Abstract
Although it has been established that sexually-immature goldfish and their relatives recognize members of their own species by using chemicals that they release, the identity of this cue(s) and whether it might be produced and used by other life stages is not yet known. To address this question, this study tested the behavioral responses of sexually immature and mature goldfish to each other’s body washings, their sensitivity to this cue, the role of the olfactory sense in detecting it, and whether it is comprised of either polar and/or non-polar compounds. Tests that used two-choice mazes discovered that juvenile, immature, mature male, and mature female goldfish all release and respond to a common chemical cue(s). Dilution studies next demonstrated that this cue is active when diluted over 10 times and thus capable of functioning as a short range attractant/identifier. Olfactory occlusion demonstrated that it is detected by the olfactory sense. Finally, chemical fractionation demonstrated that it is comprised of both polar and non-polar components but likely does not include bile acids. Together, these results suggest that all life stages of goldfish use a complex multicomponent pheromonal odor to discern species identity, and that this odor has the potential to function with hormonal metabolites to identify sexual condition in behaviorally active fish of many species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pheromones have been defined as chemical cues that convey information among individuals of the same species (Karlson and Lüscher, 1959; Wyatt, 2003, 2009). Cues that fit this definition are commonly used by teleost (‘bony’) fishes to mediate alarm, aggregation, and reproductive synchrony in the vast expanses of dark and/or turbid waters that they inhabit (Liley, 1982; Stacey et al., 1986; Stacey and Sorensen, 2009). All fish pheromones identified to date are body metabolite(s), a likely consequence of life in water (Sorensen and Hoye, 2010). Studies of the biological activity and identity of pheromonal odors in fish have taken two approaches. Several dozen studies have tested the actions of unpurified whole odors of immature and mature fish (body washings) and found that when tested, they generally elicit behavioral activity in a species-specific manner (Liley, 1982; Stacey et al., 1986; Baker and Montgomery, 2001; Sisler and Sorensen, 2008). Another set of studies has tested the activity of key hormonal metabolites (‘hormonal pheromones’) commonly produced by fishes, and found that they frequently stimulate olfactory and behavioral activity among closely related taxa (Stacey and Sorensen, 2005, 2009; Olsen et al., 2006). One possible explanation for these seemingly divergent results is that fish pheromones may function naturally as complex mixtures of metabolites, some of which identify species identity, while others (e.g., hormonal pheromones) change with maturity and time, thus adding information about physiological state.
The goldfish, Carassius auratus, is an excellent model to address whether and how a fish might use a species-identifying pheromone because it is easily studied, well-understood, and seemingly typical of many other fishes (Stacey and Sorensen, 2009). Behavioral studies of immature (sexually-regressed) goldfish and the closely related common carp (Cyprinus carpio) show that these species both recognize their own species (Saglio and le Martret, 1982; le Martret and Saglio, 1982) and readily distinguish between each other and many other species by using water-borne chemicals that do not appear to be learned (Sisler and Sorensen, 2008). Further, a laboratory spawning study suggests that goldfish and carp do not readily hybridize (Sorensen et al., 1992) despite the fact that they use a common set of five hormonal compounds that mediate pre-spawning hormonal surges and reproductive behavior (Kobayashi et al., 2002; Stacey and Sorensen, 2009). Comparisons of the olfactory sensitivity and reproductive physiology of these species have yet to show any differences (Sorensen and Stacey, 2004). Intriguingly, a single study also has demonstrated that while male goldfish respond behaviorally to a metabolite of prostaglandin F2α when it is added to goldfish holding water, males of another species (the blue gourami, Trichogaster trichopterus) do not become sexually aroused when the prostaglandin metabolite is added to their holding water (Sorensen et al., 2000, unpublished data). Although these data suggest that common goldfish body metabolites are perceived as a species-identifying cue, it has not yet been tested directly whether all life stages of any fish release a common subset of odorous compounds that might serve this function. This is an especially relevant question because physiological (reproductive) condition is known to modify many aspects of fish metabolism, sex hormonal pheromone release, and behavior (Stacey and Sorensen, 2009).
