peaks was obtained (Fig. 2). To facilitate further structure elucidation, preparative HPLC was employed to purify fractions for NMR spectroscopy. A preparation of alliarinoside obtained using this protocol was essentially pure (Online Resource 1). After solvent change to CD3OD, the alliarinoside spectra were identical to spectra of a synthetic sample of alliarinoside in that solvent (Olsen et al. 2014). Additional results obtained by NMR, LC/MS, and GC/MS are reported in the following sections.
Detection of alliarinoside, sinigrin, petiolatamide, and a hexoside of a hydroxybutenoic acid (HHBA) by LC/MS. Extracted ion chromatograms (EIC) and MS2 spectra of the newly discovered metabolites are shown in the middle and lower panels, respectively. Interpretation of the prominent MS2 fragments is stated in brackets
Identification of Petiolatamide
In the LC/MS metabolite profile of A. petiolata rosette leaves from Danish and North American populations, an unknown metabolite (m/z 286, [M + Na]+) was found to elute 2.1 min earlier than alliarinoside (m/z 268, [M + Na]+) (Fig. 2). The m/z value was 18 amu higher than that of alliarinoside, which indicated that it might be a related hydration product, and this was our original reason for isolating the compound. However, the 1H NMR spectrum of the purified compound showed signals of three protons at a terminal C C double bond, which allowed us to conclude that the compound was not the result of hydration of the double bond or the nitrile group of alliarinoside. The 1H NMR spectrum also showed signals of a putative monosaccharide moiety including distinct signals of H1′ (d, J = 8Hz) and H6′a (Table 2, Online Resource 1). From the COSY spectrum, the signal of H2 (hidden under the solvent signal) was revealed by coupling to H3, and the HSQC spectrum gave the chemical shifts of C2-C4. An HMBC correlation from H2 to a carbon at ca. 176 ppm (and a very weak signal at 176.5 ppm in 13C NMR detected after 31571 scans) demonstrated the final aglucon carbon (C1) suspected to be an amide C from the value of chemical shift. From these data, the aglucon moiety was concluded to be 2-hydroxy-3-buteneamide.
Glycosylation at the aglucon hydroxyl group was confirmed by HMBC correlations between H2 and C1’ and between H1’ and C2 (primes indicate glycosyl numbering). From the molecular mass and MS2, a hexoside moiety was deduced, which was identified as a β-glucopyranoside by extensive use of 2D NMR spectra. Starting from the anomeric H1′, the signals of H2′ and C2′ were extracted from the COSY and HSQC spectra. The critical coupling constants of H2′, H3′, and H4′ were found to be 8 Hz as expected for a glucoside by inspection of the J-resolved proton NMR spectrum, meaning that the hexoside moiety was a β-glucoside. Logically, two coupled protons could not have the same chemical shift. This constraint implied assignment of 3.35 ppm to H4′ and 3.5 ppm to H3′ (Table 2). The H5′ signal would be expected to be a complex multiplet at the intensity level of the impurities in the J-resolved spectrum, and accordingly, this signal was not identified with certainty. The remaining C′ chemical shifts were 70.3, 76.4, and 77.0 ppm, to be assigned to position 3′, 4′, and 5′. This assignment could not be done conclusively from the spectral data alone. Hence, the chemical shifts in the 3′-5′ positions were assigned using authentic β-glucopyranosides as a guide (Roslund et al. 2008) and agreed well with these values. As expected, a strong HMBC correlation was observed between H6′ and C4′, confirming the assignment of this chemical shift. In contrast, no HMBC correlation was observed between the anomeric proton and C4′, supporting the deduced presence of a pyranoside ring as opposed to a furanoside ring. Assuming the deduced glucose to be the usual natural D-isomer, we concluded the structure of the isolated metabolite to be β-D-2-glucopyranosyloxy-3-buteneamide (stereochemistry at C2 of the aglucon unresolved) (Fig. 1). This structure was in agreement with the accurate molecular mass (Found: 286.0916, calculated for best match [C10H17O7N + Na]+: 286.0897). As the aglucon structure is somewhat different from that of alliarinoside, we suggest naming this metabolite from the species name rather than the genus name of Alliaria petiolata and propose the common name petiolatamide.
The MS2 spectrum of petiolatamide showed intense fragment ions at m/z =201 and m/z =108 corresponding to [Glc-H2 + Na]+ and [C4H7ON + Na]+, i.e., sodium adducts of an oxidized Glc fragment and a reduced aglucon (Fig. 2). The often observed m/z =185 fragment ion ([Glc-H2O + Na]+) from glucosides was detected as expected (Bjarnholt et al. 2008), and a minor fragment with m/z =124 likely represents a sodium adduct of the aglucon, [C4H7O2N + Na]+ (Fig. 2). The yield of isolated petiolatamide was approximately. 0.5 mg after correction of the dry weight for ca. 25 % contamination with an unidentified aliphatic glycoside as quantified by 1H NMR. The yield of isolated, pure alliarinoside from the same fractionations was 5.3 mg. Hence, petiolatamide was a medium abundant metabolite in garlic mustard leaves, present in around one tenth of the level of the dominating alliarinoside.
