Abstract
This manuscript reviews the chemical ecology of two of the major aquatic animal models, fish and crustaceans, in the study of chemoreception. By necessity, it is restricted in scope, with most emphasis placed on teleost fish and decapod crustaceans. First, we describe the nature of the chemical world perceived by fish and crustaceans, giving examples of the abilities of these animals to analyze complex natural odors. Fish and crustaceans share the same environments and have evolved some similar chemosensory features: the ability to detect and discern mixtures of small metabolites in highly variable backgrounds and to use this information to identify food, mates, predators, and habitat. Next, we give examples of the molecular nature of some of these natural products, including a description of methodologies used to identify them. Both fish and crustaceans use their olfactory and gustatory systems to detect amino acids, amines, and nucleotides, among many other compounds, while fish olfactory systems also detect mixtures of sex steroids and prostaglandins with high specificity and sensitivity. Third, we discuss the importance of plasticity in chemical sensing by fish and crustaceans. Finally, we conclude with a description of how natural chemical stimuli are processed by chemosensory systems. In both fishes and crustaceans, the olfactory system is especially adept at mixture discrimination, while gustation is well suited to facilitate precise localization and ingestion of food. The behaviors of both fish and crustaceans can be defined by the chemical worlds in which they live and the abilities of their nervous systems to detect and identify specific features in their domains. An understanding of these worlds and the sensory systems that provide the animals with information about them provides insight into the chemical ecology of these species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chemical Senses in the Aquatic Medium
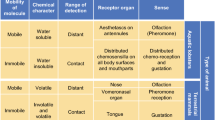
Aquatic organisms evolved in a world of dissolved chemicals, and this resulted in the appearance of numerous types of chemosensory systems. As in terrestrial organisms, the senses of olfaction and gustation are usually identified as the main chemosensory systems in aquatic organisms, although the distinctions between them are somewhat blurred. This is because the primary distinction between these senses in terrestrial organisms—the physical medium through which chemical molecules are delivered to animals (air for olfaction and water for gustation)—does not apply in aquatic systems. Thus, a second basis for distinguishing between olfaction and gustation—neuroanatomy—becomes particularly relevant for aquatic animals. However, this distinction is more useful for vertebrates than invertebrates, whose neuroanatomy differs. In aquatic vertebrates, like all vertebrates, olfaction is defined as the sense mediated by neurons with axons in the olfactory nerve (cranial nerve I). In addition, a common feature of olfaction in vertebrates and many invertebrates is that the first-order processing regions in their brains are organized into glomeruli, which contain the synapses between the olfactory receptor neurons and interneurons, and that these brain regions have a chemotopic organization such that different odorants generate distinctive patterns of glomerular activity (Hildebrand and Shepherd 1997; Eisthen 2002; Ache and Young 2005). Gustation in vertebrates, on the other hand, is mediated by non-neuronal, modified epithelial cells that are innervated by the facial (cranial nerve VII), glossopharyngeal (cranial nerve IX), and vagal (cranial nerve X) nerves that project to different brain structures and appear to have different functions (Atema 1977; Caprio et al. 1993). Another way that olfaction and gustation can be distinguished is by their function: Gustation is more apt to mediate simple and reflexive behaviors, food consummatory behaviors in particular, whereas olfaction tends to mediate more complex behaviors such as searching for distant sources of chemicals, courtship behavior, and learning about odors (Atema 1977).
Besides olfaction and gustation, both fish and crustaceans have a diversity of other, less understood chemical senses. Fish have a trigeminal system and solitary chemoreceptor cells that cover their bodies (Kapoor and Finger 2003), whose functions are not yet clearly established. Crustaceans have a diversity of chemoreceptor neurons that differ in their packaging within sensilla, their connections and organization in the central nervous system, and the behaviors that they mediate (Horner et al. 2006, 2008b). One pathway of crustaceans—the aesthetasc sensilla and the olfactory lobe pathway—is considered ‘olfactory’ because of organizational similarities between it and the olfactory pathways of vertebrates and insects. While other crustacean chemosensors are typically packaged with mechanosensors into sensilla, these sensilla are extremely diverse in structure and distributed differently across the animal’s body surface where they serve different behavioral functions (see “Processing of Natural Chemical Stimuli”). Regardless of how chemosensory systems are defined, it is important to recognize that aquatic organisms have a variety of chemosensory systems whose neuroanatomical structures and functions vary dramatically.
The Chemical World Perceived by Fish and Crustaceans
Aquatic animals detect, discriminate, and respond to a wealth of chemicals in their natural environment. This diversity is immense, as aquatic organisms in general release literally thousands of small and soluble products that can carry information. Notably, most of the compounds found in aquatic environments are relatively unspecialized metabolic products (Atema 1988; Carr 1988). With the possible exception of some pheromones (Sorensen and Stacey 1999), there is little evolutionary pressure for organisms to produce specialized chemicals that facilitate their discrimination. Chemoreception is basic to meeting most biological needs of organisms, including those related to reproduction, social interactions, acquiring food and shelter, and defense from predators. Of course, this is particularly true in waters with low light levels. For the most part, chemoreception in aquatic ecosystems requires the detection of small differences in mixture composition in complex backgrounds, as opposed to detection of a few specialized compounds. In the next section, we give select examples of the discriminatory abilities of fish and crustaceans that focus on the nature of their chemical worlds and their abilities to detect, discriminate, and respond to them. A discussion of the molecular identity of important chemicals and how they are neurally processed follows later.
Fish
Fish use chemicals that mediate many key aspects of their lives, most of which are poorly understood but appear highly complex. Among the most important are habitat recognition, food finding, conspecific identification, and predator avoidance. Fish are the most diverse group of vertebrates, represented by more than 26,000 species that live in an immense variety of habitats. The type, concentration, and distribution of chemicals in their environments are all important factors in determining the chemical ecology and life history strategies. Because of this vast diversity, our review considers only a small number of fish species and situations, so we have selected representative examples.
Most species of fish are highly mobile and exhibit a variety of complex behaviors, many of which depend on them having information about their environment. Ablation studies often demonstrate that the type and location of habitat is determined by using environmental chemicals as cues. Although some species, such as freshwater bass, live in and compete for limited territories, other species are migratory and travel great distances only to return to very specific locations to reproduce. For example, Pacific salmon are born in small inland streams and then migrate to oceans to feed but eventually return ‘home’ by using olfactory cues (Hasler and Scholz 1983). A variety of studies demonstrate that the chemical nature of ‘home stream’ odor is learned. Salmon select between proximate streams when returning home by choosing the stream in which they were raised many years earlier. Given the proximity and ecological similarities of the streams among which Pacific salmon must choose, it has been suggested that the odor that they learn is complex and comprised of mixtures of compounds from minerals, plants, and animals (Hasler and Wisby 1951; Nordeng 1977). Salmon can identify stream odors after many years at sea and environmental change, demonstrating their remarkable ability to recognize patterns. Another interesting example of olfactory-driven migration is the parasitic sea lamprey, which has a similar life history to salmon except that it does not return to home streams but rather selects streams that contain young lamprey. Lamprey recognize home streams by using innately recognized pheromones released by larval lamprey in combination with other unknown compounds found in all stream waters (Sorensen et al. 2003, 2005; Sorensen and Hoye 2007). A third example is the use of olfactory cues by non-migratory fish to identify home ranges within streams—difference in the odor mixtures they discriminate must be subtle (Gunning 1959; Arnesen and Stabell 1992). These three examples demonstrate that fish discriminate complex matrices of water-borne chemicals and can remember and track them through space and time by using their olfactory systems that demonstrate sophisticated perceptual abilities.
As most fishermen know, fish are also adept at using chemicals to identify and locate food, even in turbid, deep, or dark waters. This highlights the outstanding ability of fish to perceive chemicals associated with food and may even be partially responsible for the evolutionary success of fish in exploiting diverse feeding niches (Moyle and Cech 2000). Both olfaction and gustation are used in distinguishing between similar types of food. Most fish are ‘feeding generalists’ with keenly developed abilities to identify and locate a range of foods based on their nutritive values, even in changing environments in which the relative abundance of specific prey may dramatically shift. Notably, fish have evolved multiple suites of feeding behaviors, including appetitive and consummatory behaviors (Jones 1992; Valentinčič 2005). These behaviors are mediated by both olfaction and gustation, which can detect overlapping sets of relatively common metabolic products. l-Amino acids are the most important of these, but other classes are known (see below). Species differences in sensitivity to these classes of chemical stimuli are common, and almost certainly, there are innate abilities to learn certain types of stimuli (Jones 1992). These abilities may be mediated by the gustatory sense, which is generally more narrowly tuned and often linked to mechanistic responses. Nevertheless, in the natural world, most feeding chemical stimuli appear to be discriminated as complex mixtures. For example, Carr (1982) reports that mixtures of 17 to 22 amino acids plus betaine could account for the majority, though not all, of the feeding activity elicited by four natural food items for the pigfish but not the related pinfish. Finally, as one might expect, selection of food by at least some marine fish is influenced by their detection of deterrent molecules, such as alkaloids, tetrodotoxin, and acids. This subject has received considerable attention from chemical ecologists but, unfortunately, little work from neuroscientists (Hara 1994; Hay 1996; Kicklighter et al. 2005; Hayden et al. 2007; Kamio et al. 2007; Cohen et al. 2008).
Of course, fish do more than hide and eat. Pheromones, here defined as sets of chemicals that convey information about an individual’s identity and condition to other members of its species, play essential roles in the sexual and social life histories of most fishes (Stacey and Sorensen 2005). Olfactory blocking studies consistently demonstrate that olfaction mediates the perception of sex pheromones, which are often so important that anosmic fish simply fail to mate (Stacey and Kyle 1983). Finding and identifying conspecifics of appropriate maturity in complex natural environments can be challenging, so mating in even highly visual species such as swordtails is almost always assisted by pheromones (Wong et al. 2005). Intraspecific cues and pheromones can have multiple functions. Among these, species recognition for the purpose of aggregation or schooling is paramount. Although the chemical basis of aggregation is not clear, variations in metabolite production—rather than production of novel, species-specific compounds—are likely responsible (Sorensen and Stacey 1999). Odor recognition systems tuned to small variations in mixture composition should provide species-specific information. In addition to using olfaction for species recognition, some fish, including sticklebacks and salmon, use odors to determine kinship. These odors may include major histocompatibility complex-related peptides (Reusch et al. 2002; Ward and Hart 2002). Finally, almost all fish are able to discriminate reproductive state by using sex pheromones that they detect with high sensitivity and specificity (Stacey and Sorensen 2005). Hormonally derived signals are especially important, presumably because of their inherent relevance (Stacey and Sorensen 2005). However, hormone systems are highly conserved, thus providing little latitude for the evolution of novel hormonal products and their receptors, and some species have evolved to use a wider range of products (Li et al. 2002; Yambe et al. 2007).