The chemical identity and biological significance of the compounds employed by goldfish to identify species also await resolution. Unique mixtures of bile acids may serve this role, as many fishes, including goldfish and related carps, are known to release and detect these relatively distinctive compounds with high sensitivity and specificity (Døving et al., 1980; Sorensen et al., 1987; Li and Sorensen, 1997; Derby and Sorensen, 2008). Complex mixtures of amino acids also could contribute because they too are released in quantity, and are detected with high sensitivity and specificity (Saglio and Blanc, 1989). Of course, other types of metabolites could also be involved. Notably, it has yet to be resolved whether the body washings (metabolites) of fishes are detected by the olfactory sense at concentrations low enough that they could function as pheromones in open waters.
The present study tested the possibility that all life stages of goldfish produce and perceive a common species-identifying pheromone by addressing five questions: 1) Does responsiveness of goldfish to conspecific washings remain constant with time as might be expected of a pheromone?; 2) Do all maturational stages of goldfish produce and respond to conspecific washings in the same manner?; 3) Are conspecific washings recognized by the sense of smell?; 4) Is the cue detected at low-enough concentrations for it to function as a pheromone?; and 5) Is this cue comprised of a single or multiple components, and might either bile acids and/or amino acids be involved?
Methods and Materials
Fish and Fish Care
Goldfish were obtained from outdoor ponds (Hunting Creek Fish Farm, PA, USA) and shipped to the laboratory where they were held in 1000-l flow-through holding tanks supplied with well water (3-l/min; 17°C) on a 16:8 hr (L:D) photoperiod. Fish were fed a mixture of flake food (Aquatic Ecosystems, Apopka, FL, USA) and brine shrimp (San Francisco Bay Brand, CA, USA). Four groups of fish were used (Table 1). Briefly, juvenile fish were 3–6-mo-old and sexually undifferentiated. Sexually-immature fish were sexually regressed (previously mature) individuals that had been held in the laboratory for several years and had been previously used by Sisler and Sorensen (2008). Sexually-mature male and female fish were obtained from ponds several weeks prior to spawning, and were held in the laboratory as single sex groups to prevent them from stimulating each other (recent reproductive behavior or exposure to pheromones strongly enhances sexual receptivity and pheromone release; see Table 1 for more details on fish conditions). With the exception of the olfactory occlusion experiments, all fish were tested only once. Odor donors came from the same groups as the behavioral subjects.
Behavioral Assay
Responses of goldfish were studied in the same circular mazes employed (and described) by Sisler and Sorensen (2008). Briefly, these mazes were 1.5 m diam and divided into two semi-circular sections that were connected by a 35 cm long central drained region. Each maze was illuminated by a set of dim (<1 lux) overhead incandescent bulbs and infrared light sources. A low-light overhead camera with a DVD recorder also was placed overhead. Well water (100 ml/min, 18°C) entered the central region of each maze and was maintained at a depth of 20 cm by using a drain. When appropriate (see below), odor stimuli were added to the semi-circular test sections through silicon tubes attached to air stones (to evoke mixing) using a peristaltic pump (10 ml/ min; Gilson, France). Control experiments have shown these mazes to be without bias (Sisler and Sorensen, 2008; Levesque, unpublished data). The day before each test, groups of 5 goldfish were moved into the mazes and allowed to acclimate. Experimental trials were conducted one at a time and started the next morning when the inflowing well water was turned off, and then 1 hr later, a 15-min pretest was conducted during which time blank well water (10 ml/min) was pumped into both sides of each maze. Pretests were followed by 15-min tests during which time either well water (control) or test odor (see below) were added to opposite sides of each maze while fish distribution was monitored. Test odor was added to the side that had been used least during the pretest to minimize possible bias. Trials concluded with a food odor control in which food odor (see below) was added to the least-used side for 15-min to confirm that fish were responsive to odors. All experiments were conducted 10 times with different groups of fish.