An Unidentified Hexoside of a Hydroxybutenoic Acid (HHBA) in A. petiolata
In addition to petiolatamide, rosette leaves of 2-3-months-old A. petiolata plants with 3–5 fully unfolded leaves from North American populations contained minute amounts of a metabolite detected in LC/MS at a retention time 0.4 min longer than petiolatamide. The compound was also present in rosette leaves collected from Danish A. petiolata plants of similar size, but was undetectable in Danish plants older than 4 months (3 populations, N >60). This suggests ontogenetic regulation of the metabolite, and obstructed purification for NMR spectral structure elucidation. In LC/MS, the compound was detected as m/z 287 and m/z 309 representing [M + Na]+ and [M +2Na - H]+, respectively, which is characteristic for carboxylic acids in electrospray ion trap MS with sodium ions in the mobile phase (Fig. 2). The accurate masses were in accordance with M = C10H16O8 (Found: 287.0721, calculated for [C10H16O8 + Na]+: 287.0737, error: 5.8 ppm; found: 309.0542, calculated for [C10H15O8 + Na2]+: 309.0557, error: 4.8 ppm). MS2 of the monosodium adduct showed a prominent fragment ion m/z =185 (Fig. 2) characteristic for glucosides and probably other hexosides as well. MS2 of the disodium adduct showed a prominent fragment at m/z 105 (disodium adduct of acetate), which we have also observed from disodium adducts of other carboxylic acids, and a prominent fragment at m/z 147 corresponding to the disodium adduct of an aglucon of the formula C4H5O3. The molecular formula and MS2 fragmentation is compatible with several isomeric carboxylic acids, of which two may likely be present in A. petiolata: the carboxylic acid derivative of alliarinoside (formed by hydrolysis of the nitrile functional group) and the carboxylic acid derivative of petiolatamide. In conclusion, the m/z 287 ([M + Na]+) metabolite eluting after petiolatamide was a hexoside (possibly a glucoside) of a hydroxybutenoic acid. In the rest of this paper, it will be referred to as “hexoside of a hydroxybutenoic acid”, abbreviated HHBA.
Search for Biosynthetic Precursors of Petiolatamide
The identification of petiolatamide in the present study fostered questions regarding its biosynthesis. The presence in petiolatamide of a C4 aglucon and a nitrile-derived functional group suggested biosynthesis from the sinigrin-derived simple nitrile, 3-butenenitrile. A possible biosynthetic pathway for petiolatamide via 3-butenenitrile would include hydroxylation at C2 followed by glucosylation, either before or after conversion of the nitrile functional group to an amide. Based on comparison of the GS/MS elution profiles and fragmentation patterns to the authentic standards, both 3-butenenitrile and the possible hydroxylation product 2-hydroxy-3-butenenitrile were identified in leaf homogenate from plants containing petiolatamide (data not shown). The endogenous 2-hydroxy-3-butenenitrile in A. petiolata rosette leaf homogenate (defined as 100 % at t =0 min) decreased to 13 % ±4 % (mean standard deviation (SD), N = 3 biologically independent leaf pools) following incubation for 2 h at pH 8.0. Attempts to determine presence of 2-hydroxy-3-butenamide were obstructed by unavailability of a standard. Search for a compound with the corresponding mass was unsuccessful.
Variation of Specialized Metabolites in A. petiolata Populations
Knowledge of biological variations in the contents of sinigrin, alliarinoside, petiolatamide, and HHBA may further the understanding of biosynthetic relations and biological relevance of these specialized metabolites. For this purpose, the youngest fully unfolded rosette leaves from fifteen North American populations were subjected to targeted metabolite profiling by LC/MS. Alliarinoside and sinigrin were quantified using authentic standards, and the content was determined to 22 ± 10 μmol g FW−1 for alliarinoside and 11 ± 5 μmol g FW−1 for sinigrin (mean SD of 120 individuals, regardless of population). Compared to alliarinoside, the EIC peak area relative to the internal standard was 17 and 120 fold lower for petiolatamide (m/z 286) and HHBA (m/z 287), respectively. Hence, these compounds are minor metabolites in A. petiolata.
Within most populations, the contents of the four metabolites varied considerably among individuals (Fig. 3). Interestingly, whereas alliarinoside and sinigrin were present in all individuals of the fifteen analyzed populations, petiolatamide was undetectable in the NY7 and OH7 populations, while HHBA was undetectable in the OH8 and UK2 populations. In addition, two populations exhibited a mixed chemotype for either petiolatamide or HHBA (IL21 and IN1, Fig. 3). Differences among populations were analyzed by One-Way ANOVA, and population content means differed significantly for all four metabolites (For all, P < 0.001; alliarinoside: F 14, 103 = 3.507; sinigrin: F 14, 103 = 6.13; petiolatamide: F 14, 103 = 14.32; HHBA: F 14, 103 = 16.36). Tukey′s Multiple Comparison Test was used to compare metabolite contents among populations (Fig. 4). As expected for petiolatamide and HHBA, most of the overall variation among populations was attributable to populations completely lacking petiolatamide or HHBA, but also populations that did contain the metabolites differed significantly in content. The fewest content differences between populations were found for alliarinoside.
Some Alliaria petiolata populations differ significantly in leaf contents of alliarinoside, sinigrin, petiolatamide and HHBA. Results from Tukey’s Multiple Comparison Tests, asterisks signify the significance level of the difference (*, P < 0.05; **, P < 0.01; ***, P < 0.001). IL6, IL19, …, UK3: population ID. Grey shading indicates populations with mixed chemotype or negative chemotype (i.e. lack of compound)
To address questions regarding biosynthetic relationships between the metabolites, analysis of correlations in contents was performed (Fig. 5). In the majority of populations (11 out of 15), there was no significant correlation, positive or negative, between sinigrin and any of the other three metabolites. Only two populations exhibited a significant, strong positive correlation between sinigrin and alliarinoside (Fig. 5a: populations IN1 and NJ1). In most populations (9 out of 15), alliarinoside strongly correlated positively with HHBA or petiolatamide (Fig. 5a: populations IL6, IL19, IL28, IN2, NJ1, NY8, OH8, OH17, and UK3). Of the 9 populations containing both HHBA and petiolatamide, 7 populations showed a strong positive correlation between these two compounds (Fig. 5a: populations IL6, IL28, NJ1, NY8, OH17, VT1, and UK3). In summary, populations clearly differed with respect to metabolite correlations, but a general pattern of positive correlation between alliarinoside, petiolatamide and HHBA was seen. This was supported by correlation analysis of the pool of individual plants (N =75) disregarding the underlying populations (Fig. 5b).