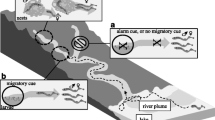
In addition to sex pheromones, many fish can detect intraspecific chemicals that indicate danger, in particular, chemicals from conspecifics injured by predators (Smith 1992; Døving et al. 2005). However, injured fish release a variety of chemicals, some of which (e.g., amino acids) may be food cues in some contexts. Since the first description of the alarm response in European minnows in the field by von Frisch (1938), at least six types of compounds or their mixtures have been proposed to mediate alarm responses, with the purine hypoxanthine-3-N-oxide receiving the most attention (Pfeiffer et al. 1985; Brown et al. 2000, 2003). However, none of the suggested bioactive molecules has received supporting evidence from electrophysiological recordings or chemical measurements. Interestingly, several studies suggest that the alarm cues can be learned (Wisenden 2000), raising the possibility that multiple types or mixtures of chemicals may be involved. The observation that fish recognize chemicals released by predators that have eaten conspecifics—presumably conspecific alarm cues that remain functional after being digested by the predators (Brown et al. 1993)—supports this possibility. Alarm cues from injured conspecifics are mediated by the olfactory system (Maniak et al. 2000; Døving et al. 2005).
In summary, fish live in complex environments wherein they face extreme challenges in finding shelter, mates, and food, while at the same time avoiding predators. They use their chemical senses in these behaviors by discriminating complex (though, at present, often incompletely defined) chemical mixtures of relatively common molecules. These abilities have allowed fish to succeed and diversify, becoming the majority of planet’s vertebrate biomass and biodiversity.
Crustaceans
Crustaceans, like fish, rely on combinations of sophisticated chemosensory systems to identify and locate food, mates, and predators in noisy chemical environments filled with a multitude of products. The best studied chemosensory behavior in crustaceans is the selection and acquisition of food. Crustaceans use antennular chemoreception to identify attractive food (Derby 2000; Derby et al. 2001) and locate it from a distance (Atema 1996; Zimmer and Butman 2000; Grasso and Basil 2002; Weissburg et al. 2002; Keller and Weissburg 2004). Amino acids and nucleotides are two major sets of molecules that they use. Once near the food, ingestion is based on input from their gustatory systems on legs and mouthparts (Derby 2000; Derby et al. 2001). Food selection and ingestion is influenced by the blend of attractive and deterrent compounds, although we know more about the former than the latter (Derby et al. 2001; Prusak et al. 2005; Kamio et al. 2007). Some crustaceans can learn to avoid food associated with gastric malaise (Wight et al. 1990).
Crustaceans make use of chemical signals in most aspects of their reproduction. They use sex pheromones to identify and locate conspecifics of the opposite sex. Copepods, amphipods, shrimp, crabs, lobsters, and crayfish are leading examples (Gleeson 1991; Asai et al. 2000; Hardege et al. 2002; Kamio et al. 2002, 2008; Stebbing et al. 2003; Ting and Snell 2003; Caskey and Bauer 2005; Ekerholm and Hallberg 2005; Belanger and Moore 2006; Atema and Steinbach 2007). Some sex pheromones are detected from a distance, others seem to be used in close range, even requiring contact. Chemical cues are also used in other aspects of reproduction. For example, many female crustaceans incubate their fertilized eggs, and chemicals released from hatching eggs induce abdominal pumping, fanning, and other behaviors from the females that facilitate the rapid and synchronized hatching of eggs and release of larvae (Tankersley et al. 2002; Rittschof and Cohen 2004).
Crustaceans use chemical cues during intraspecific interactions and social behavior. These chemicals are often in their urine and under controlled release so that they can be used at appropriate times during behavioral interactions (Breithaupt 2001; Breithaupt and Atema 2000; Breithaupt and Eger 2002; Moore and Bergman 2005; Moore 2007). Some crustaceans, such as lobsters and hermit crabs, use cues to recognize individual conspecifics (Johnson and Atema 2005; Gherardi et al. 2005). Others, such as crayfish, use cues to determine social status (Moore and Bergman 2005; Moore 2007). Lobsters use chemical information in aggressive interactions with conspecific (Breithaupt and Atema 2000). Spiny lobsters, which are highly social animals that often live in aggregations, use chemicals to identify each other and find safe shelter (Zimmer-Faust et al. 1985; Nevitt et al. 2004; Briones-Fourzán and Lozano-Álvarez 2005; Horner et al. 2006, 2008b). Spiny lobsters even recognize diseased conspecifics through chemical cues and avoid aggregating with them (Behringer et al. 2006). Young crayfish, which associate with their mother for some days after hatching, can locate her, as well as the shelter that she provides, by means of chemicals that she releases around the time of hatching (Little 1975). This cue appears not be specific to mother but is sex specific (Little 1976).
Crustaceans use chemicals to locate high-quality shelter or places to live. A well-known and long-studied example is the selection of sites to settle by larval barnacles (Dreanno et al. 2006a, b). Crustaceans such as pea crabs that live as commensals or symbionts with other organisms use chemical cues to locate their future hosts (Grove and Woodin 1996). Chemical cues are also used by hermit crabs to recognize shells as future homes (Rittschof and Cohen 2004).
Crustaceans also use chemoreception to avoid predators. Some can sense predators from a distance and thereby avoid them. Examples include crayfish and spiny lobsters (Berger and Butler 2001; Bouwma and Hazlett 2001). Predator avoidance can also be mediated through a less direct mechanism. Some crustaceans release chemicals when damaged, via leakage of body fluids, or when disturbed, via controlled release in urine, and these chemicals are avoided by conspecifics. Examples of species that use alarm cues include crayfish, spiny lobsters, and hermit crabs (Hazlett 1994; Zimmer-Faust et al. 1985; Rittschof et al. 1992; Nevitt et al. 2000; Zulandt Schneider and Moore 2000; Shabani et al. 2006; Bouwma 2007).
The Molecular Identity of Chemical Cues and Signals
Methods of Identification
The identity of bioactive molecules can be elucidated by using natural products chemistry techniques together with bioassays, based on any of several experimental approaches. Bioassay-guided fractionation is a standard technique that makes no assumptions about the nature of the bioactive substances. By this method, a natural product is separated into fractions based on any of a number of properties, including solubility in solvents of different polarity, molecular mass, and molecular charge. One method is a Kupchan partition scheme or modifications thereof, which is based on partitions differing in solubility—hexanes, chloroform, ethyl acetate, butanol, or water (Kupchan et al. 1975). With each separation, the resultant fractions are tested for bioactivity, usually with behavioral or electrophysiological assays. Comparison of the bioactivity of the fractions vs. the original material and a negative control allows identification of fractions that contain active molecules. When a natural product has more than one active ingredient, more than one fraction may have activity. It is often possible to separate bioactive molecules to sufficient purity to identify them through mass spectroscopy, nuclear magnetic resonance (NMR), or other analytical procedures. Databases such as Marinlit (http://www.chem.canterbury.ac.nz/marinlit/marinlit.shtml) and Chenomx NMR suite (http://www.chenomx.com), which contain known molecules, can be searched using known features of the bioactive molecules to identify possible molecular structures. Potential problems with this approach include degradation of bioactive molecules during separation and purification, and synergistic interactions among bioactive molecules that partition into different fractions such that their activity cannot be followed.
A second experimental approach to the molecular identification of chemical cues and signals is to determine which molecules are in relatively high concentration in the natural extracts that contain them. For example, when seeking a female sex pheromone in crustaceans, one might identify molecules in higher concentration in water from reproductive females compared to water from conspecifics that do not produce the pheromone. An example of this is the use of metabolomics to identify sex pheromones in blue crabs (Kamio et al. 2006). Metabolomics is a high-throughput approach to identify molecules enriched in or unique to one stimulus vs. another, usually focusing on small metabolites. Metabolomics has the advantage of not requiring purification of a component but can be based on spectra from mixtures (Daviss 2005). It can use data from either mass spectroscopy or NMR. This approach does not guarantee identification of bioactive molecules, in part because the bioactive molecules are not necessarily those in high concentrations, especially in the fishes whose pheromones can be common hormonal products (Sorensen and Scott 1994).
A third approach is searching for specific types of chemicals based on knowledge of the chemistry or biology of a system. For example, when ink secretions from sea hares were found to excite lobster chemosensory neurons, knowing that many of those neurons are sensitive to amino acids prompted amino acid analysis of the sea hare secretions and the eventual demonstration that amino acids in those secretions play an important defensive role (Kicklighter et al. 2005; Derby et al. 2007).
Fish
Fish perceive complex mixtures that contain a diversity of types of chemicals. Although there is presently no complete explanation for these perceptual abilities, electrophysiological studies have identified seven major classes of chemical stimuli that explain some of these abilities. These classes are amino acids, amines, nucleotides, bile acids (reduced steroids produced by the liver), aminosterols (a special class of bile steroids conjugated with amines), sex steroids, and prostaglandins. These compounds are all small (<8 kD), polar, and ubiquitous (Carr 1988; Hara 1994, 2007; Carr et al. 1996; Caprio and Derby 2007). There is overlap among classes—for example, both bile acids and sex steroids are steroids, and the relationship between olfactory receptor type and ligand class is not yet clear, so these categories of chemicals may mean more to biochemists than they do to the fish. Almost certainly, more classes of chemical stimuli await identification. Here, we briefly review our current understanding of known stimuli and how they are perceived by fish.
The chemical nature of food cues is best understood. Amino acids appear to be universally used by fish in this regard, likely because most are predators and have high protein requirements, and thus, amino acids are good indicators of high-quality food. Laboratory studies consistently demonstrate that specific mixtures of l-amino acids can attract many fish species and that single amino acids sometimes trigger reflexive snapping and biting behaviors, which at least on occasion are linked with the gustatory sense (Mackie 1982; Caprio et al. 1993; Hara 1994; Carr et al. 1996). Electrophysiological recordings demonstrate that fish olfactory systems detect all primary l-amino acids with high (nanomolar) sensitivity and specificity (Hara 1994, 2007). Fish external gustatory systems also detect l-amino acids, although often with much narrower ranges of sensitivity and, generally, in species-specific manners (Hara 2007; see below). This difference in the sensitivity of fish olfactory and gustatory systems is correlated with the different but overlapping functions of these systems (see “Processing of Natural Chemical Stimuli”).
Although amino acids are critical to food recognition, other molecules also are employed. The detailed studies on food recognition and ingestion show that l-amino acids rarely can explain all behaviors (Atema et al. 1980; Carr 1982; Carr et al. 1996). Indeed, in the case of marine fishes, betaine (a methylated amino acid) and d-amino acids, both present in marine environments, are important gustatory stimulants that can enhance the actions of amino acids (Carr 1982; Carr et al. 1996; Sorensen and Caprio 1998). Polyamines, which are protein breakdown products, are powerful stimulants of feeding arousal and search in goldfish due to their activation of specific olfactory pathways (Rolen et al. 2003). Nucleotides, which stimulate the olfactory and gustatory systems of many fish, have roles too (Carr 1988; Carr et al. 1996). Other bioactive chemicals will almost certainly come to light with further investigation. The blend of chemicals in a mixture is important to its efficacy, especially in olfactory-driven arousal and search behaviors. In summary, a wide variety of nitrogenous products serve as feeding cues for fish, although other bioactive chemicals, some yet to be identified, surely contribute.