For data analysis, the numbers of fish on each side of each maze were counted at 15-sec intervals across each 15-min test period (pilot studies showed this to give a good representation of fish distribution). The total number of fish on each side was then summed for each test period and divided by the total number of times fish were seen on both sides after which the difference between the percent time fish spent on the test side during the pre-test period was subtracted from the percent time they spent during the test period to yield ‘relative attraction’. Data then were summed for each experiment, evaluated for normality (Kolmogorov Smirnov test), transformed if appropriate (data for experiment 3 were arc-sin transformed), and then analyzed with one-way ANOVA (Instat, Graphpad Software, CA, USA). If significance was indicated (P < 0.05), individual values were compared with the control in that experiment using Tukey Kramer post-hoc tests. In addition, well water and food odor controls within each experiment were compared to an expected value of zero using Student t-tests (Instat, Graphpad Software, CA) to ensure that there was no bias.
Test Odor Preparation
Odor stimuli were prepared fresh each day following established protocols (Sisler and Sorensen, 2008). Briefly, approximately 100 g of goldfish (a value previously deemed to reflect that of a dense shoal of fish (Saglio and Blanc, 1989)) were carefully captured by hand net, weighed, and placed into clean plastic buckets containing 5- l of well water, and aerated by using a silicon air stone for 1-h. Blank control was prepared the same way without fish. Food odor was prepared by placing 1 g of fish food (tropical flake food, Aquatic Ecosystems Inc., Apopka, FL, USA) into 100 ml of well water for 1-h and then filtering it through filter paper (Grade 3,Whatman, International Ltd, Maidstone, England). Stimuli were used within 5 hr.
Experiment 1: Does Responsiveness of Juvenile Goldfish to Conspecific Washings Change with Time?
This initial experiment sought to determine whether juvenile goldfish exhibit the same responses to conspecific washings as the sexually-immature goldfish that had been held for over a year in the laboratory. Not only did this question address whether this cue is a pheromone, but it allowed us to address whether we could use immature and juvenile fish interchangeably. Responses of both juvenile and regressed immature goldfish were tested to each other’s body washings and blank water control in a randomized order. In a final test, juvenile goldfish were offered simultaneously the choice of juvenile and immature washings to confirm that they were equally attractive (thus reducing the possibility that fish may simply have appeared to have been equally attracted because these odors had been tested against a blank (a very weak competing stimulus).
Experiment 2: Do All Maturational Stages of Goldfish Produce and Respond to Conspecific Washings in the Same Manner?
Experiment 1 indicated that immature and juvenile goldfish release and respond to the same chemical stimuli; this experiment extended this approach to include sexually-mature males and females. All combinations of mature and juvenile fish and their body washings were tested. In addition, mature female goldfish were offered a choice of mature male vs. immature washings in one experiment, and mature male vs. mature female washings in another. The later experiments sought to confirm that these odors were perceived as being the same.
Experiment 3: Are Conspecific Body Washings Detected by the Olfactory Sense?
To test the role of the olfactory sense in conspecific recognition, responses of immature goldfish were tested to body washing after their nares had been occluded, and then again after their occlusions had been removed. Immature fish were used because their large size made them easier to handle. For occlusion, fish were anesthetized in MS222® (tricaine methanesulfonate; 1 g/ 10 l), placed onto a wet paper, and an inert dental impression material (3M Express vinyl polysiloxane, St. Paul, MN, USA) was injected carefully into their nares following protocols previously shown not to affect fish swimming behavior or health (Maniak et al., 2000). Fish were tested with goldfish body washings within a day of occlusion, then anesthetized to remove their nasal occlusions using forceps, and given 3 week to recover before being re-tested.
Experiment 4: How Potent Is the Species-identifying Cue?
Different groups of juvenile goldfish fish were tested to full strength body washings (20 g/ l), a 10-fold dilution of the former, and then a 100-fold dilution. The order of testing was randomized, and a well water control was included in the test matrix.