Metabolite correlations in leaves of a 15 Alliaria petiolata populations (ID indicated in top left corner of each correlation matrix) and b pool of 75 individuals containing all four metabolites regardless of population. Numbers indicate Pearson’s product–moment correlations coefficients (r) for alliarinoside [A, (μmol g FW−1)], sinigrin [S, (μmol g FW−1)], petiolatamide [P, (relative peak area g FW−1)] and HHBA [H, (relative peak area g FW−1)]. For significant correlations, the degree of green shading indicates strength of positive correlation (very strong, 0.90 ≤ r ≤1.00; strong, 0.70 ≤ r ≤0.89; modest, 0.40 ≤ r ≤0.69). Stripes signify mixed chemotype in the population resulting in misleading correlation coefficient. Significance levels: ***, P < 0.001; **, P < 0.01; *, P < 0.05. a, Mixed chemotype: Petiolatamide was not detected in 3 out of 5 individuals in the population. b, Mixed chemotype: HHBA was not detected in 6 out of 7 individuals in the population. c, Petiolatamide was not detected in the population. d, HHBA was not detected in the population
Developmental differences in metabolite contents were investigated by analyzing rosette leaf 1 (youngest fully unfolded leaf) to leaf 3 in five populations with four individuals (Fig. 6, Table 3). For all four metabolites, the amount per g FW was highest in the youngest leaf. To determine the extent of variation caused by leaf age and population, respectively, repeated measures Two-Way ANOVA was performed (Table 3). Leaf age was the strongest source of variation in the content of alliarinoside, petiolatamide, and HHBA. For sinigrin, the population factor was the major contributor to variation. The significance of differences in leaf contents in each population was determined by Bonferroni posttests. Generally, and for alliarinoside in particular, the content differences were more significant for leaf 1 to 2 than for leaf 2 to 3. Not all populations showed significant differences between leaf ages, which may partly be due to the low sample size (N =4), but most likely also reflect true similarities in leaf contents in some populations. For example, population IN2 showed no significant difference with leaf age for any of the four metabolites (Table 3), which reflected small population variation and very little decrease with leaf age (Fig. 6). In summary, the leaf content of alliarinoside, sinigrin, petiolatamide and HHBA decreased with leaf development, and considerable differences among populations existed.
Investigation of Plant-Insect Interactions and A. petiolata β-Glucosidase Activities
In order to analyze the fate of the four metabolites in A. petiolata following ingestion of plant material by P. rapae caterpillars, a no-choice feeding experiment was performed with different types of leaf material. To investigate the separate effect of alliarinoside, chemically synthesized alliarinoside was painted on leaves of Brassica juncea, another Brassicaceae species accepted by P. rapae as food source (Renwick and Lopez 1999). The predominant glucosinolates present in B. juncea are sinigrin and 3-butenylglucosinolate (Li et al. 2000; Palmer et al. 1987), and the sinigrin content of the B. juncea leaves was 4 ± 2 μmol g FW−1 (mean SD, N =20), which was similar to previous reports (assuming a FW:DW ratio of 10:1) (Müller and Sieling 2006; Palmer et al. 1987). Starved third instar caterpillars of P. rapae were allowed to feed for 24 h on the alliarinoside-treated B. juncea leaves or leaves of A. petiolata or B. juncea. For evaluation of the digestive handling of alliarinoside, sinigrin, petiolatamide, and HHBA, the content of the four phytochemicals in caterpillars, frass, and food plants was determined by LC/MS. Figure 7 shows the amounts in caterpillars and frass relative to the consumed amount, and thus reflect, respectively, sequestration and simple passage of intact phytochemicals through the digestive system. Considerable fractions of the ingested phytochemicals were unaccounted for in frass or larvae, which indicates metabolic conversion by plant or insect enzymes. Caterpillars fed A. petiolata contained petiolatamide (30 % ±40 % of consumption (mean SD, N =10)), which demonstrated sequestration of this phytochemical by 9 out of 10 of the individuals. Smaller amounts (8 % ±6 %) of petiolatamide were detected in frass. The metabolic fate of alliarinoside was very different. Only trace amounts of alliarinoside were detected in caterpillars fed A. petiolata or alliarinoside-treated B. juncea. Hence, sequestration of alliarinoside was negligible. Whereas no alliarinoside was detected in frass from A. petiolata-fed caterpillars, considerable amounts (26 % ±26 %) of the ingested alliarinoside were present in the frass from caterpillars fed alliarinoside-treated B. juncea. This difference in excretion of alliarinoside indicated differences in the specificity of the β-glucosidases present in the two plant species. The data would imply that B. juncea does not produce a β-glucosidase capable of hydrolyzing alliarinoside efficiently, if at all, thereby making large amounts of the intact phytochemical available for uptake, excretion or metabolic conversion by larval enzymes.