With the exception of the sea lamprey (see below), little is known about the identities of chemical cues employed by fish to recognize the location or type of habitat. For species such as landlocked masu salmon that learn locational odors, the challenge is especially complex, although behavioral tests suggest that mixtures of l-amino acids are an important part of home stream odor recognition (Shoji et al. 2003). It is difficult to envision that amino acids are the sole contributors to home stream odor in more complex ecosystems because they are ubiquitous and their concentrations change with time and location. Suggestions that bile acids that originate from fish contribute to home stream odor (Døving et al. 1980) are intriguing, especially because they are detected by fish olfactory systems with great sensitivity and specificity. However, they are relatively generic and vary little among species (Sorensen, unpublished). Complex mixtures that contain many classes of chemicals are most likely to be important. Even for the relatively simple sea lamprey, mixtures are important. Using bioassay-guided fractionation, Sorensen et al. (2005) discovered three new sulfated steroids—petromyzonamine disulfate, petromyzosterol disulfate, and petromyzonol sulfate—that are released by larval lamprey and attract adults at concentrations below 10−13 M. All three components stimulate the olfactory system with great specificity, and while they will attract migratory adults on their own, they synergize each other’s actions, especially in the context of stream water (Sorensen et al. 2003). Other unidentified minor components exist in this mixture (Fine et al. 2004, unpublished). Why the ancient and relatively simple lamprey has evolved a multi-component pheromone is unclear, but it may relate to the challenges they face in locating home streams at great distances.
The vast majority of fish species appear able to detect and discriminate sex steroids and F-series prostaglandins as hormonal pheromones (Stacey and Sorensen 2005). Indeed, the olfactory systems of dozens of species from a variety of taxa (cyprinids, salmonids, and gobids) are able to detect hormonal products with high sensitivity and specificity, and in about half a dozen instances, biological responses have been noted as well (Stacey and Sorensen 2005). Critical to the detection of sex steroids is the number of carbons in the steroid nucleus, absence or presence of a conjugating group, and side-chain position and structure (Sorensen et al. 1990; Stacey and Sorensen 2005). Most sex steroid products are relatively common, and for the most part, species from the same genus share the same sensitivities. Thus, sex steroids alone cannot be responsible for species specificity. To date, only prostaglandin F2α and its immediate metabolites have been shown to be especially active stimulants for an entire taxonomic group (e.g., minnows; Stacey and Sorensen 2005). We speculate that signal specificity resides with contextual cues such as body odor and acute sensitivity to the composition of odorant mixtures,
To address how fish employ sex pheromones, we briefly review the goldfish, the best understood example. Similar to many external-fertilizing temperate freshwater fish, goldfish ovulate in the spring in response to a surge in a luteinizing hormone, which triggers a series of gonadal hormonal surges whose by-products function as potent sex pheromones. This system may have evolved because males that could predict female spawning by detecting released hormonal metabolites had a reproductive advantage. Three sets of female cues are known. The first is released by mature females and is associated with estradiol (Kobayashi et al. 2002). The second is released 12 h before spawning by ovulatory females and functions as a preovulatory primer with endocrinological actions. This pheromone is comprised of a mixture of at least two common sex steroid hormones, androstenedione and 17,20β-dihdroxy-4-pregnen-3-one (17,20βP), and one metabolite, 17,20β-dihdroxy-4-pregnen-3-one-20-sulfate (17,20βP-S), the ratio of which shifts during the course of the reproductive cycle during which time dozens of steroids are released (Sorensen and Scott 1994). All three components are potent olfactory stimulants and detected by different, specific receptor sites. Male fish identify these chemicals as a mixture and exhibit a strong endocrine response (leading to sperm production) when 17,20βP is highest and a behavioral response when 17,20βP-S is highest (Sorensen et al. 1995; Poling et al. 2001). The third, post-ovulatory pheromone is a mixture of F prostaglandins that is released by ovulated females in their urine in pulses, thus suggesting that odor concentration and context are also important to cue function (Sorensen et al. 1988; Appelt and Sorensen 2007). Recent data also suggest that cue specificity is determined by critical but unknown components in goldfish body odor, for if this odor is modified, responses to hormonal pheromones are greatly reduced (Sorensen et al. 2000). The reliance of goldfish on common hormonal mixtures together with context is so striking that we believe that this strategy is probably commonplace in fish.
Finally, several studies have investigated the chemistry of alarm signals, without clear resolution. Hypoxanthine-3-N-oxide, peptides, purines, proteins, and their mixtures have all been suggested to function as alarm signals (Brown et al. 2000, 2003; Døving et al. 2005). Some evidence exists that mixtures of nitrogen oxides may mediate alarm responses in somewhat taxon-specific manners (Brown et al. 2003), but data on release of these chemicals by fish and chemosensitivity to them are lacking.
In summary, substantial subsets of various metabolic products, some specialized and most not, are identified by fish olfactory and gustatory systems as meaningful chemicals that are then used to mediate an array of behaviors. Other unidentified chemicals are undoubtedly important. These stimuli occur and only have biological relevance as mixtures.
Crustaceans
Feeding stimulants are some of the most studied and best understood molecules in the chemical ecology of crustaceans. Trophic level accounts for some of the differences in the identity of feeding stimuli of crustaceans. Carnivores such as lobsters (Homarus) and spiny lobsters (Panulirus) respond best to small, nitrogen-containing compounds that are prevalent in tissues of their animal prey. These compounds include many that are also feeding stimulants for fish, including amino acids, amines, nucleotides, and peptides (Zimmer-Faust and Case 1982a; Carr 1988; Derby and Atema 1988; Zimmer-Faust 1993). Carnivorous crustaceans are relatively insensitive to carbohydrates and sugars. Herbivores and omnivores, such as fiddler crabs, ghost crabs, kelp crabs, and crayfish, are often sensitive to sugars common to plants, bacteria, and diatoms, as well as to some amino acids (Robertson et al. 1981; Zimmer-Faust and Case 1982b; Trott and Robertson 1984; Weissburg and Zimmer-Faust 1991; Archdale and Anraku 2005; Corotto et al. 2007). Selection of food by crustaceans is also controlled by the presence of feeding deterrents, yet the molecular identity of these deterrents is much more poorly understood than for the stimulants. An example of identified feeding deterrents is in crayfish, which are deterred from ingesting plants that contain phenylpropanoid-based natural products and a C-18 acetylenic acid (Prusak et al. 2005; Lane and Kubanek 2006; Parker et al. 2007).
Larval settlement factors have been studied with great interest for decades due to their economic impact. Therefore, the recent molecular identification of the factor was highly anticipated and gratefully received (Dreanno et al. 2006a, b). The bioactive molecule is a 169-kD glycoprotein called settlement-inducing protein complex, which has 30% similarity to α2-macroglobulins. The observation that small and specific peptides evoke settlement (Rittschof and Cohen 2004) makes sense in light of knowing the identity of the protein.
Sex pheromones in crustaceans also have been intensely investigated but without much success in identifying the active molecules. The pheromone used by male copepods (Tigriopus japonicus) to recognize females has been partially characterized—and interestingly, it too has similarity to α2-macroglobulin (Ting and Snell 2003). Efforts to identify sex pheromones in decapod crustaceans, such as crabs, crayfish, clawed lobsters, and spiny lobsters, have not led to the conclusive identification of the molecular structures (Gleeson 1991; Asai et al. 2000; Hardege et al. 2002; Kamio et al. 2002, 2006; Atema and Steinbach 2007). Efforts to identify pheromones on these and other species continue (J. Hardege, personal communication; M. Kamio, personal communication).
Plasticity
The Importance of Plasticity
The ability to adaptively modify chemically mediated behaviors through experience is critical to the survival of most animals. This is particularly true for animals that are long-lived, mobile, and omnivorous, which is the case for many fish and crustaceans. Plasticity occurs in several contexts in chemoreception of fish and crustaceans, including selection of food, social interactions with conspecifics, and even in responses to pheromones. Some examples are given in the next sections.
Fish
Fishes have well-developed abilities to recognize and learn natural chemical cues. The most impressive example is that of olfactory learning in migratory pacific salmonids, which imprint to bouquets of stream odors during sensitive developmental periods (Dittman et al. 1996). Although this ability is partially attributable to developmental changes in their olfactory epithelia (Nevitt et al. 2004), the ability of adults to recall olfactory memories many years later presumably involves higher brain centers. Surely pattern recognition is paramount to this process, which appears sophisticated enough that fish can discriminate and remember the presence of trace amounts of chemicals to which they are relatively insensitive, such as morpholine for salmon (Hasler and Scholz 1983; Hara et al. 1984). It is also apparent that combinations of odorants can be identified as distinct ‘bouquets’ that may give the impression of mixture synergism. For example, goldfish cannot be trained to respond to morpholine alone, but they can learn to respond to odorant mixtures that contain morpholine (Dodson and Bitterman 1989). Mixture perception and the ability to learn unique odors clearly are important to place location in salmon, which do not have a particularly notable olfactory system from an anatomical perspective. Presumably, other fishes are similar, as suggested by the examples of food recognition in pinfish and sex pheromone discrimination in goldfish. Glomerular processing in the olfactory bulb probably plays a role in this process as described below.
Feeding studies also provide evidence of the ability of fish to discriminate and learn complex chemical mixtures. Fishermen and behavioral ecologists enumerate examples of fish that develop the ability to recognize and select especially desirable food items as their abundance fluctuates (Jones 1992). Olfaction plays a major role in this process. For example, responses of yellowfin tuna to prey odors shift as their diet changes (Atema et al. 1980). Ablation studies suggest that olfaction alone mediates this response through formation of ‘chemical search images’ (Atema et al. 1980). In this case, olfaction appears to guide arousal and search, and vision assumes the final role of food location. In other species, gustation rather than vision plays the consummatory role. This plasticity does not signify a lack of innate predisposition to respond to particular types of prey. There are several anecdotal examples of naive young fish responding to specific food items (Jones 1992), a phenomenon once termed ‘specific appetite,’ which may be a gustatory attribute (see below). The interplay between these senses warrants specific investigation by using natural cues.
Not surprisingly, laboratory studies of associative and instrumental learning show that some fish are adept at learning to discriminate among different amino acids. The channel catfish is the best understood in this regard, and many studies demonstrate its ability to learn to discriminate among amino acids when presented individually or in mixtures (Valentinčič et al. 2000; Valentinčič 2005). Channel catfish can identify binary mixtures as unique entities and as the sum of their components. Other studies suggest that channel catfish can discriminate mixtures that contain up a half a dozen amino acids (Valentinčič 2005), an important ability in the natural world where feeding stimuli are complex. While two behavioral studies suggest these abilities are mediated by the olfactory system alone (Atema 1977; Valentinčič and Caprio 1994), another that used different techniques suggests that fish can learn to recognize single amino acids via their external gustatory system (Holland and Teeter 1981). Nevertheless, many young fish that have not fed before respond to individual amino acids with increased biting and swallowing, suggesting at least that the internal gustatory system is innately responsive (Jones 1992; Valentinčič and Caprio 1997; Hara 2007). Catfish also can learn to avoid eating palatable food items that have been previously associated with distasteful chemicals (Little 1977). Olfactory-blocked goldfish can be trained to preferentially acquire more nutritious food (Bitterman 1982) and to touch funnels through which amino acids are injected, although in the latter case, their sensitivity is reduced (Zippel et al. 1993). In summary, the roles of olfaction and gustation in feeding can differ with the former being more flexible. However, how experience modifies responsiveness to natural odors is incompletely known at present.
Finally, a few studies suggest that olfaction can mediate learning to a wide variety of relevant stimuli. For example, wild minnows recall the odor of predators more than 1 year after experiencing them (Smith 1992). Goldfish may be able to associate simple organic molecules, acids, and even steroidal sex pheromones with feeding (Zippel et al. 1993; Sorensen 2007).
