Abstract
In social insects, cuticular hydrocarbons (CHCs) play a central role in nestmate recognition. CHCs have proved to be useful for identifying species and differentiating populations. In combination with CHCs, isoprenoid soldier defensive secretions (SDSs) have been previously used in some termite species for chemotaxonomic analyses. This study compared the levels of chemical variation within and among introduced (French) and native (U.S.) populations of the subterranean termite, Reticulitermes flavipes. Worker CHCs and soldier SDSs from termites collected from colonies in nine populations in Florida, Louisiana, and France were analyzed. Discriminant analyses revealed that both localities and populations can be distinguished by using the variation in CHC profiles. Principal component analyses of CHC profiles as well as the calculation of two distance parameters (Nei and Euclidean) revealed remarkable chemical homogeneity within and among French populations. These analyses also showed that the CHC profiles of French populations were closer to termite populations from Louisiana than to those from Florida. Of the six distinct SDS chemotypes, one was common to populations in France and Louisiana. The possibility that populations in France originated from Louisiana, and the potential causes and consequences of chemical homogeneity within introduced populations are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many arthropods have hydrocarbons on the surface of their cuticle (i.e., cuticular hydrocarbons or CHCs). Although the primary function of these CHCs is to provide protection against desiccation, many studies have demonstrated that CHCs also are essential in insect recognition and communication systems (Howard and Blomquist, 2005; Blomquist and Bagnères, 2010). Furthermore, CHCs have been shown to play a central role in the evolution and cohesion of insect societies (Howard, 1993). In social insects, colony members typically share a common chemical signature given by the overall proportion of hydrocarbons on the cuticle created by the admixture of individual profiles (Clément and Bagnères, 1998; Howard and Blomquist, 2005). Consequently, variation in cuticular hydrocarbons has been used to identify species (Bagnères and Wicker-Thomas, 2010) and also to differentiate populations (Haverty et al., 1990; Nowbahari et al., 1990).
In termites (Isoptera), CHCs have been studied extensively, in particular with respect to taxonomy (Howard et al., 1982; Bagnères et al., 1990; Kaib et al., 1991; Haverty et al., 1997, 2000; Clément et al., 2001; Page et al., 2002; Uva et al., 2004). Although several studies have suggested a genetic basis for CHC variation (Carlin and Hölldobler, 1986; Dronnet et al., 2006), other research has shown that environmental factors such as food, temperature, and social environment can affect the composition of CHC profiles (Florane et al., 2004; Dronnet et al., 2006). Although the processes underlying the production of chemical signatures are not well understood, analysis of these signatures has proved to be effective for termite classification. Qualitative differences in hydrocarbons can discriminate between species, whereas quantitative differences can discriminate between populations and colonies (Haverty et al., 1997; Page et al., 2002; Bagnères and Wicker-Thomas, 2010). Analysis of CHCs among Rhinotermitidae has helped to clarify the taxonomy of the Asian Reticulitermes (Takematsu and Yamaoka, 1999); the Australian Heterotermes and Coptotermes (Watson et al., 1989; Brown et al., 1990); the American Coptotermes and Reticulitermes (Bagnères et al., 1990; Haverty et al., 1991, 1996, 1997, 2000); and the European Reticulitermes (Clément et al., 2001).
In the Rhinotermitidae and Termitidae, soldiers have a frontal gland that secretes defensive compounds. These soldier defensive secretions (SDSs) are believed to play a role in defending colonies against predators and competitors (Zalkow et al., 1981), and they also may play a role in the production of primer pheromones in Reticulitermes (Henderson, 1998; Tarver et al., 2009). SDSs can be composed of alkanes, aldehydes, ketones, and terpenes, as well as more complex compounds (Quintana et al., 2003; Piskorski et al., 2007). They have often been studied in combination with CHC profiles as their composition differs geographically and among taxa (Bagnères et al., 1990), and they can be useful for identifying species (Bagnères et al., 1990; Haverty et al., 1996; Nelson et al., 2001, 2008; Clément et al., 2001; Piskorski et al., 2009).