Recovery in Pieris rapae caterpillars and frass of ingested phytochemicals. Stacked bars show the mean SD (N = 10) of the phytochemical content in caterpillars (bottom blue striped bars) and frass (top green bars) relative to the content in the consumed leaf mass of Alliaria petiolata, Brassica juncea (Bj) or B. juncea treated with alliarinoside (Bj + A). Tr, trace amounts; ND, not detectable
To investigate this further, the β-glucosidase and myrosinase activities of A. petiolata and B. juncea towards the four investigated phytochemicals were analyzed (Fig. 8). The midgut pH of P. rapae is pH 7.3, but Lepidoptera species vary widely with respect to midgut pH (Berenbaum 1980). Hence, the assay was performed at a pH range from 6 to 10. Incubation of alliarinoside at alkaline conditions (pH 9–10) in the absence of any plant material was found to result in chemical degradation due to isomerization to the (E)-isomer and conversion of the nitrile group to the amide, not because of hydrolysis of the β-glucosidic bond, which would require acidic conditions (Fig. 8 and data not shown). When incubated with a homogenate of A. petiolata leaves, endogenous alliarinoside was degraded completely during the 2 h assay at all tested pH values indicating efficient β-glucosidase activity towards alliarinoside at pH 6–8, where chemical degradation was negligible. In contrast, no degradation of alliarinoside was detected at pH 6–8 upon incubation with a homogenate of B. juncea leaves. This latter experiment demonstrated that B. juncea leaves did not contain β-glucosidases able to catalyze hydrolysis of alliarinoside. Additional experiments with β-glucosidases isolated from other plant species documented that alliarinoside was not hydrolyzed at pH 6 by linamarase purified from Manihot esculenta, by Lotus japonicus BGD2 and BGD4 heterologously expressed in Tobacco benthamiana, by β-glucosidase from almonds (www.sigmaaldrich.com) or by Viscozyme® (Novozymes, Bagsvaerd, Denmark) (data not shown). In both A. petiolata and B. juncea, sinigrin was completely degraded at all tested pH values, whereas neither petiolatamide nor HHBA was degraded chemically or by A. petiolata enzyme activities. Thus, the absence of HHBA in caterpillars and frass indicates efficient digestive degradation of this phytochemical by P. rapae.
Analysis of enzymatic and chemical degradation of phytochemicals in Alliaria petiolata and Brassica juncea. By LC-MS, the content of endogenous or exogenous alliarinoside (a), petiolatamide and HHBA (b) and sinigrin (c) was determined in samples containing leaf homogenate of A. petiolata, B. juncea or no plant material and incubated at different pH for 2 h. The plant metabolite content was quantified relative to the content of control samples extracted at t =0 min. Bars represent mean SD (N =3, except no-plant-samples: N = 1). Metabolite contents <1.0 in samples devoid of plant material reflect chemical degradation. In plant samples, metabolite breakdown was the sum of any chemical degradation and enzymatic hydrolysis of β-glucosidic bonds catalyzed by β-glucosidase and myrosinase activities
There were no significant correlations between the A. petiolata leaf content of the four phytochemicals and the caterpillars’ relative consumption (consumed leaf mass * caterpillar mass−1) (N =10, Pearson’s product–moment correlation coefficient (r), alliarinoside: r = 0.5079, P = 0.134, sinigrin: r = 0.4802, P = 0.160, petiolatamide: r = 0.4923, P = 0.148, HHBA: r = 0.5245, P = 0.120). Thus, none of these specialized metabolites in A. petiolata function as feeding deterrents to P. rapae, which is in accordance with observations that P. rapae will feed on A. petiolata in the field in North America and can reach pupation on it in the laboratory (unpublished results reported in Huang et al. 1994; Davis and Cipollini, unpublished data).
Discussion
The success of A. petiolata as an invasive species in North America is incompletely understood and may be attributed to specialized metabolites displaying allelopathic properties and acting as strong deterrents against endemic herbivores and pathogens (Cipollini et al. 2012; Haribal and Renwick 1998; Keeler and Chew 2008; Renwick et al. 2001). Quantifications in the present study showed that alliarinoside (22 μmol g FW−1; 5.4 mg g FW−1; 0.5 % FW) is a major specialized metabolite in young rosette leaves of A. petiolata. Stoichiometrically, this is twice as much as sinigrin (11 μmol g FW−1 (110 μmol g DW−1, assuming a DW:FW ratio of 10:1 for leaf material); 3.9 mg g FW−1 (39 mg g DW−1); 0.4 % FW), which we observed at higher levels than previously reported, most likely partly due to developmental differences in the analyzed plant material (rosette leaves in May: 13.6 mg g DW−1 (Nielsen et al. 1979); rosette leaves in October: 2.8 mg g DW−1 (Vaughn and Berhow 1999); cauline leaves: 35 μmol g DW−1 (Agerbirk et al. 2010)). If the large amount of alliarinoside produced in A. petiolata is derived from sinigrin, this implies considerable turnover of this glucosinolate in living tissue. Here, we show that generally the content of sinigrin did not correlate, neither negatively nor positively, with that of alliarinoside, petiolatamide, or HHBA. Thus, alliarinoside is not biosynthesized at the expense of the sinigrin pool size. This indicates that the sinigrin content is not a major factor in regulation of sinigrin degradation in vivo and subsequent biosynthesis of alliarinoside. Rather, the lack of correlations involving sinigrin indicates that the rate of sinigrin biosynthesis is regulated to allow production of alliarinoside without reducing the sinigrin pool.