Crustaceans
Crustaceans provide many interesting cases of experience-dependent plasticity and learning (Krasne 1973), including several varieties of chemosensory learning. These include habituation, classical and operant conditioning, aversive associative learning, and one-trial flavor avoidance learning (Abramson and Feinman 1988; Daniel and Derby 1988; Fine-Levy et al. 1988; Wight et al. 1990; Derby 2000; Shuranova et al. 2005). Such learning can influence the selection of food by crustaceans.
Learning is involved in identifying and remembering the odor of conspecifics, including individual recognition in crayfish, lobsters, hermit crabs, and mantis shrimp (Caldwell and Dingle 1985; Karavanich and Atema 1998; Breithaupt and Atema 2000; Hazlett 2003; Gherardi et al. 2005; Atema and Steinbach 2007). The formal nature of this learning has not been fully characterized, but memories of these odors can last for many days.
Experience can also influence selection of hosts by commensal crabs (Derby and Atema 1980). This ability might be useful for these crabs to relocate hosts after leaving them to mate.
The ability of spiny lobsters to learn food odors has been explored intensively, largely within the context of defining the neural basis of olfactory discrimination of mixtures (Derby 2000; Derby et al. 2001). Spiny lobsters are able to learn to associate food odors with danger and subsequently to avoid searching for those odors. Differential conditioning shows that spiny lobsters can learn to discriminate chemical mixtures that differ only in the ratios of their components. They can remember these odors and perform discrimination tasks for several days (Fine-Levy et al. 1988).
A form of plasticity in chemical communication in blue crabs has recently been demonstrated (Kamio et al. 2008). This is not experience-dependent plasticity but context-dependent plasticity. In this case, male crabs detect reproductive females, move toward them, and quickly pair-bond with them if they are immediately accessible. If they can sense but not grab the female, however, the males will perform a courtship display behavior that directs his pheromone toward her. This context-dependent chemical signaling is a form of behavioral plasticity that should enhance reproductive success.
Processing of Natural Chemical Stimuli
Many studies of neural processing in fish and crustaceans have used natural products and their components as chemical stimuli. Tested stimuli include single compounds of known biological importance and synthetic mixtures of these components in varying degrees of complexity (Derby 2000; Valentinčič 2005). Much is known about processing of individual food-related chemicals because their molecular identity is defined. Coding of natural products other than food has been less thoroughly examined for sex pheromones and chemical deterrents. Little work has been conducted on natural mixtures. In this section, we describe the organization of the chemosensory systems of fish and crustaceans, and how they code natural stimuli.
Fish
The olfactory and gustatory systems of fish are distinctly different senses that mediate different aspects of chemical recognition and drive different behaviors. Olfaction, not gustation, is used to identify and locate pheromones. On the other hand, both olfaction and gustation mediate aspects of feeding, though the specific roles of each appear to depend on the species, feeding experience, and other factors. Information from the olfactory and gustatory systems is probably integrated at higher neural levels, driving what to an outside observer might seem to be a single continuum of behaviors. Exactly where this occurs in the brain is not yet known. Here, we describe the neurobiology of these systems, elements of which are recently reviewed by Caprio and Derby (2007), with the aim of outlining how natural chemical mixtures are perceived by fish.
The basic neural structure of the fish olfactory sense is fundamentally similar to that of other vertebrates. It is comprised of three elements: olfactory receptor neurons (ORNs) located in the olfactory epithelium, glomeruli in the olfactory bulb into which ORNs converge, and output neurons (mitral cells) that convey information from the glomeruli to the forebrain where further processing occurs (Michel 2006; Caprio and Derby 2007). Fish have three major types of ORNs: ciliated, microvillar, and crypt cells. These cells express particular types of olfactory receptor molecules, second messengers, channels, and other molecules associated with transduction, suggesting that they have specific and different roles. Current evidence suggests that microvillar cells detect amino acids and that ciliated cells detect all other cues and perhaps some amino acids. Nevertheless, the coding of olfactory information commences with the olfactory receptors, which are seven-transmembrane G-protein linked proteins, and the receptor cells containing them (Caprio and Derby 2007; Saraiva and Korsching 2007). Although the process of receptor discovery is ongoing, fish species are thought to express over 100 members from three families of receptors (ORs, V1Rs, and V2Rs; Ngai et al. 1993; Saraiva and Korsching 2007). Of these, only one class has been functionally expressed to date. It detects amino acids, with a specific bias to arginine, and its activity can be predictably altered with site-directed mutations (Luu et al. 2004). The current view is that fish olfactory systems encode most chemical stimuli by using a combinatorial code, whereby particular attributes of molecules are discriminated by the activity of specific combinations of receptors, the pattern of which conveys identity of the molecules. Such a code should be both versatile and specific, but it would require neural mechanisms capable of encoding complex patterns of receptor binding—an attribute of the olfactory bulb. In addition to a combinational scheme for identifying general odors, such as those associated with food, a simpler scheme based on one or a few receptor types may be used to identify sex pheromones (Friedrich and Korsching 1998; Sorensen and Sato 2005).
All ORNs that share the same receptor type converge on the same glomeruli in the olfactory bulb, where the neural signal is enhanced and modulated by interneurons that extend from other glomeruli or by centrifugal input from higher levels of the central nervous system. Because glomeruli with similar chemosensitivity are located close to each other, the result is a systematic three-dimensional chemotopic map of chemical identities across the bulb (Friedrich and Korsching 1998; Nikonov and Caprio 2001). Odorants such as sex pheromones appear to be detected by fewer, specialized neurons (Friedrich and Korsching 1998), although electroencephalogram recordings from goldfish bulb suggest that these units receive significant inhibitory input (Hanson 2001), so they are not true ‘labeled lines.’ Indeed, glomeruli in the olfactory bulb are extensively interconnected, as mitral cells project to more than one glomerulus and local interneuronal connections are extensive. Thus, there is considerable potential for complex but orderly processing of mixture information, perhaps from multiple classes of odorants, in the fish olfactory bulb (Hara 1970), likely creating distinctive olfactory ‘fingerprints’ for natural odor sources including conspecifics, as occurs in the mouse (Schaefer et al. 2001)
Chemical information is passed from the olfactory bulb to the forebrain through a series of lateral and medial olfactory tracts whose functions have been studied in the carps by using single odorants, as described below. Behavioral and electrophysiological studies in the goldfish show that the medial tracts convey sex pheromone information (Stacey and Kyle 1983; Sorensen et al. 1991), and the lateral tracts convey amino acid and food odor information (Stacey and Kyle 1983; Hamdani et al. 2001). Responses to alarm cues appear to be conveyed by special parts of the medial tract in the closely related crucian carp (Hamdani et al. 2000). Interestingly, some amino acid information is also conveyed by the medial tracts in the goldfish (Sorensen et al. 1991), suggesting that amino acids might be perceived as part of the background body odor component of pheromones. The forebrain of catfish contains a chemotopic map (Nikonov et al. 2005). Connections from the forebrain to higher brain regions would allow integration of olfactory information with that from visual and other systems, including gustatory, as is required for driving multimodal behaviors such as feeding and mating. In summary, the olfactory system contains a network of connections that encodes the identities of complex odor mixtures and perhaps their concentrations in highly precise manners that should permit learning and memory. Tests of natural odors are now needed to ascertain the full potential of this system and whether it can explain all of the behaviors observed in wild animals to natural stimuli.
The anatomy and neural functions of the gustatory system in fish are remarkably different from the olfactory system except that the chemosensitivities of the two overlap for food stimuli. These differences emphasize what seem to be important distinctions in their respective roles in food procurement. Gustation is mediated by taste buds, groupings of receptor cells that are derived from epithelial tissue. These cells synapse onto primary gustatory neurons, with each neuron typically receiving input from cells in many buds. Several classes of genes for gustatory receptors have been cloned in fish (Ishimaru et al. 2005), and their chemosensitivity is beginning to be understood (Oike et al. 2007). Some taste cells express more than one type of receptor, which distinguishes them from olfactory cells (Ishimaru et al. 2005). The expression and connectivity properties of taste cells result in gustatory fibers that respond to multiple classes of chemical stimuli but still with specificity. Responses are concentration-dependent, and stimulus quality seems to be encoded by overall firing rate. There is no evidence of special processing of mixture information (Caprio and Derby 2007). Taste bud abundance and distribution vary enormously with fish species, especially for the external gustatory system (facial nerve; VII), which innervates the barbels (if present) and/or the lips, and projects to the highly structured facial lobe. Taste buds in the oropharyngeal cavities and gill arches of all fish are innervated by the vagal (X) and glossopharyngeal nerves (IX), which project to the vagal lobe and other regions of the hindbrain. This topographic mapping scheme appears to reflect an emphasis on processing the spatial distribution of chemical concentrations, an attribute correlated with catfish’s ability to track chemical plumes by using gustation alone (Bardach et al. 1967). Similar topographic maps are found in the carp vagal lobe and appear to mediate food sorting in the mouth (Finger and Morita 1985; Finger 2008). Swallowing and ingestion do not appear to be highly specific processes, as most detected amino acid will evoke the response (Caprio and Derby 2007).
In summary, the olfactory and gustatory systems are both designed to process natural chemical mixtures but in quite different manners. While olfaction is organized to adaptively integrate information associated with a wide range of chemical stimuli for various functions, gustation is specialized for sensitivity and localization of food source. Tests of the natural function of these neural systems in free-swimming animals to complex, natural stimuli await. It will be particularly important to examine function in species other than catfish, which are somewhat unusual in their possession of a highly developed and broadly tuned external gustatory system but for which sex pheromones are not yet available for testing.
Crustaceans
A Diversity of Organs Mediating a Diversity of Behaviors
The chemical sensors of crustaceans differ in their sensillar organization, central connections, and behavioral role. One pathway that is conserved in many crustaceans is that of the aesthetasc sensilla and olfactory lobe. This is considered an ‘olfactory’ pathway because the aesthetascs are innervated only by chemosensory (and not mechanosensory) neurons and because of structural similarities with the insect and vertebrate olfactory pathways (Schachtner et al. 2005). All other sensilla that contain chemosensory neurons also contain mechanosensory neurons. But these bi-modal chemosensilla are extremely diverse in structure, are distributed differently across the animal’s body surface, and serve different behavioral functions. This suggests that crustacean chemoreceptors cannot be categorized according to a dichotomous olfactory–gustatory classification.
Functions of Different Chemosensory Pathways
Together, the diverse chemosensors across the body of crustaceans gather information about conspecifics, predators, attractive and defensive properties of prey, and more. These sensors connect differentially within the central nervous system, leading to the control of different aspects of behavior. The olfactory (aesthetasc) and gustatory pathways differ in the behaviors controlled. In spiny lobsters, the antennules drive detection of and orientation to distant, attractive chemicals, including food-related chemicals and intraspecific chemicals such as aggregation or alarm cues (Derby et al. 2001; Horner et al. 2004, 2008b; Shabani et al. 2006). In blue crabs, however, leg chemoreceptors can play a major role in mediating orientation to distant odors, as the wide spacing between legs on the animal’s two sides provides spatial information that helps the crabs locate the plume’s boundaries (Keller and Weissburg 2004). In lobsters, chemosensors on the legs of lobsters are used to locate food once the animal has reached the source (Derby and Atema 1982). Mouthpart chemoreceptors then assess the food for phagostimulants and phagodeterrents that lead to its ingestion or rejection (Derby and Atema 1982; Derby et al. 2001; Garm et al. 2005; Kicklighter et al. 2005; Garm and Høeg 2006).