Experiment 5: Is this Cue Comprised of a Single or Multiple Components, and Might Either Bile and/or Amino Acids Be Involved?
This experiment proceeded in two steps. The first tested whether the cue was polar and/or non-polar, while the second tested the role of bile acids found in the non-polar fraction. For the first step, 1-l aliquots of juvenile goldfish holding water (20 g/ l) were filtered (Whatman Grade 3, International Ltd, Maidstone, England), passed through activated reversed-phase C18 columns (Sep-pak Plus, Waters Corp., Milford, MA, USA), and eluted with 5 ml of methanol following established protocols (Polkinghorne et al., 2001). The eluate (i.e., the non-polar fraction) then was dried under a stream of nitrogen, reconstituted in well water to its original concentration, and its behavioral activity tested with juveniles. The filtrate (polar fraction) was tested directly in the maze. A mixture of the two then was tested to confirm that activity had not been lost. Pilot studies using C18 resins have shown that they do not release unknown compounds that influence fish behavior.
For the second step, the bile acid composition of goldfish holding water was examined following established protocols (Polkinghorne et al., 2001), and the bile acids found therein were tested for behavioral activity. Briefly, three 1-l aliquots of goldfish holding water were extracted as described above, dried, spiked with standards, injected onto a reversed-phase C18 column (Nova-pak C18, 4 μm column; Waters Corp., Milford, MA, USA) eluted with a gradient of acetonitrile and ammonium dihydrogen phosphate, and treated with 3α-hydroxysteroid dehydrogenase while fluorescence was monitored. Bile acids were identified based on retention times and mass spectrometry (Fine and Sorensen, 2008). The available compound (taurocholic acid) was purchased (Sigma Chemical Co, MO, USA), while the unavailable compound (cyprinol sulfate) was isolated and purified (99% purity by LC-MS) from goldfish gall bladders. These bile acids then were tested in our maze (in methanol carrier) at the concentration (10−10 Molar) estimated to be present in body washings.
Results
Experiment 1 found that while immature goldfish were not attracted to well water control (P > 0.10), they were attracted to the body washings of both immature goldfish (P < 0.01) and juvenile goldfish (P < 0.01) (Fig. 1a). Similarly, juvenile goldfish were not attracted to well water (P > 0.10) but were attracted to both washings of immature (P < 0.01) and juvenile goldfish (P < 0.01) (Fig. 1b). When juvenile goldfish were offered the choice of juvenile and immature body washings, they did not choose between them (P > 0.10) (Fig. 1c). Food odor control was highly attractive to both groups (P < 0.01) (Figs. 1a, b).
a Changes in the distribution (‘Relative attraction’) of immature goldfish after the addition of either well water control, juvenile goldfish washings, or immature goldfish washings (mean percent change ± Standard error [SE]; N = 10 groups of 5 fish in each case). Different letters denote statistical differences between treatments (P < 0.05 by ANOVA). A summation of all food odor control experiments is shown to the right (*P < 0.01 vs. 0 by paired t-test). b Relative attraction (mean percent change) of juvenile after addition of well water control, juvenile and immature goldfish washings (N = 10). A summation of all food odor control experiments is shown to the right (*P < 0.01 vs. 0.0 by paired t-test). c Changes in the relative distribution of immature goldfish offered a choice between juvenile and immature goldfish washings (N = 10 groups of 5 fish)
Experiment 2 demonstrated that all life stages of goldfish produced and discerned similar conspecific aromas. Juvenile goldfish were not influenced by well water control (P > 0.10) but were highly attracted to washings of mature males (P < 0.01) and mature females (P < 0.01) (Fig. 2a). Mature females also were not attracted to the well water control but were attracted to washings of juveniles (P < 0.01), mature females (P < 0.01), and mature males (P < 0.01). Further, when females were offered the choice of mature male vs. immature washings, and mature male vs. mature female washings, they showed no preference for either (P > 0.10; Fig. 2c). Finally, mature males were attracted to the washings of juveniles (P < 0.01), mature females (P < 0.01) and mature males (P < 0.01) (Fig. 2d).