We provide quantitative investigations of variation in alliarinoside and sinigrin among populations and in the course of plant development. The large number of populations studied and analysis of individual plants revealed that the contents of alliarinoside and sinigrin differ significantly among some of the fifteen analyzed populations, and considerable variation was seen between individual plants from the same population. As the investigated plant material originated from plants germinated and grown under standardized environmental conditions in the growth room, all observed population differences were attributable to genetic variation. In comparison, a previous study demonstrated environmental regulation by showing that differences in unspecified glucosinolate content in A. petiolata plants collected from different sites in the same forest were harmonized when field-collected seedlings were transplanted to pots and grown under controlled conditions (Cipollini 2002). Previous studies of the relative content of alliarinoside and total glucosinolates in A. petiolata reported no significant differences between two (Haribal and Renwick 2001) and four (Cipollini et al. 2005) North American populations grown under controlled conditions. The lower number of analyzed populations in those studies may explain this discrepancy.
To gain more knowledge about the existence of hitherto undiscovered allelochemicals in A. petiolata, we investigated the metabolite profile and discovered the presence of petiolatamide (β-D-2-glucopyranosyloxy-3-butenamide), which is a novel metabolite according to a structural search in SciFinder by April 23, 2014. The C4 aglucon of petiolatamide containing a terminal double bond and a nitrile group suggests sinigrin as a likely biosynthetic precursor, and we propose a pathway for biosynthesis, which includes the sinigrin-derived simple nitrile, 3-butenenitrile, as the first intermediate (Fig. 9). In the biosynthetic studies of A. petiolata, we identified 3-butenenitrile in leaf homogenate as well as trace amounts of 2-hydroxy-3-butenenitrile. Hence, the biosynthetic route to petiolatamide may proceed from 3-butenenitrile via 2-hydroxy-3-butenenitrile and subsequent nitrilase-catalyzed hydrolysis of the nitrile group to form the derived amide (Fig. 9), although we did not detect production of the petiolatamide aglucone.
Suggested biosynthetic pathway for petiolatamide. Different routes from 3-butenenitrile may exist, possibly in parallel. Green lines: compound detected in Alliaria petiolata. Red dashed line: compound not detected in A. petiolata. The structure of HHBA detected in A. petiolata in this study is unresolved, but may be the carboxylic acid corresponding to petiolatamide. The dotted box and dotted arrows show possibly pathway(s) to this suggested metabolite. Suggested enzyme activities are indicated. Moieties resulting from the indicated enzymatic conversion are shown on a yellow background. P450, cytochrome P450; FMO, flavin monooxygenase; UGT, UDP-glycosyl transferase
In addition, an unidentified hexoside of a hydroxybutenoic acid (HHBA) was demonstrated to be present in A. petiolata. A likely candidate structure of this metabolite is the carboxylic acid derivative of alliarinoside or the isomeric carboxylic acid derivative of petiolatamide. If the structure of HHBA is identified as the petiolatamide-derived carboxylic acid, 3-butenoic acid may be an intermediate in the route to this hexoside (Fig. 9, dotted box). For both these possible structures, HHBA is likely a product of nitrilase activity.
The nitrilase(s) involved in biosynthesis of petiolatamide and HHBA are likely to be NIT1 homologs, which in other Brassicaceous species have been shown to be bi-functional enzymes possessing nitrilase activity as well as nitrile hydratase activity (EC 4.2.1.84) converting nitriles to the corresponding amide (Agerbirk et al. 2008; Piotrowski 2008). The product ratio among carboxylic acid and amide varies between species, and at least one Brassicaceous species (Sinapis arvensis) contains a nitrilase that produces mainly amides (Agerbirk et al. 2008). We observed chemotype differences among A. petiolata populations with respect to petiolatamide and HHBA. In most populations containing both metabolites, the contents of these correlated strongly, which is in agreement with shared steps of biosynthesis. However, a few populations showed no such correlation, and some completely lacked one metabolite, but not the other. Taken together with the observed indications that HHBA is differently developmentally regulated than petiolatamide, this suggests that more than one nitrilase with different ontogenetic regulation are involved in their biosynthetic pathway(s). At least one, but very often two or more NIT1 homologs have been identified in all analyzed Brassicaceous species (Janowitz et al. 2009). Alliaria petiolata remains to be subjected to such investigations, and in comparison to diploid Brassicaceae species, the hexaploid genome of A. petiolata may contain more alleles offering ample room for recruitment to biosynthesis of petiolatamide and HHBA. Biosynthetic studies investigating the production and metabolism of the suggested intermediates remain to be performed. Experimental elucidation of the pathway(s) for biosynthesis of petiolatamide and HHBA may bring increasing evidence to the suggested involvement of nitrilases in glucosinolate metabolism in living tissue.
Any biological functions of petiolatamide and HHBA are unknown, and their absence in some North American populations indicate that they are not absolutely required for the invasive success of A. petiolata. The detectable amounts of these specialized metabolites were low compared to alliarinoside and sinigrin, which likely play more critical ecological roles. Also considering the negative chemotype found in some populations, this suggests facultative roles in the plant, or that they result from recent neofunctionalization events still in their infancy. Based on the observed insect deterring effects of alliarinoside (Haribal et al. 2001; Renwick et al. 2001), we suggest that alliarinoside may have evolved as means to modify the chemical defense to target glucosinolate-adapted specialists. If alliarinoside is biosynthesized from sinigrin, this evolution would at the same time confer a metabolic plasticity enabling allocation of sulfur from sinigrin to primary metabolism. The high content of alliarinoside suggests considerable turnover of sinigrin in intact tissue. Petiolatamide and possibly HHBA may be product(s) of an in vivo pathway involving nitrilase-catalyzed metabolism of glucosinolate-derived simple nitriles. Cytochrome P450-catalyzed hydroxylation and glycosylation by glycosyl transferases are common detoxification mechanisms in plants (Jones and Vogt 2001), and the suggested pathway may have evolved from such unspecific detoxification reactions (Fig. 9). Due to the observed absence in A. petiolata of β-glucosidase activity towards petiolatamide and HHBA, remobilization of the aglucons seems unlikely.