The antennules are the most intensively studied of all of the crustacean chemosensory pathways. The two major antennular chemosensory pathways—the aesthetasc/olfactory lobe pathway and non-aesthetasc/lateral antennular lobe pathway—of spiny lobsters have some functional redundancy: Both assess the quality of food, both mediate olfactory learning, and both enable orientation to distant food odors (Derby et al. 2001; Steullet et al. 2001, 2002; Horner et al. 2004). Unique functions of the aesthetasc pathway include encoding intraspecific cues used in sexual and social interactions (Gleeson 1982, 1991; Johnson and Atema 2005; Horner et al. 2008a, b). Unique functions of the non-aesthetasc pathway include mediating antennular sensory-motor tasks. One example is the class of non-aesthetasc sensilla—the asymmetric sensilla—that are necessary and sufficient for mediating chemically evoked antennular grooming behavior (Schmidt and Derby 2005). Detectors of pheromones and social cues are not exclusively located in the aesthetasc/olfactory pathway, however. For example, male crayfish have sensors on their claws that detect female odors (Belanger and Moore 2006).
How are mixtures coded by the peripheral olfactory system of decapod crustaceans? Information about different classes of chemicals is carried by different types of chemoreceptor neurons (CRNs). CRNs have different specificities, with some being most sensitive to one or several amino acids, others most sensitive to one or several adenine nucleotides, others most sensitive to ammonium ions, and so on (Voigt and Atema 1992; Derby 2000; Garm et al. 2005). Single CRNs can be stimulated by compounds that belong to different classes of molecules. For example, taurine-best CRNs can also be sensitive to other amino acids, AMP, ammonium, and other compounds, although the thresholds for taurine are 100 to 10,000 times lower than other stimuli (Cromarty and Derby 1997). Stimulus quantity, at least for food-related chemicals and their mixtures, is coded by CRN population responses or across-fiber patterns rather than labeled lines (Derby 2000; Caprio and Derby 2007). Such codes enable discrimination of highly similar mixtures, such as binary mixtures or 30+ component mixtures that contain the same components but at different blend ratios (Steullet and Derby 1997; Derby 2000; Derby et al. 2001).
Our understanding of how crustaceans process sex pheromones, social cues, anti-feeding compounds, and other chemical stimuli besides feeding stimulants is in its infancy. Not knowing the identity of these bioactive molecules is a major reason, and such identification is urgently needed. One example of how chemical defenses are processed is a set of recent studies on spiny lobsters’ responses to defensive secretions of sea hares (Kicklighter et al. 2005; Kamio et al. 2007). The studies suggest that these secretions function by ‘phagomimicry,’ in which the defensive secretion stimulates the feeding pathway to deceive spiny lobsters into attending to a false food stimulus, and by sensory disruption, in which the sticky and potent secretions cause high-amplitude, long-lasting sensory stimulation. Distasteful feeding deterrents also occur in these secretions, although their identity and neural processing are just now being studied (Kamio et al. 2007). Thus, chemical defenses may act in complex ways, even against a single predatory species.
General Conclusions and Future Directions
The chemical milieu of aquatic environments shapes how animals living there perceive their chemical world. Our paper discussed the chemosensory abilities of two major groups of aquatic animals—fish and crustaceans—within the context of the chemical ecology of the aquatic environment. Fish and crustaceans use chemoreception in many of their inter- and intraspecific interactions, including identifying and locating high-quality food, mates, and suitable habitat and shelters, avoiding predators and low-quality or toxic food, and interacting with conspecifics in social and agonistic encounters. The compounds that mediate these interactions are diverse but typically are mixtures of small, water-soluble compounds. The responses of many fish and crustaceans to some chemicals are somewhat hard wired, but others, particularly for species that live in complex and diverse habitats, and may live for decades, show enormous plasticity. Aquatic animals have a diversity of chemical senses to detect these chemicals, including but not limited to those that can be placed into the classical categories of ‘olfaction’ and ‘gustation.’ In general, olfactory systems are well adapted for discrimination of complex stimuli, and gustatory systems control proximate identification, location, and ingestion of palatable food and rejection of distasteful or toxic food.
A conceptualization of the approaches to studying the chemical ecology of chemoreception is presented in Fig. 1. Individual disciplines can be used to make important contributions. For example, current techniques in analytical chemistry can determine what molecules are present in organisms and their environment and, thus, might serve as chemical cues or signals. Neuroscience brings molecular, cellular, and systems level analyses that can be used to ascertain what chemicals are detected, as well as mechanisms of reception and integration of these chemicals. Ethology allows evaluation of the behavioral relevance of detected chemicals. Evolutionary ecological approaches allow investigations of phylogenetic relationships, and how the habitat in which organisms live can influence the nature of their chemical senses. Reliance upon only one of these approaches can lead to, at best, partial understanding of a system and often leads to misunderstandings. We and others (e.g., Zimmer and Derby 2007; Zimmer and Zimmer 2008) advocate using multiple approaches and working at the intersection of these disciplines using the integrated approaches of neuroethology, neuroecology, and chemical ecology.
Although our understanding of neural processing of fish and crustaceans has advanced in recent years, we still have many challenges facing us. We have almost no information on how entire categories of chemicals are processed. For example, how pheromones and other intraspecific cues are processed by crustacean nervous systems is virtually unknown, and scant information exists for fishes. In the former case, this is largely a result of not knowing the molecules involved. In general, our knowledge of the identity of ecologically relevant chemicals is limited to a few contexts (especially feeding) and a few species (often the commercially important ones). However, with the advent of sensitive and sophisticated analytical chemical equipment and techniques, the challenge of identifying bioactive chemicals is not nearly so daunting, and we expect major advances to be made in this area.
Advances in molecular techniques also have given us tools to elucidate mechanisms of sensory processing. These tools have helped in identifying olfactory and gustatory receptors and other molecules involved in sensory transduction, especially in animals whose genomes have been sequenced, most notably zebrafish (Oike et al. 2007; Saraiya and Korsching 2007). The first crustacean with a sequenced genome is Daphnia pulex (Colbourne et al. 2005; see Daphnia Water Flea Genome Database at http://wfleabase.org), which provides a useful tool for studying the chemical senses of this and other crustaceans. Nonetheless, having a sequenced genome for a more established model organism, such as a decapod crustacean, will be a prized contribution. In the meantime, other molecular tools will have to suffice to identify molecules involved in chemical sensing (McClintock et al. 2006). Advances in methods of simultaneously recording from ensembles of neurons by using multielectrode arrays or imaging with voltage- and calcium dyes opens up exciting possibilities for understanding how sensory systems process and discriminate mixtures.
On a final note, we urge that mechanistic approaches to the study of chemical sensing be applied within the framework of chemical ecology and with an eye toward broader contributions of this type of work. What we learn about neural processing of ecologically and economically important species, such as salmon, lamprey, lobsters, and crabs, can and should have broader applications to be used by community and systems ecologists and fisheries scientists.
References
Abramson, C. I., and Feinman, R. D. 1988. Classical conditioning of the eye withdrawal reflex in the green crab. J. Neurosci 8:2907–2912.
Ache, B. W., and Young, J. M. 2005. Olfaction: diverse species, conserved principles. Neuron 48:417–430.
Appelt, C. A., and Sorensen, P. W. 2007. Female goldfish signal spawning readiness by altering when and where they release a urinary pheromone. Anim. Behav 74:1329–1338.
Archdale, M. V., and Anraku, K. 2005. Feeding behavior in Scyphozoa, Crustacea and Cephalopoda. Chem. Senses 30:i303–i304.
Arnesen, A. M., and Stabell, O. B. 1992. Behaviour of stream-dwelling brown trout towards odours present in home stream water. Chemoecology 3:94–100.
Asai, N., Fusetani, N., Matsunaga, S., and Sasaki, J. 2000. Sex pheromones of the hair crab Erimacrus isenbeckii. Part 1: Isolation and structures of novel ceramides. Tetrahedron 56:9895–9899.
Atema, J. 1977. Functional separation of smell and taste in fish and Crustacea, pp. 165–174, in J. Le Magnen, and P. Mac Leod (eds.). Olfaction and Taste VI. Information Retrieval, Paris.
Atema, J. 1988. Distribution of chemical stimuli, pp. 29–56, in J. Atema, R. R. Fay, A. N. Popper, and W. N. Tavolga (eds.). Sensory Biology of Aquatic Animals. Springer, New York.
Atema, J. 1996. Eddy chemotaxis and odor landscapes: exploration of nature with animal sensors. Biol. Bull 191:129–138.
Atema, J., and Steinbach, M. A. 2007. Chemical communication in the social behavior of the lobster, Homarus americanus, and other decapod Crustacea, pp. 115–144, in E. Duffy, and M. Thiel (eds.). Ecology and Evolution of Social Behavior: Crustaceans as Model Systems. Oxford University Press, Oxford, UK.
Atema, J., Holland, K., and Ikehara, W. 1980. Olfactory responses of yellowfin tuna (Thunnus albacares) are prey odors: chemical search image. J. Chem. Ecol 6:457–465.
Bardach, J. E., Todd, J. H., and Crickmer, R. 1967. Orientation by taste in fish of the genus Ictalurus. Science 155:1276–1278.
Behringer, D. C., Butler, M. J., and Shields, J. D. 2006. Avoidance of disease by social lobsters. Nature 441:421.
Belanger, R. M., and Moore, P. A. 2006. The use of the major chelae by reproductive male crayfish (Orconectes rusticus) for discrimination of female odours. Behaviour 143:713–731.
Berger, D. K., and Butler, M. J. 2001. Octopuses influence den selection by juvenile Caribbean spiny lobster. Mar. Freshw. Res 52:1049–1053.
Bitterman, M. E. 1982. Migration and learning in fishes, pp. 397–420, in J. D. McCleave, G. P. Arnold, J. J. Dodson, and W. Neill (eds.). Mechanisms of Migration in Fishes. Plenum, New York.
Bouwma, P. 2007. Should I stay or should I go? The ontogeny of spiny lobster responses to alarm odor cues. Abstract at the 2007 Benthic Ecology meeting.
Bouwma, P., and Hazlett, B. A. 2001. Integration of multiple predator cues by the crayfish Orconectes propinquus. Anim. Behav 61:771–776.
Breithaupt, T. 2001. The fan organs of crayfish enhance chemical information flow. Biol. Bull 200:150–154.
Breithaupt, T., and Atema, J. 2000. The timing of chemical signaling with urine in dominance fights of male lobsters (Homarus americanus). Behav. Ecol. Sociobiol 49:67–78.
Breithaupt, T., and Eger, P. 2002. Urine makes the difference: chemical communication in fighting crayfish made visible. J. Exp. Biol 205:1221–1231.
Briones-fourzán, P., and Lozano-álvarez, E. 2005. Seasonal variations in chemical response to conspecific scents in the spotted spiny lobster, Panulirus guttatus. N. Z. J. Mar. Freshw. Res 39:383–390.