a Relative attraction (mean percent change ± Standard error [SE]) of juvenile goldfish after the addition of either well water control, mature female, or mature male washings (N = 10 groups of 5 fish; letters denote differences between treatments at p < 0.05); A summated food odor experiment is shown to the right (*P < 0.01 vs. 0.0 by paired t-test). b Relative attraction of mature female goldfish to well water control, juvenile, mature female, or mature male goldfish washings (N = 10; P < 0.05). The summated food odor control experiment is shown to the right (*P < 0.01); c Changes in the relative distribution of mature female goldfish offered a choice between mature male and immature goldfish washings, and between mature male and mature female washings (N = 10 for each; P < 0.05); d Relative attraction of mature male goldfish to well water control, juvenile, mature females, or mature male washings (N = 10 groups of 5, P < 0.05). The food odor control is shown to the right (*P < 0.05)
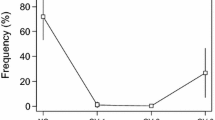
Experiment 3 demonstrated that immature goldfish are attracted to conspecific washings because of their odor; they were not attracted to this stimuli when their nares were occluded (P > 0.10), but they were attracted when occlusions were removed (P < 0.01; Fig. 3). Experiment 4 showed this cue to be fully potent when diluted 10 times (P < 0.01) but not 100 times (Fig. 4). The food odor control was attractive for all groups (P < 0.01).
Changes (mean percent change ± Standard error [SE]) in the relative distribution of immature goldfish with occluded nostrils (black bar) and the same fish after their occlusions had been removed (clear bar) to immature goldfish body washings (N = 5 groups of 10; *P < 0.01 vs. 0 for both treatments). The food odor control for the post-occluded group (*P < 0.05 vs. 0) is shown to the right
Finally, experiment 5 showed that both the non-polar C18 eluate and the polar filtrate of juvenile holding water were attractive to juvenile goldfish (P < 0.05; Fig. 5). A combination of the eluate and filtrate had potency equivalent to untreated juvenile holding water (P > 0.10), and also was more attractive than either the filtrate or eluate alone (P < 0.05; Fig. 5). HPLC analysis of goldfish water found large quantities of two identifiable bile acids, cyprinol sulfate (CS) and taurocholic acid (TCA) (Fig. 6a). The former was released at a rate of 4.2 μg / l / 20 g body weight of goldfish for CS and the later at a rate of 0.085 μg / l / 20 g. A mixture of these bile acids had no measurable effect on the behavior of juvenile goldfish (P > 0.10; Fig. 6b).
Relative attraction (mean percent change ± Standard error [SE]) of juvenile goldfish after the addition of well water, juvenile goldfish, juvenile goldfish C18 extract, C18 filtrate, or a mixture of C18 extract and filtrate [N = 10 groups of 5; significant differences between treatments (P < 0.05) noted by letters]. The summated food control is noted to the right (*P < 0.05 vs. 0)
a A representative chromatogram showing the bile acids found in goldfish holding water. Y-axis shows relative fluorescence and x-axis time. CS, cyprinol sulfate; TCA, Taurocholic acid; Std1, standard 1 (0.1 μg hyocholic acid); Std2, standard 2 (0.1 μg lithocholic acid); c. unknown non-bile acid contaminant. b Changes (mean percent change ± Standard error [SE]) in the relative distribution of juvenile goldfish exposed to either well water control or a mixture of CS and TCA (no differences when compared to each other by t-test)
Discussion
This study confirms the existence of a species-identifying cue in immature goldfish body washings (Saglio and le Martret, 1982; Sisler and Sorensen, 2008), while demonstrating that juveniles, mature males, and mature females all produce and respond to a similar conspecific cue. The fact that neither juvenile nor female goldfish distinguished between mature or juvenile fish washings in head-to-head behavioral tests, argues strongly that maturity does not influence the production or discrimination of this cue, whose specificity has already been described in some detail by Sisler and Sorensen (2008). Because the species-identifying cue is active at low concentrations and it is detected by the olfaction, it should be considered a pheromone. Fractionation suggests that it has multiple non-polar and polar components. This appears to be the first systematic description of the ability of a fish species to identify all life history stages of conspecifics by using odor alone. Previous work allows us to apply this finding to the closely-related common carp (Sisler and Sorensen, 2008).