Alliarinoside, a γ-hydroxynitrile glucoside, acts as a feeding deterrent to early instars of P. oleracea by a post-ingestive mechanism (Renwick et al. 2001). The chemical details of this inhibitory activity remain elusive. The defensive activity of other hydroxynitrile glucosides have been established for the α-hydroxynitrile glucosides (cyanogenic glucosides), which release toxic hydrogen cyanide (HCN) from the aglucon after hydrolysis of the β-glucosidic bond, but the biological functions of β- and other γ-hydroxynitrile glucosides are yet to be resolved (Frisch and Møller 2012; Morant et al. 2008b). The aglucons of γ-hydroxynitrile glucosides have been suggested to rearrange to furanones with antifungal and antimicrobial properties (Bjarnholt and Møller 2008; Saito et al. 2012). This also may apply to alliarinoside, and thus may contribute to the inhibition of mycorrhizal fungi by A. petiolata (Frisch and Møller 2012). Based on knowledge from cyanogenic glucosides and the suggested furanone formation, it is reasonable to assume that β-glucosidase-catalyzed hydrolysis releasing the aglucon is a prerequisite for the defensive activity of alliarinoside. In accordance with this, β-glucosidase activity towards alliarinoside was observed in A. petiolata. Consequently, the presence of β-glucosidase activity is most likely an additional key parameter to analyze, when designing experiments investigating the ecological relevance of alliarinoside. In our work to establish a bioassay furthering this purpose, no such enzymatic activity was detected in B. juncea. Application of an exogenous β-glucosidase could allow experimental control of hydrolysis, but apart from the β-glucosidase activity detected in A. petiolata, such an enzyme remains to be identified, as almond β-glucosidase, M. esculenta and L. japonicus β-glucosidases with specificity for other aliphatic hydroxynitrile glucosides (Mkpong et al. 1990; Morant et al. 2008a), and Viscozyme®, a commercial multi-enzyme product with broad glycosidase activity, did not show activity towards alliarinoside.
The reported deterring effect of alliarinoside on early instars of P. oleracea was determined using cabbage (unspecified Brassica oleracea) as a substrate on which alliarinoside was applied (Haribal et al. 2001; Renwick et al. 2001). Investigations of the β-glucosidase activity in this species may substantiate the assumed hydrolysis requirement for the deterring effect of alliarinoside. However, cleavage of the exogenous alliarinoside in that bioassay could also have been mediated by insect-encoded β-glucosidases present in P. oleracea caterpillars. Counter-adaptations among insect herbivores to overcome toxicity of phytochemicals are widespread and include, among other things, inhibition of plant- and insect-encoded β-glucosidases (Pentzold et al. 2013). For example, the highly alkaline environment in the midgut of many larval Lepidioterans – excluding P. rapae – greatly reduces plant β-glucosidase activity (Berenbaum 1980; Pentzold et al. 2013). Interestingly, the sensitivity of P. oleracea towards alliarinoside was found to decrease with larval development until 4th instar, which was not deterred by alliarinoside (Renwick et al. 2001). Furthermore, populations of P. oleracea now show variation in the ability to consume and grow on A. petiolata, with those having more lengthy experience with this plant displaying increased tolerance (Keeler and Chew 2008). This suggests that adaptation to A. petiolata is occurring in this multivoltine butterfly, which may involve alterations in metabolism of alliarinoside or other metabolites. In contrast, the related, but univoltine, butterfly P. virginiensis suffers complete and rapid mortality on A. petiolata, and no variation among populations in the ability to tolerate A. petiolata has yet been detected (Davis and Cipollini 2014). Variations in inhibition of β-glucosidase activities or in alliarinoside detoxification mechanisms may explain the developmental changes in P. oleracea sensitivity as well as differences between herbivorous insect species.
Our study demonstrated that the complete content of alliarinoside in A. petiolata was degraded following ingestion by P. rapae. In light of the tolerance of P. rapae to A. petiolata and thus alliarinoside (unpublished results reported in Huang et al. 1994; Davis and Cipollini unpublished data), this indicates that P. rapae may efficiently detoxify product(s) of alliarinoside hydrolysis. Thus, investigations of such detoxification mechanisms in P. rapae and mechanisms for regulation of hydrolysis in other insect herbivores are highly relevant in future investigations of the defensive effects of alliarinoside.
The unintentional application of a control plant species without β-glucosidase activity facilitating alliarinoside hydrolysis enabled us to detect differential digestive handling of intact alliarinoside and petiolatamide in P. rapae. Considerable amounts of petiolatamide (30 % of consumption) were sequestered by the caterpillars. In comparison, 0.5 to 50 % of ingested phytochemicals have been recovered from sequestering larvae in the relatively few studies that have quantified larval sequestration of phytochemicals (Bjarnholt et al. 2012, and references herein; Zagrobelny et al. 2014). In contrast, intact alliarinoside was not sequestered, but excreted in the frass (26 %). This indicates different specificity for alliarinoside and petiolatamide in the uptake system of the larval gut. The minor content of petiolatamide detected in frass (8 %) may be residuals from inefficient uptake or may result from excretion of sequestered compounds, which were not useful or in surplus and hence excreted from the haemolymph via the Malpighian tubules to the frass. Extending the time period between feeding of test plant material to the caterpillar and extraction of caterpillar and frass for quantification by LC/MS may clarify if sequestered petiolatamide is stored or metabolized in the caterpillar or excreted to the frass. Furthermore, additional investigations of the differences in sequestration and excretion of petiolatamide and alliarinoside may potentially further our understanding of the specificity of the transport system facilitating uptake of phytochemicals from the larval gut.