Brown, G. E., Chivers, D. P., and Smith, R. J. F. 1993. Localized defecation by pike: a response to labeling by cyprinid alarm pheromone? Behav. Ecol. Sociobiol 36:105–110.
Brown, G. E., Adrian, J. C., Smyth, E., Leet, H., and Brennan, S. 2000. Ostariophysan alarm pheromones: laboratory and field tests of the functional significance of nitrogen oxides. J. Chem. Ecol 26:139–154.
Brown, G. E., Adrian, J. C., Naderi, N. T., Harvey, M. C., and Kelly, J. M. 2003. Nitrogen oxides elicit antipredator responses in juvenile catfish but not convict cichlids or rainbow trout: conservation of the ostariophysan alarm pheromone. J. Chem. Ecol 29:1781–1796.
Caldwell, R., and Dingle, J. 1985. A test of individual recognition in the stomatopod Gonodactylus festae. Anim. Behav 33:101–106.
Caprio, J., and Derby, C. D. 2007. Aquatic animal models in the study of chemoreception, in A. Basbaum, M. Bushnell, D. Smith, G. Beauchamp, S. Firestein, P. Dallos, D. Oertel, R. Masland, T. Albright, J. Kaas, and E. Gardner (eds.). The Senses: A Comprehensive Reference, Six-Volume Set. Elsevier, New York.
Caprio, J. C., Brand, J. B., Teeter, J. H., Valentinčič, T., Kalinoski, D. L., Kohbara, J., Kamazawa, T., and Wegert, S. 1993. The taste system of the channel catfish: from biophysics to behavior. Trends Neurosci 16:192–197.
Carr, W. E. S. 1982. Chemical stimulation of feeding behavior, pp. 259–274, in T. J. Hara (ed.). Chemoreception in Fishes. Elsevier, New York.
Carr, W. E. S. 1988. The molecular nature of chemical stimuli in the aquatic environment, pp. 3–27, in J. Atema, R. R. Fay, A. N. Popper, and W. N. Tavolga (eds.). Sensory Biology of Aquatic Animals. Springer, New York.
Carr, W. E. S., Netherton, J. C. III, Gleeson, R. A., and Derby, C. D. 1996. Stimulants of feeding behavior in fish: analyses of tissues of diverse marine organisms. Biol. Bull 190:149–160.
Caskey, J. L., and Bauer, R. T. 2005. Behavioral tests for a possible contact pheromone in the caridean shrimp Palaemonetes pugio. J. Crustac. Biol 25:571–576.
Cohen, S. A. P., Hatt, H., Kubanek, J., and Mccarty, N. A. 2008. Reconstitution of a chemical defense signaling pathway in a heterologous system. J. Exp. Biol 211:599–605.
Colbourne, J. K., Singan, V. R., and Gilbert, D. G. 2005. wFleaBase: the Daphnia genome database. BMC Bioinformatics 6:45.
Corotto, F. S., Mckelvey, M. J., Parvin, E. A., Rogers, J. L., and Williams, J. M. 2007. Behavioral responses of the crayfish Procambarus clarkii to single chemosensory stimuli. J. Crustac. Biol 27:24–29.
Cromarty, S. I., and Derby, C. D. 1997. Multiple receptor types on individual excitatory olfactory neurons: implications for coding of mixtures in the spiny lobster. J. Comp. Physiol. A 180:481–492.
Daniel, P. C., and Derby, C. D. 1988. Behavioral olfactory discrimination of mixtures in the spiny lobster (Panulirus argus) based on a habituation paradigm. Chem. Senses 13:385–395.
Daviss, B. 2005. Growing pains for metabolomics. Scientist 19:25–28.
Derby, C. D. 2000. Learning from spiny lobsters about chemosensory coding of mixtures. Physiol. Behav 69:203–209.
Derby, C. D., and Atema, J. 1980. Induced host odor attraction in the pea crab Pinnotheres maculatus. Biol. Bull 158:26–33.
Derby, C. D., and Atema, J. 1982. The function of chemo- and mechanoreceptors in lobster (Homarus americanus) feeding behaviour. J. Exp. Biol 98:317–327.
Derby, C. D., and Atema, J. 1988. Chemoreceptor cells in aquatic invertebrates: peripheral mechanisms of chemical signal processing in decapod crustaceans, pp. 365–385, in J. Atema, R. R. Fay, A. N. Popper, and W. N. Tavolga (eds.). Sensory Biology of Aquatic Animals. Springer, New York.
Derby, C. D., Steullet, P., Horner, A. J., and Cate, H. S. 2001. The sensory basis to feeding behavior in the Caribbean spiny lobster Panulirus argus. Mar. Freshw. Res 52:339–1350.
Derby, C. D., Kicklighter, C. E., Johnson, P. M., and Zhang, X. 2007. Chemical composition of inks of diverse marine molluscs suggests convergent chemical defenses. J. Chem. Ecol 33:1105–1113.
Dittman, A. D., Quinn, T. P., and Nevitt, G. A. 1996. Timing of imprinting to natural and artificial odors by coho salmon (Oncorhynchus kisutch). Can. J. Fish Aquat. Sci 53:434–442.
Dodson, J. J., and Bitterman, M. E. 1989. Compound uniqueness and the interactive role of morpholine in fish chemoreception. Biol. Behav 14:13–27.
Døving, K. B., Selset, R., and Thommesen, G. 1980. Olfactory sensitivity to bile acids in salmonid fishes. Acta Physiol. Scand 108:123–131.
Døving, K. B., Hamdani, E. H., Hoglund, E., Kasumyan, A., and Tuvikene, A. O. 2005. Review of the chemical and physiological basis of alarm reactions in cyprinids, pp. 131–163, in G. von der Emde, J. Mogdans, and B. G. Kapoor (eds.). Senses of Fish. Narosa, New Delhi.
Dreanno, C., Matsumura, K., Dohmae, N., Takio, K., Hirota, H., Kirby, R. R., and Clare, A. S. 2006a. An a2-macroglobulin-like protein is the cue to gregarious settlement of the barnacle Balanus amphitrite. Proc. Natl. Acad. Sci. U.S.A 103:14396–14401.
Dreanno, C., Kirby, R. R., and Clare, A. S. 2006b. Smelly feet are not always a bad thing: the relationship between cyprid footprint protein and the barnacle settlement pheromone. Biol. Lett 2:423–425.
Eisthen, H. L. 2002. Why are olfactory systems of different animals so similar? Brain Behav. Evol 59:273–293.
Ekerholm, M., and Hallberg, E. 2005. Primer and short-range releaser pheromone properties of pre-moult female urine from the shore crab Carcinus maenas. J. Chem. Ecol 31:1845–1864.
Fine, J. M., Vrieze, L. A., and Sorensen, P. W. 2004. Evidence that petromyzontid lampreys employ a common migratory pheromone that is partially comprised of bile acids. J. Chem. Ecol 30:2091–2110.
Fine-levy, J. B., Girardot, M.-N., Derby, C. D., and Daniel, P. C. 1988. Differential associative conditioning and olfactory discrimination in the spiny lobster Panulirus argus. Behav. Neural Biol 49:15–331.
Finger, T. A. 2008. Sorting food from stones: the vagal taste system in goldfish Carassius auratus. J. Comp. Physiol. A 194:135–143.
Finger, T. A., and Morita, Y. 1985. Two gustatory system: facial and vagal gustatory nuclei have different brainstem connections. Science 227:776–778.
Friedrich, R. W., and Korsching, S. I. 1998. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J. Neurosci 18:9977–9900.
Garm, A., and Høeg, J. T. 2006. Ultrastructure and functional organization of mouthpart sensory setae of the spiny lobster Panulirus argus: new features of putative mechanoreceptors. J. Morphol 267:464–476.
Garm, A., Shabani, S., Høeg, J. T., and Derby, C. D. 2005. Chemosensory neurons in the mouthparts of the spiny lobsters Panulirus argus and Panulirus interruptus (Crustacea: Decapoda). J. Exp. Mar. Biol. Ecol 314:175–186.
Gherardi, F., Tricarico, E., and Atema, J. 2005. Unraveling the nature of individual recognition by odor in hermit crabs. J. Chem. Ecol 31:2877–2896.
Gleeson, R. A. 1982. Morphological and behavioral identification of the sensory structures mediating pheromone reception in the blue crab Callinectes sapidus. Biol. Bull 163:162–171.
Gleeson, R. A. 1991. Intrinsic factors mediating pheromone communication in the blue crab, Callinectes sapidus, pp. 17–32, in R. T. Bauer, and J. W. Martin (eds.). Crustacean Sexual Biology. Columbia University Press, New York.
Grasso, F. W., and Basil, J. A. 2002. How lobsters, crayfishes, and crabs locate sources of odor: current perspectives and future directions. Curr. Opin. Neurobiol 12:721–727.
Grove, M. W., and Woodin, S. A. 1996. Conspecific recognition and host choice in a pea crab, Pinnixa chaetopterana (Brachyura: Pinnotheridae). Biol. Bull 190:359–366.
Gunning, G. E. 1959. The sensory basis of homing in the longear sunfish, Lepomis megalotis megalotis (Rafinsque). Invest. Indiana Lakes Streams 5:103–130.
Hamdani, E. H., Stabell, O. B., Alexander, G., and Døving, K. B. 2000. Alarm reaction in the crucian carp is mediated by the medial bundle of the medial olfactory tract. Chem. Senses 25:103–109.
Hamdani, E. H., Alexander, G., and Døving, K. B. 2001. Projection of sensory neurons with microvilli to the lateral olfactory tract indicates their participation in feeding behaviour in the crucian carp. Chem. Senses 26:1134–1144.
Hanson, L. H. 2001. Coding of pheromonal information in the goldfish olfactory bulb. Ph.D. dissertation, University of Minnesota.
Hara, T. J. 1970. An electrophysiological basis for olfactory discrimination in homing salmon: a review. J. Fish. Res. Board Can 27:565–586.
Hara, T. J. 1994. The diversity of chemical stimulation in fish olfaction and gustation. Rev. Fish Biol. Fish. 4:1–35.
Hara, T. J. 2007. Gustation, pp. 45–96, in T. J. B. S. HaraZielinski (ed.). Fish Physiology Series (A. P. Farrell and C. J. Brauner, eds.). Sensory Systems Neuroscience. Elsevier, New York.
Hara, T. J., Macdonald, S., Evans, R. E., Marui, T., and Arai, S. 1984. Morpholine, bile acids, and skin mucous as possible chemical cues in salmonid homing: electrophysiological re-evaluation, pp. 363–378, in J. D. McCleave, G. P. Arnold, J. J. Dodson, and W. Neill (eds.). Mechanisms of Migration in Fishes. Plenum, New York.
Hardege, J. D., Jennings, A., Hayden, D., Muller, C. T., Pascoe, D., Bentley, M. G., and Clare, A. S. 2002. Novel behavioural assay and partial purification of a female-derived sex pheromone in Carcinus maenas. Mar. Ecol. Prog. Ser 244:179–189.
Hasler, A. D., and Wisby, W. J. 1951. Discrimination of stream odors by fishes and relation to parent stream behavior. Am. Nat 85:223–238.
Hasler, A. D., and Scholz, A. T. 1983. Olfactory Imprinting and Homing in Salmon. Investigations in the Mechanism of the Imprinting Process. p. 134. Springer, Berlin.