This species-identifying pheromone should be adaptive to the goldfish and its relatives that live in turbid waters, and forage and spawn in groups (McCrimmon, 1968). Our dilution experiments suggest that the primary role of the pheromone may be as a close range identifier for shoaling and spawning. Although the present study did not examine the full range or complexity of pheromonal compounds released by goldfish (the specific role of sexual cues was not examined), it nevertheless does demonstrate that different life history stages consistently release common compounds that are consistently perceived as a distinctive species-specific entity by all life stages. The presence of a species-specific odor could explain previous observations that goldfish and carp do not readily hybridize despite the fact that they share the same hormonal pheromones (Sorensen et al., 1992), and that altering body odor background could alter the function of hormonal >pheromones (Sorensen et al., 2000; Sorensen and Levesque, unpublished data). If true, this phenomenon would answer the paradox of how various fish species can employ common hormonal products as species-specific sex pheromones (Sorensen and Stacey, 1999; Stacey and Sorensen, 2005, 2009). A similar scenario can be envisaged for the fish alarm cue, which some suggest to be a simple purine (Brown et al., 2000), but which detailed cross-species comparisons using whole odors often show to be species-specific (Smith, 1992). The sophisticated neural architecture of the fish olfactory system appears capable of discriminating odor complexes (Friedrich and Korsching, 1998; Michel, 2006; Derby and Sorensen, 2008). Further study of pheromone mixtures would be timely.
The present study demonstrates that the species-identifying pheromone recognized by the goldfish is comprised of both non-polar and polar components, and all components need to be present for full activity. Somewhat surprisingly, the two bile acids we isolated from goldfish waters, cyprinol sulfate (a bile acid novel to the cyprinid minnows; Tammar, 1974) and taurocholic acid, seemed unable to explain the biological activity of the non-polar fraction although both are detected by the goldfish with picomolar sensitivity (Sorensen et al., 1987; Sorensen and Stacey, 2004; Sorensen, unpublished data). However, while bile acids and related compounds have been shown to exhibit pheromonal activity in an ancient cartilaginous fish, the sea lamprey (Petromyzon marinus; Li et al., 2002; Fine and Sorensen, 2008), their roles as behavioral stimuli have to date been equivocal in bony fishes (Baker et al., 2006). More detailed tests are needed. Although our study did not address the chemical identity of the active component(s) in the polar fraction, it remains possible that it is comprised of mixtures of amino acids as suggested by Saglio and Blanc (1989). Future work should fully fractionate goldfish and carp body washings to determine the identity of these pheromones.
In summary, this study has demonstrated that all life stages of goldfish produce and use a common species-specific pheromone. The pheromone awaits identification although pilot data suggest that bile acids may not play a prominent role. Because the goldfish has proven to be a good model for many fish, it seems likely that that other fish species use similar cues. It will be especially interesting to test directly how this species-identifying pheromone influences perception and function of hormonal pheromones.
References
Baker, C. F., and Montgomery, J. C. 2001. Species-specific attraction of migratory banded kokopu juveniles to adult pheromones. J. Fish Biol. 58:1221–1229.
Baker, C. F., Carton, A. G., Fine, J. M., and Sorensen, P. W. 2006. Can bile acids function as a migratory pheromone in banded kokopu, Galaxias fasciatus (Gray)? Ecol. Freshw. Fish 15:275–283.