In summary, we demonstrated in an extensive study of A. petiolata populations from North America that variations in contents of alliarinoside, sinigrin, the novel metabolite petiolatamide, and an unidentified hexoside of a hydroxybutenoic acid, HHBA, were attributable to genetic population differences as well as ontogenetic regulation. The metabolic fate of these four glycosides after ingestion by the adapted specialist P. rapae differed from sequestration over conversion to passage of intact compounds through the digestive system. The differences in metabolic fate were influenced by plant β-glucosidases, insect-mediated degradation, and the specificity of the larval transport system mediating uptake of phytochemicals.
The demonstrated presence of petiolatamide and HHBA in A. petiolata is proposed to result from sinigrin turnover as may also be the case for alliarinoside. Further investigations of A. petiolata specialized metabolites and the emerging picture of diversified glucosinolate metabolism in this species may offer significant new knowledge about metabolic plasticity and allelopathic properties enabling the success as invasive species in North America.
References
Agerbirk N, Chew FS, Olsen CE, Jørgensen K (2010) Leaf and floral parts feeding by Orange Tip Butterfly larvae depends on larval position but not on glucosinolate profile or nitrogen level. J Chem Ecol 36:1335–1345
Agerbirk N, Olsen CE (2012) Glucosinolate structures in evolution. Phytochemistry 77:16–45
Agerbirk N, Warwick SI, Hansen PR, Olsen CE (2008) Sinapis phylogeny and evolution of glucosinolates and specific nitrile degrading enzymes. Phytochemistry 69:2937–2949
Bednarek P (2012) Chemical warfare or modulators of defence responses - The function of secondary metabolites in plant immunity. Curr Opin Plant Biol 15:407–414
Berenbaum M (1980) Adaptive significance of midgut pH in larval Lepidoptera. Am Nat 115:138–146
Bjarnholt N, Møller BL (2008) Hydroxynitrile glucosides. Phytochemistry 69:1947–1961
Bjarnholt N, Nakonieczny M, K-Ödziorski A, Debinski D, Matter S, Olsen C, Zagrobelny M (2012) Occurrence of sarmentosin and other hydroxynitrile glucosides in Parnassius (Papilionidae) butterflies and their food plants. J Chem Ecol 38:525–537
Bjarnholt N, Rook F, Motawia MS, Cornett C, Jørgensen C, Olsen CE, Jaroszewski JW, Bak S, Møller BL (2008) Diversification of an ancient theme: Hydroxynitrile glucosides. Phytochemistry 69:1507–1516
Brown PD, Tokuhisa JG, Reichelt M, Gershenzon J (2003) Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry 62:471–481
Cipollini D (2002) Variation in the expression of chemical defenses in Alliaria petiolata (Brassicaceae) in the field and common garden. Am J Bot 89:1422–1430
Cipollini D, Mbagwu J, Barto K, Hillstrom C, Enright S (2005) Expression of constitutive and inducible chemical defenses in native and invasive populations of Alliaria petiolata. J Chem Ecol 31:1255–1267
Cipollini D, Rigsby C, Barto E (2012) Microbes as targets and mediators of allelopathy in plants. J Chem Ecol 38:714–727
Davis S, Cipollini D (2014) Do mothers always know best? Oviposition mistakes and resulting larval failure of Pieris virginiensis on Alliaria petiolata, a novel, toxic host. Biol Invasions. doi:10.1007/s10530-013-0637-2
Falk KL, Tokuhisa JG, Gershenzon J (2007) The effect of sulfur nutrition on plant glucosinolate content: Physiology and molecular mechanisms. Plant Biol 9:573–581
Frisch T, Møller BL (2012) Possible evolution of alliarinoside biosynthesis from the glucosinolate pathway in Alliaria petiolata. FEBS J 279:1545–1562
Haribal M, Renwick J (2001) Seasonal and population variation in flavonoid and alliarinoside content of Alliaria petiolata. J Chem Ecol 27:1585–1594
Haribal M, Renwick JA (1998) Isovitexin 6''-o-[beta]-d-glucopyranoside: a feeding deterrent to Pieris napi oleracea from Alliaria petiolata. Phytochemistry 47:1237–1240
Haribal M, Yang Z, Attygalle AB, Renwick JA, Meinwald J (2001) A cyanoallyl glucoside from Alliaria petiolata, as a feeding deterrent for larvae of Pieris napi oleracea. J Nat Prod 64:440–443
Hopkins RJ, van Dam NM, van Loon JJA (2008) Role of glucosinolates in insect-plant relationships and multitrophic interactions. Annu Rev Entomol 54:57–83
Huang X, Renwick JAA, Chew FS (1994) Oviposition stimulants and deterrents control acceptance of Alliaria petiolata by Pieris rapae and P. napi oleracea. Chemoecology 5:79–87
Janowitz T, Trompetter I, Piotrowski M (2009) Evolution of nitrilases in glucosinolate-containing plants. Phytochemistry 70:1680–1686
Jones P, Vogt T (2001) Glycosyltransferases in secondary plant metabolism: Tranquilizers and stimulant controllers. Planta 213:164–174
Keeler M, Chew F (2008) Escaping an evolutionary trap: Preference and performance of a native insect on an exotic invasive host. Oecologia 156:559–568
Khakimov B, Motawia MS, Bak S, Engelsen SB (2013) The use of trimethylsilyl cyanide derivatization for robust and broad-spectrum high-throughput gas chromatography–mass spectrometry based metabolomics. Anal Bioanal Chem 405:9193–9205