Hay, M. E. 1996. Marine chemical ecology: what’s known and what’s next? J. Exp. Mar. Biol. Ecol 200:103–134.
Hayden, D., Jennings, A., Müller, C., Pascoe, D., Bublitz, R., Webb, H., Breithaupt, T., Watkins, L., and Hardege, J. 2007. Sex-specific mediation of foraging in the shore crab, Carcinus maenas. Horm. Behav 52:162–168.
Hazlett, B. A. 1994. Alarm responses in the crayfish Orconectes virilis and Orconectes propinquus. J. Chem. Ecol 20:1525–1535.
Hazlett, B. A. 2003. Predator recognition and learned irrelevance in the crayfish Orconectes virilis. Ethology 109:765–780.
Hildebrand, J. G., and Shepherd, G. M. 1997. Mechanisms of olfactory discrimination: converging evidence for common principals across phyla. Annu. Rev. Neurosci 20:595–561.
Holland, K. M., and Teeter, J. H. 1981. Behavioral and cardiac reflex assays of the chemosensory acuity of channel catfish to amino acids. Physiol. Behav 27:699–707.
Horner, A. J., Weissburg, M. J., and Derby, C. D. 2004. Dual chemosensory pathways can mediate orientation of spiny lobsters to distant food odors. J. Exp. Biol 207:3785–3796.
Horner, A. J., Nickles, S. P., Weissburg, M. J., and Derby, C. D. 2006. Source and specificity of chemical cues mediating shelter preference of Caribbean spiny lobsters (Panulirus argus). Biol. Bull 211:128–139.
Horner, A. J., Schmidt, M., Edwards, D. H., and Derby, C. D. 2008a. Role of the olfactory pathway in agonistic behavior of crayfish Procambarus clarkii. Invertebr. Neurosci 8:11–18.
Horner, A. J., Weissburg, M. J., and Derby, C. D. 2008b. The olfactory pathway mediates sheltering behavior of Caribbean spiny lobsters, Panulirus argus, to conspecific urine signals. J. Comp. Physiol. A 194:243–253.
Ishimaru, Y., Okada, S., Naito, H., Nagai, T., Yasuoka, A., Matsumoto, I., and Abe, K. 2005. Two families of candidate taste receptors in fishes. Mech. Dev 122:1310–1321.
Johnson, M. E., and Atema, J. 2005. The olfactory pathway for individual recognition in the American lobster Homarus americanus. J. Exp. Biol 208:2865–2872.
Jones, K. A. 1992. Food search and behaviour in fish and the use of chemical lures in commercial and sports fishing, pp. 288–320, in T. J. Hara (ed.). Fish Chemoreception. Chapman and Hall, New York.
Kamio, M., Matsunaga, S., and Fusetani, N. 2002. Copulation pheromone in the crab Telmessus cheiragonus (Brachyura: Decapoda). Mar. Ecol. Prog. Ser 234:183–190.
Kamio, M., Kubanek, J., and Derby, C. 2006. N-Acetylglucosamino-1,5-lactone is a candidate sex pheromone in female blue crabs. Chem. Senses 31:A82.
Kamio, M., Kicklighter, C., Ko, K.-C., Nusnbaum, M., Aggio, J., Hutchins, M., and Derby, C. 2007. Defense through chemoreception: an l-amino acid oxidase in the ink of sea hares deters predators through their chemical senses. Chem. Senses 32:A37.
Kamio, M., Reidenbach, M. A., and Derby, C. D. 2008. To paddle or not: context dependent courtship display by male blue crabs, Callinectes sapidus. J. Exp. Biol 211:1243–1248.
Kapoor, B. G., and Finger, T. E. 2003. Taste and solitary chemoreceptor cells, pp. 753–769, in G. Arratia, B. G. Kapoor, M. Chardon, and R. Diogo (eds.). Catfishes, vol. 2. Science, Enfield, New Hampshire.
Karavanich, C., and Atema, J. 1998. Individual recognition and memory in lobster dominance. Anim. Behav 56:1553–1560.
Keller, T. A., and Weissburg, M. J. 2004. Effects of odor flux and pulse rate on chemosensory tracking in turbulent odor plumes by the blue crab, Callinectes sapidus. Biol. Bull 207:44–55.
Kicklighter, C. E., Shabani, S., Johnson, P. M., and Derby, C. D. 2005. Sea hares use novel antipredatory chemical defenses. Curr. Biol 15:549–554.
Kobayashi, M., Sorensen, P. W., and Stacey, N. E. 2002. Hormonal and pheromonal control of spawning in goldfish. Fish Physiol. Biochem 26:71–84.
Krasne, F. B. 1973. Learning in Crustacea, pp. 49–130, in W. C. Corning, J. A. Dyal, and A. O. D. Willows (eds.). Invertebrate Learning. Vol. 2: Arthropods and Gastropod Mollusks. Plenum, New York.
Kupchan, S. M., Britton, R. W., Lacadie, J. A., Ziegler, M. F., and Sigel, C. W. 1975. The isolation and structural elucidation of bruceantin and cruceantinol. J. Org. Chem 40:648–654.
Lane, A. L., and Kubanek, J. 2006. Structure–activity relationship of chemical defenses from the freshwater plant Micranthemum umbrosum. Phytochemistry 67:1224–1231.
Li, W., Scott, A. P., Siefkas, M. J., Yan, H., Liu, Q., Yun, S.-S., and Gage, D. A. 2002. Bile acid secreted by male sea lamprey that acts as sex pheromone. Science 296:138–141.
Little, E. E. 1975. Chemical communication in maternal behaviour of crayfish. Nature 255:400–401.
Little, E. E. 1976. Ontogeny of maternal behavior and brood pheromone in crayfish. J. Comp. Physiol. A 112:133–142.
Little, E. E. 1977. Conditioned aversion to amino acid flavors in the catfish, Ictalurus punctatus. Physiol. Behav 19:743–747.
Luu, P., Achner, F., Bertrand, H.-O., Fan, J., and Ngai, J. 2004. Molecular determinants of ligand selectivity in a vertebrate odorant receptor. J. Neurosci 24:10128–10137.
Mackie, A. M. 1982. Identification of the gustatory feeding stimulants, pp. 275–291, in T. J. Hara (ed.). Chemoreception in Fishes. Elsevier, New York.
Maniak, P. J., Lossing, R., and Sorensen, P. W. 2000. Injured Eurasian ruffe, Gymnocephalus cernuss, release an alarm pheromone which may prove useful in their control. J. Great Lakes Res 26:183–195.
Mcclintock, T. S., Ache, B. W., and Derby, C. D. 2006. Lobster olfactory genomics. Integr. Comp. Biol 46:940–947.
Michel, W. C. 2006. Chemoreception, pp. 471–497, in D. H. Evans, and J. B. Claiborne (eds.). The Physiology of Fishes. 3rd edn.CRC, Boca Raton, FL.
Moore, P. A. 2007. Agonistic behavior in freshwater crayfish: the influence of intrinsic and extrinsic factors on aggressive behavior and dominance, pp. 90–114, in J. E. Duffy, and M. Thiel (eds.). Evolutionary Ecology of Social and Sexual Systems: Crustacea as Model Organisms. Oxford University Press, Oxford, UK.
Moore, P. A., and Bergman, D. A. 2005. The smell of success and failure: the role of intrinsic and extrinsic chemical signals on the social behavior of crayfish. Integr. Comp. Biol 45:650–657.
Moyle, P. B., and Cech, J. J. 2000. Fishes: An Introduction to Ichthyology. 3rd edn.Prentice Hall, New York.
Nevitt, G., Pentcheff, N. D., Lohmann, K. J., and Zimmer, R. K. 2000. Den selection by the spiny lobster Panulirus argus: testing attraction to conspecific odors in the field. Mar. Ecol. Prog. Ser 203:225–231.
Nevitt, G. A., Dittman, A. D., Quinn, T. P., and Moody, W. J. 2004. Evidence for a peripheral olfactory memory in imprinted salmon. Proc. Nat Acad. Sci. U.S.A. 91:4288–4292.
Ngai, J., Dowling, M. M., Buck, L., Axel, R., and Chess, A. 1993. The family of genes encoding odorant receptors in the channel catfish. Cell 72:657–666.
Nikonov, A. A., and Caprio, J. 2001. Electrophysiological evidence for a chemotopy of naturally relevant odors in the channel catfish. J. Neurophysiol 86:1869–1876.
Nikonov, A. A., Finger, T. A., and Caprio, J. 2005. Beyond the olfactory bulb: an odotopic map in the forebrain. Proc. Natl. Acad. Sci. U.S.A 102:18688–18693.
Nordeng, H. 1977. A pheromone hypothesis for homeward migration in anadromous salmonids. Nature 233:411–413.
Oike, H., Nagai, T., Furuyama, A., Okada, S., Aihara, Y., Ishimaru, Y., Marui, T., Matsumoto, I., Misaka, T., and Abe, K. 2007. Characterization of ligands for fish taste receptors. J. Neurosci 27:5584–5592.
Parker, J. D., Burkepile, D. E., Collins, D. O., Kubanek, J., and Hay, M. E. 2007. Stream mosses as chemically-defended refugia for freshwater macroinvertebrates. Oikos 116:302–312.
Pfeiffer, W., Riegelbauer, G., Meier, G., and Scheibler, B. 1985. Effect of hypoxanthine-3-N-oxide on central nervous excitation of the black tetra, Gymnocorymbus ternetzi (Characidae, Ostariophysi, Pisces) indicated by dorsal light response. J. Chem. Ecol 11:507–523.
Poling, K. R., Fraser, E. J., and Sorensen, P. W. 2001. The three steroidal components of the goldfish preovulatory pheromone signal evoke different behaviors in males. Comp. Biochem. Physiol 129B:645–651.
Prusak, A. C., O’neal, J., and Kubanek, J. 2005. Prevalence of chemical defenses among freshwater plants. J. Chem. Ecol 31:1145–1160.
Reusch, T. B., Haberli, M. A., Aeschlimann, P. B., and Milinski, M. 2002. Female sticklebacks in a strategy of sexual selection explaining MHC polymorphism. Nature 414:300–302.
Rittschof, D., and Cohen, J. H. 2004. Crustacean peptide and peptide-like pheromones and kairomones. Peptides 25:1503–1516.
Rittschof, D., Tsai, D. W., Massey, P. G., Blanco, L., Kueber, G. L., and Haas, R. J. 1992. Chemical mediation of behavior in hermit crabs: alarm and aggregation cues. J. Chem. Ecol 18:959–984.
Robertson, J. R., Fudge, J. A., and Vermeer, G. K. 1981. Chemical and live feeding stimulants of the sand fiddler crab, Uca pugilator (Bose). J. Exp. Mar. Biol. Ecol 53:47–64.
Rolen, S. H., Sorensen, P. W., Mattson, D., and Caprio, J. 2003. Polyamines as olfactory stimuli in the goldfish, Carassius auratus. J. Exp. Biol 206:1683–1696.
Saraiva, L. R., and Korsching, S. I. 2007. A novel olfactory receptor gene family in teleost fish. Genome Res 17:1448–1457.
Schachtner, J., Schmidt, M., and Homberg, U. 2005. Organization and evolutionary trends of primary olfactory brain centers in Tetraconata (Crustacea plus Hexapoda). Arthropod. Struct. Dev 34:257–299.