Brown, G. E., Adrian, J. C., Smyth, E., Leet, H., and Brennan, S. 2000. Ostariophysan Alarm pheromones: Laboratory and field tests of the functional significance of nitrogen oxides J. Chem. Ecol. 26:139–154,
Derby, C. D., and Sorensen, P. W. 2008. Neural processing, perception and behavioral responses to natural chemical stimuli by fish and crustaceans. J. Chem. Ecol. 34:898–914.
Døving, K. B., Selset, R., and Thommsen, G. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Phisiol. Scand. 108:123–131.
Fine, J. M., and Sorensen, P. W. 2008. Isolation and biological activity of the multi-component sea lamprey migratory pheromone and new information in its potency. J. Chem. Ecol. 34:1259–1267
Friedrich, R. W., and Korsching, S. I. 1998. Chemotopic, combinatorial, and noncombinatorial odorants representations of the olfactory bulb revealed by voltage-sensitive axon tracer. J. Neurosci. 18:87–95.
Karlson, P., and Lüscher, M. 1959. “Pheromones”, a new term for a class of biologically active substances. Nature 183:55–56.
Kobayashi, M., Sorensen, P. W., and Stacey, N. E. 2002. Hormonal and pheromonal control of spawning in goldfish. Fish Physiol. Biochem. 26:71–84.
Le Martret, M. A., and Saglio, P. 1982. Communication intraspécifique d’origine phéromonale chez le carassin doré immature (Carassius auratus L.). Mise en évidence olfactométrique du phéromone. Biol Behav. 7:41–47.
Li, W., and Sorensen, P. W. 1997. Highly independent olfactory receptor sites for conspecific bile acids in the sea lamprey, Petromyzon marinus. J. Comp. Physiol. A. 180:429–438.
Li, W., Scott, A. P., Siefkes, M. J., Yan, H., Liu, Q., and Yun, S.-S. 2002. Bile acid secreted by male sea lamprey that acts as a sex pheromone. Science. 296:138–141.
Liley, N. R. 1982. Chemical communication in fish. Can. J. of Fish. Aquat. Sci. 39:22–35.
Maniak P. J., Lossing, R., and Sorensen, P. W. 2000. Injured Eurasian ruffe, Gymnocephalus cernuss, release an alarm pheromone which may prove useful in their control. J. Great Lakes Res. 26:183–195.
Mccrimmon, H. R. 1968. Carp in Canada. Bull Fish. Res, Bd. Can. 165, 93p.
Michel, W. C. 2006. Chemoreception, pp. 471–498, in D. H. Evans and J. B. Clairborne (eds.). The Physiology of Fishes, 3rd Ed. CRC Press, Boca Raton, Florida.
Olsen, K., Swaski, J. R., and Stacey, N. E. 2006. Endocrine and milt responses of male crucian carp (Carassius carassius L.) to periovulatory females under field conditions. Gen Comp. Endocrinol. 149:249–302.
Polkinghorne, C. A., Olson, J. M., Gallaher, D. G., and Sorensen, P. W. 2001. Larval sea lamprey release two unique bile acids to the water at a rate sufficient to produce detectable riverine pheromonal plumes. Fish Physiol. Biochem. 24:15–30.
Saglio, P., and Blanc, J. M. 1989. Intraspecific chemocommunication in immature goldfish, Carassius auratus L.: attraction in olfactometer to free amino acid fractions from skin extract. Biol. Behav. 14:132–147.
Saglio, P., and Le Martinet, M. -A. 1982. Communication intraspecifique d’origine phéromonale chez le carassin doré immature, Carassius auratus L. 2. Etude en olfactomètre de l’activité comportementale d’extraits d’origine épidermique. Biol. Behav. 7:41–54.
Scott, A. P., and Sorensen, P. W. 1994. Time course of release of pheromonally active gonadal steroids and their conjugates by ovulatory goldfish. Gen. Comp. Endocrin. 96:309–323.
Sisler, S. P., and Sorensen, P. W. 2008. Common carp and goldfish discern conspecific identity using chemical cues. Behaviour 145:1409–1425.
Smith, R. J. F. 1992. Alarm signals in fish. Rev. Fish Biol. Fish. 2:33–63.