Li Q, Eigenbrode S, Stringam GR, Thiagarajah MR (2000) Feeding and growth of Plutella xylostella and Spodoptera eridania on Brassica juncea with varying glucosinolate concentrations and myrosinase activities. J Chem Ecol 26:2401–2419
Mkpong OE, Yan H, Chism G, Sayre RT (1990) Purification, characterization, and localization of linamarase in cassava. Plant Physiol 93:176–181
Møller BL (2010) Functional diversifications of cyanogenic glucosides. Curr Opin Plant Biol 13:337–346
Morant AV, Bjarnholt N, Kragh ME, Kjærgaard CH, Jørgensen K, Paquette SM, Piotrowski M, Imberty A, Olsen CE, Møller BL, Bak S (2008a) The β-glucosidases responsible for bioactivation of hydroxynitrile glucosides in Lotus japonicus. Plant Physiol 147:1072–1091
Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sanchez-Perez R, Møller BL, Bak S (2008b) β-Glucosidases as detonators of plant chemical defense. Phytochemistry 69:1795–1813
Müller C, Sieling N (2006) Effects of glucosinolate and myrosinase levels in Brassica juncea on a glucosinolate-sequestering herbivore - and vice versa. Chemoecology 16:191–201
Nielsen JK, Larsen LM, Sørensen H (1979) Host plant selection of the horseradish flea beetle Phyllotreta armoraciae (Coleoptera: Chrysomelidae): identification of two flavonol glucosides stimulating feeding in combination with glucosinolates. Entomol Exp Appl 26:40–48
Nuzzo V (2000) Element stewardship abstract for Alliaria petiolata. The Nature Conservancy, Virginia, USA
Olsen CE, Møller BL, Motawia MS (2014) Synthesis of the allelochemical alliarinoside present in garlic mustard (Alliaria petiolata), an invasive plant species in North America. Carbohydrate Res 394:13–16
Palmer MV, Yeung SP, Sang JP (1987) Glucosinolate content of seedlings, tissue cultures, and regenerant plants of Brassica juncea (indian mustard). J Agric Food Chem 35:262–265
Pentzold S, Zagrobelny M, Rook F, Bak S (2013) How insects overcome two-component plant chemical defence: Plant β-glucosidases as the main target for herbivore adaptation. Biol Rev. doi:10.1111/brv.12066
Petersen B, Chen S, Hansen C, Olsen C, Halkier B (2002) Composition and content of glucosinolates in developing Arabidopsis thaliana. Planta 214:562–571
Piotrowski M (2008) Primary or secondary? Versatile nitrilases in plant metabolism. Phytochemistry 69:2655–2667
Renwick JAA, Lopez K (1999) Experience-based food consumption by larvae of Pieris rapae: Addiction to glucosinolates? Entomol Exp Appl 91:51–58
Renwick JA, Zhang W, Haribal M, Attygalle AB, Lopez KD (2001) Dual chemical barriers protect a plant against different larval stages of an insect. J Chem Ecol 27:1575–1583
Roslund MU, Tähtinen P, Niemitz M, Sjöholm R (2008) Complete assignments of the 1H and 13C chemical shifts and JH, H coupling constants in NMR spectra of D-glucopyranose and all D-glucopyranosyl-D-glucopyranosides. Carbohydrate Res 343:101–112
Saito S, Motawia MS, Olsen CE, Møller BL, Bak S (2012) Biosynthesis of rhodiocyanosides in Lotus japonicus: Rrhodiocyanoside A is synthesized from (Z)-2-methylbutanaloxime via 2-methyl-2-butenenitrile. Phytochemistry 77:260–267
Svanem P-J, Bones AM, Rossiter JT (1997) Metabolism of [α-14C]-desulphophenethylglucosinolate in Nasturtium officinale. Phytochemistry 44:1251–1255
Vaughn SF, Berhow MA (1999) Allelochemicals isolated from tissues of the invasive weed garlic mustard (Alliaria petiolata). J Chem Ecol 25:2495–2504
Winde I, Wittstock U (2011) Insect herbivore counteradaptations to the plant glucosinolate-myrosinase system. Phytochemistry 72:1566–1575
Zagrobelny M, Olsen CE, Pentzold S, Fürstenberg-Hägg J, Jørgensen K, Bak S, Møller BL, Motawie MS (2014) Sequestration, tissue distribution and developmental transmission of cyanogenic glucosides in a specialist insect herbivore. Insect Biochem Mol Biol 44:44–53
Acknowledgments
Kasper Brink-Jensen, Section for Biostatistics, University of Copenhagen, provided statistical guidance. Analysis of large batches of LC/MS data was facilitated by Bruker Data Analysis scripts developed by Lasse Nielsen. Heterologously expressed BGD2 and BGD4 from L. japonicus were provided by Daniela Lai. We thank Rick Landau for seeds of some A. petiolata populations. Our work was financially supported by the VILLUM Research Center for Plant Plasticity, by Center for Synthetic Biology “bioSYNergy” funded by the UCPH Excellence Program for Interdisciplinary Research, and by the Ohio Plant Biotechnology Consortium. TF acknowledges a PhD stipend granted by the Faculty of Life Sciences, University of Copenhagen.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Frisch, T., Agerbirk, N., Davis, S. et al. Glucosinolate-Related Glucosides in Alliaria petiolata: Sources of Variation in the Plant and Different Metabolism in an Adapted Specialist Herbivore, Pieris rapae . J Chem Ecol 40, 1063–1079 (2014). https://doi.org/10.1007/s10886-014-0509-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0509-y