Schaefer, M. L., Young, D. A., and Restrepo, D. 2001. Olfactory fingerprints for major histocompatibility body odors. J. Neurosci 21:2481–2487.
Schmidt, M., and Derby, C. D. 2005. Non-olfactory chemoreceptors in asymmetric setae activate antennular grooming behavior in the Caribbean spiny lobster Panulirus argus. J. Exp. Biol 208:233–248.
Shabani, S., Kamio, M., and Derby, C. D. 2006. Chemicals released by injured or disturbed conspecifics mediate defensive behaviors via the aesthetasc pathway in the spiny lobster Panulirus argus. Chem. Senses 31:A81–A82.
Shoji, T., Yamamoto, Y., Nishikawa, D., Kurihara, K., and Ueda, H. 2003. Amino acids in stream waters are essential for salmon homing migration. Fish Physiol. Biochem 28:249–251.
Shuranova, Z., Burmistrov, Y., and Abramson, C. I. 2005. Habituation to a novel environment in the crayfish (Procambarus cubensis). J. Crustac. Biol 25:488–494.
Smith, R. J. F. 1992. Alarm signals in fish. Rev. Fish Biol. Fish 2:33–63.
Sorensen, P. W. 2007. Goldfish can be conditioned to respond to a sex pheromone. Chem. Senses 32:A19.
Sorensen, P. W., and Scott, A. P. 1994. The evolution of hormonal sex pheromones in teleost fish: poor correlation between the pattern of steroid release by goldfish and olfactory sensitivity suggests that these cues evolved as a result of chemical spying rather than signal specialization. Acta Scand. Physiol 152:191–205.
Sorensen, P. W., and Caprio, J. 1998. Chemoreception in fish, pp. 375–406, in R. E. Evans (ed.). The Physiology of Fishes. 2nd edn.CRC, Florida.
Sorensen, P. W., and Stacey, N. E. 1999. Evolution and specialization in fish hormonal pheromones, pp. 15–48, in R. E. Johnston, D. Müller-Schwarze, and P. W. Sorensen (eds.). Advances in Chemical Signals in Vertebrates. Plenum, New York.
Sorensen, P. W., and Sato, K. 2005. Second messenger systems mediating sex pheromone and amino acid sensitivity in goldfish olfactory receptor neurons. Chem. Senses 30:Suppl. 1315–316.
Sorensen, P. W., and Hoye, T. R. 2007. A critical review of the discovery and application of a migratory pheromone in an invasive fish, the sea lamprey Petromyzon marinus L. J. Fish Biol 71:100–114.
Sorensen, P. W., Hara, T. J., Stacey, N. E., and Goetz, F. W. 1988. F prostaglandins function as potent olfactory stimulants that comprise the postovulatory female sex pheromone in goldfish. Biol. Reprod. 39:1039–1050.
Sorensen, P. W., Hara, T. J., Stacey, N. E., and Dulka, J. G. 1990. Extreme olfactory specificity of male goldfish to the preovulatory pheromone 17a,20b-dihydroxy-4-pregnen-3-one. J. Comp. Physiol. A 166:373–385.
Sorensen, P. W., Hara, T. J., and Stacey, N. E. 1991. Sex pheromones selectively stimulate the medial olfactory tracts of male goldfish. Brain Res 558:343–347.
Sorensen, P. W., Scott, A. P., Stacey, N. E., and Bowdin, L. 1995. Sulfated 17,20b-dihydroxy-4-pregnen-3-one functions as a potent and specific olfactory stimulant with pheromonal actions in the goldfish. Gen. Comp. Endocrinol 100:128–142.
Sorensen, P. W., Scott, A. P., and Kihslinger, R. L. 2000. How common hormonal metabolites function as specific pheromones in the goldfish, pp. 125–129, in B. Norberg, O. S. Kjesbu, G. L. Taranger, E. Andersson, and S. O. Stefansson (eds.). Proceedings of the Sixth International Symposium on the Reproductive Physiology of Fish. Bergen, Norway.
Sorensen, P. W., Vrieze, L. A., and Fine, J. 2003. A multicomponent migratory pheromone in the sea lamprey. Fish Physiol. Biochem 28:253–257.
Sorensen, P. W., Fine, J. M., Dvornikovs, V., Jeffrey, C. S., Shao, F., Wang, J., Vrieze, L. A., Anderson, K. R., and Hoye, T. R. 2005. Mixture of new sulfated steroids functions as a migratory pheromone in the sea lamprey. Nat. Chem. Biol 1:324–328.
Stacey, N. E., and Kyle, A. L. 1983. Effects of olfactory tract lesions on sexual and feeding behavior in the goldfish. Physiol. Behav 30:621–628.
Stacey, N. E., and Sorensen, P. W. 2005. Hormones, pheromones, and reproductive behaviors, pp. 359–412, in K. A. Sloman, S. Balshine, and R. W. Wilson (eds.). Behaviour: Interactions with Fish Physiology, vol. 24 in Fish Physiology (W. S. Hoar, D. J. Randall, and A. P. Farrell, series eds.). Academic, New York.
Stebbing, P. D., Bentley, M. G., and Watson, G. J. 2003. Mating behaviour and evidence for a female released courtship pheromone in the signal crayfish Pacifastacus leniusculus. J. Chem. Ecol 29:465–475.
Steullet, P., and Derby, C. D. 1997. Coding of blend ratios of binary mixtures by olfactory neurons in the Florida spiny lobster, Panulirus argus. J. Comp. Physiol. A 180:123–135.
Steullet, P., Dudar, O., Flavus, T., Zhou, M., and Derby, C. D. 2001. Selective ablation of antennular sensilla on the Caribbean spiny lobster Panulirus argus suggests that dual antennular chemosensory pathways mediate odorant activation of searching and localization of food. J. Exp. Biol 204:4259–4269.
Steullet, P., Kruetzfeldt, D. R., Hamidani, G., Flavus, T., Ngo, V., and Derby, C. D. 2002. Dual parallel antennular chemosensory pathways mediate odor-associative learning and odor discrimination in the Caribbean spiny lobster Panulirus argus. J. Exp. Biol 205:851–867.
Tankersley, R. A., Bullock, T. M., Forward, R. B., and Rittschof, D. 2002. Larval release behaviors in the blue crab Callinectes sapidus: role of chemical cues. J. Exp. Mar. Biol. Ecol 273:1–14.
Ting, J. H., and Snell, T. W. 2003. Purification and sequencing of a mate-recognition protein from the copepod Tigriopus japonicus. Mar. Biol 143:1–8.
Trott, T. J., and Robertson, J. R. 1984. Chemical stimulants of cheliped flexion behavior by the western Atlantic ghost crab Ocypode quadrata (Fabricius). J. Exp. Mar. Biol. Ecol 78:237–252.
Valentinčič, T. 2005. Taste and olfactory stimuli and behavior in fishes, pp. 65–85, in G. von der Emde, J. Mogdans, and B. G. Kapoor (eds.). Senses of FishNarosa, New Delhi.
Valentinčič, T. S., and Caprio, J. C. 1994. Consummatory behavior in intact and anosmic channel catfish Ictalurus punctatus to amino acids. Physiol. Behav 55:857–863.
Valentinčič, T. S., and Caprio, J. C. 1997. Visual and chemical release of feeding behavior in adult rainbow trout. Chem. Senses 22:375–382.
Valentinčič, T. S., Kralj, M., Stenovec, A., and Caprio, J. C. 2000. The behavioral detection of binary mixtures of amino acids and their individual components by catfish. J. Exp. Biol 203:3307–3317.
Voigt, R., and Atema, J. 1992. Tuning of chemoreceptor cells of the second antenna of the American lobster (Homarus americanus) with a comparison of four of its other chemoreceptor organs. J. Comp. Physiol. A 171:673–683.
Von frisch, K. 1938. Zur Psychologie des Fisch-schwarmes. Naturwissenschaften 226:601–606.
Ward, A. J. W., and Hart, P. J. B. 2002. The effects of kin and familiarity on interactions between fish. Fish & Fisheries 4:348–358.
Weissburg, M. J., and Zimmer-faust, R. K. 1991. Ontogeny versus phylogeny in determining patterns of chemoreception: initial studies with fiddler crabs. Biol. Bull 181:205–215.
Weissburg, M. J., Ferner, M. C., Pisut, D. P., and Smee, D. L. 2002. The ecology of chemically-mediated prey perception. J. Chem. Ecol 28:1933–1950.
Wight, K., Francis, L., and Eldridge, D. 1990. Food aversion learning by the hermit crab Pagurus granosimanus. Biol. Bull 178:205–209.
Wisenden, B. D. 2000. Olfactory assessment of predation risk in the aquatic environment. Phil. Trans R. Soc. Lond. B 355:1205–1208.
Wong, B. B. M., Fisher, H. S., and Rosenthal, G. G. 2005. Species recognition of male swordtails by chemical cues. Behav. Ecol 16:818–821.
Yambe, Y., Kitamura, S., Kamio, M., Yamada, M., Matsunaga, M., Fusetani, N., and Yamazaki, F. 2007. l-Kynurenine, an amino acid identified as a sex pheromone in the urine of ovulated female masu salmon. Proc. Nat. Acad. Sci. U.S.A 103:15370–15374.
Zimmer, R. K., and Butman, C. A. 2000. Chemical signaling processes in the marine environment. Biol. Bull 198:168–187.
Zimmer, R. K., and Derby, C. D. 2007. Biological Bulletin virtual symposium: the neuroecology of chemical defense. Biol. Bull 213:205–207.
Zimmer, R. K., and Zimmer, C. A. 2008. Dynamic scaling in chemical ecology. J. Chem. Ecol. this issue
Zimmer-faust, R. K. 1993. ATP: a potent prey attractant evoking carnivory. Limnol. Oceanogr 38:1271–1275.
Zimmer-faust, R. K., and Case, J. F. 1982a. Odors influencing foraging behavior of the California spiny lobster, Panulirus interruptus, and other decapod Crustacea. Mar. Behav. Physiol 9:35–58.
Zimmer-faust, R. K., and Case, J. F. 1982b. Organization of food search in the kelp crab, Pugettia producta (Randall). J. Exp. Mar. Biol. Ecol 57:237–255.
Zimmer-faust, R. K., Tyre, J. E., and Case, J. F. 1985. Chemical attraction causing aggregation in the spiny lobster, Panulirus interruptus (Randall), and its probable ecological significance. Biol. Bull 169:106–118.
Zippel, H. P., Voigt, R., Knaust, M., and Luan, Y. 1993. Spontaneous behaviour, training, and discrimination training in goldfish using chemosensory stimuli. J. Comp. Physiol. A 172:81–90.
Zulandt schneider, R. A., and Moore, P. A. 2000. Urine as a source of conspecific disturbance signals in the crayfish Procambarus clarkii. J. Exp. Biol 203:765–771.
Acknowledgment
We thank our many colleagues who have contributed to work in this field. We also thank our current funding sources for support during preparation of this review (NIH DC00312, NSF IBN-0614685, Legislative Citizen Commission for Minnesota Resources, and the Minnesota Agriculture Experiment Station).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Derby, C.D., Sorensen, P.W. Neural Processing, Perception, and Behavioral Responses to Natural Chemical Stimuli by Fish and Crustaceans. J Chem Ecol 34, 898–914 (2008). https://doi.org/10.1007/s10886-008-9489-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9489-0