Sorensen, P. W., and Hoye, T. H. 2010. Pheromones in Vertebrates. pp. 225–262, in K Mori (ed.). Volume 4 Chemical Ecology, Comprehensive Natural products Chemistry II: Chemistry and Biology (series editors L.N. Mander; H. Liu). Elsevier Press, U.K.
Sorensen, P. W., and Stacey, N. E. 1999. Evolution and specialization of fish hormonal pheromones, pp. 15–47, in R. E. Johnston, D. Muller-Schwarze and P. W. Sorensen (eds.). Advances in Chemical Signals in Vertebrates. Kluwer Academic Plenum Publishers, New York.
Sorensen, P. W., and Stacey, N. E. 2004. Brief review of fish pheromones and discussion of their possible uses in the control of non-indigenous teleost fishes. N.Z. J. Mar. Freshwater Res. 38:399–417.
Sorensen, P.W., Hara, T. J., and Stacey, N. E. 1987. Extreme olfactory sensitivity of mature and gonadally-regressed goldfish to 17α,20β-dihydroxy-4-pregnen-3-one, a potent steroidal pheromone. J. Comp. Physiol. A 160: 05–313.
Sorensen, P. W., Irvine, I. A. S., Scott, A. P., and Stacey, N. E. 1992. Electrophysiological measures of olfactory sensitivity suggest that goldfish and other fish use species-specific mixtures of hormones and their metabolites as sex pheromones, pp. 357–364 in R. L. Doty and D. Muller-Schwarze (eds.). Chemical Signals in Vertebrates VI, Plenum Press, New York.
Sorensen, P. W., Scott, A. P., and Kihslinger, R. L. 2000. How common hormonal metabolites function as specific pheromones in the goldfish. pp. 125–129 in: B. Norberg, O.S. Kjesbu, G.L. Taranger, E. Andersson, and S.O. Stefansson (eds.). Proceedings of the Sixth International Symposium on the Reproductive Physiology of Fish. Bergen, Norway.
Sorensen, P. W., Pinollis, M., and Scott, A. P. 2005. Sexually mature male goldfish release large quantities of androstenedione to the water where it functions as a pheromone. Gen. Comp. Endocrin. 140:164–175.
Stacey, N. E., and Sorensen, P. W. 2005. Reproductive pheromones, pp. 359–412, in K. A. Sloman, R. W. Wilson, S. Balshine (eds.). Physiology and Behaviour of Fish, vol 24 in Fish Physiology Series, A. Farrell and C.J. Brauner (senior eds.). Academic Press, San Diego, California.
Stacey, N. E., and Sorensen, P. W. 2009. Hormonal pheromones in fish. Volume 2, pp.639–681, in D. W. Pfaff, A. P. Arnoldf, A. Eitgen, S, Frabach and R, Rubin (eds.). Hormones, Brain and Behavior, 2ed., Elsevier Press, San Diego.
Stacey, N. E., Kyle, A. L., and Liley, N. R. 1986. Fish reproductive pheromones, pp. 117–134, in D. Duvall, D. Muller-Schwarze and R. M. Silverstein (eds.). Chemical Signals in Vertebrates 4, Plenum Press, New York.
Tammar, A. R. 1974. Bile salts in fishes. Chem. Zool. 8:595–611.
Wyatt, T. D. 2003. Pheromones and Animal Behavior. Cambridge University Press, Cambridge.
Wyatt, T. D. 2009. Fifty years of pheromones, Nature 457:262–3.
Acknowledgments
This study was funded by the Minnesota Environment and Natural Resources Trust Fund. We thank Mario Travaline for his help. Two anonymous reviewers and Drs. Bajer and Lim kindly reviewed and commented on the manuscript
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levesque, H.M., Scaffidi, D., Polkinghorne, C.N. et al. A Multi-Component Species Identifying Pheromone in the Goldfish. J Chem Ecol 37, 219–227 (2011). https://doi.org/10.1007/s10886-011-9907-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-011-9907-6