The use of chemical compounds for taxonomy (chemotaxonomy) has been widely used and proved valuable in numerous termite species, particularly Reticulitermes. However, less attention has been paid to chemical variation within species, in particular with respect to invasive species (Haverty et al., 1990). This study analyzed both CHCs and SDSs within native and introduced populations of Reticulitermes flavipes (Kollar). In the United States, this termite is known as the eastern subterranean termite, but it has been introduced and established in other countries such as Canada, Chile, and Uruguay, as well as France and Germany (Clément et al., 2001; Austin et al., 2002, 2005; Su et al., 2006). For a long time, the introduced populations of France were considered as a European species, Reticulitermes santonensis (Feytaud, 1924). They are now considered to be introduced populations of R. flavipes on the basis of the homology of several mitochondrial and nuclear DNA sequences (Clément et al., 2001; Jenkins et al., 2001; Austin et al., 2002, 2005; Ye et al., 2004; Su et al., 2006), although the first correspondence between French and U.S. populations was revealed by chemical similarities by comparing CHCs and SDSs (Bagnères et al., 1990). This earlier study showed that one French populations had CHCs similar to those collected in Georgia (U.S.), but with some quantitative differences. Although the SDSs of French populations were clearly different from those of other European species, none of the SDS chemotypes in the Georgia population matched those of the French populations. Preliminary molecular genetic studies now have shown that populations in southeastern U.S. are closest to the French populations (Bagnères, 2006; Perdereau, 2010).
This study determined and analyzed both CHCs and SDSs from three introduced populations in France and six native populations in the southern U.S. The main aims were (1) to determine whether an analysis of the chemical variation permits the discrimination of geographical populations and localities, and (2) to evaluate the degree of chemical similarity and variability among native and introduced populations.
Methods and Materials
Field Collection and Sampling
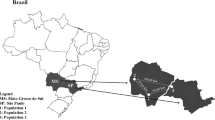
In the U.S., samples were collected in Florida and Louisiana as it has been suggested that populations in France may have originated from these areas (Bagnères, 2006; Perdereau, 2010). In Florida, 3 populations were collected from 12 collection points, 3 in the Blackwater River State Forest, 3 in the Wakulla State Forest, and 6 in the Osceola National Forest (Fig. 1). In Louisiana, 3 populations were collected from 16 collection points, 6 in New Orleans, 6 in Jean Lafitte National Historical Park and Preserve, and 4 in Baton Rouge (Fig. 1). In France, 3 introduced populations were taken from 43 collection points: 20 in the Forêt de Saint Trojan in the south of the Ile d’Oléron (Charente Maritime), 20 in the Forêt d’Olonnes (Vendée), and 3 in Tours (Indre et Loire) (Fig. 1). Termites from these 9 populations were collected from 2006 to 2009. To draw comparisons on a similar scale, the distance between each population did not exceed 200 km, and transects within each population were less than 2 km, except for the populations in Oléron and Olonnes, which were on a larger scale (4 km). Samples from the 9 populations studied were collected from wood fragments or tree stumps at the 9 localities. At least 20 workers were taken from each collection point. For these 71 samples, the species was determined by morphological and chemical identification for the French populations and DNA analysis for the U.S. populations as described previously (Clément et al., 2001; Austin et al., 2002).
Analyses of Cuticular Hydrocarbons
Twenty workers per collection point (71 in total with 12 from Florida, 16 from Louisiana and 43 from France) were pooled for chemical extraction. The CHCs in each pool were extracted by rinsing individuals in a non-polar solvent (hexane in the U.S. and pentane in France) for 5 min, and then samples were dried for transport and stored at −20°C until analysis (1 month at the latest). Before injection, extracts were re-dissolved in 200 μl of pentane with 10 μl of 10−7 g/ml of n-eicosane (n-C20) as an internal standard. Samples (2 μl) were analyzed by GC with a Delsi Nermag DN 200, flame ionization detection (FID) (Alpha MOS, Toulouse, France), and a fused silica capillary column CP Sil 5 (WCOT) (Varian Inc., Palo Alto, CA, USA) (i.d. 0.25 mm × 25 m × 0.12 μm). The injection mode was splitless (15 sec), and the carrier gas was helium at a linear flow rate of 1.5 cm/sec. The temperature was programmed from 70°C to 150°C at 30°C/min and held at 150°C for 5 min, and then raised to 320°C at 5°C/min. Compound identification was based on previously reported analyses of CHCs with GC-MS (Howard et al., 1978; Bagnères et al., 1990), and checked by us by injecting 2 μl of pooled sample of each population in a GC-MS (Hewlett Packard 5890 GC (Agilent Technologies, Santa Clara, CA, USA) coupled to a Hewlett Packard quadrupole 5889A MS (Agilent Technologies) in electron impact mode (70 eV)) with the same column and temperature program as noted above for GC-FID, with an interface temperature of 250°C. Eighteen main CHCs present in all individuals were selected for analysis: 9-tricosene (e1), x-tricosene (e2), n-tricosane (a3), 11-methyltricosane (m4), 4/2-methyltricosane (m7), 9-tetracosene (e8), 3-methyltricosane (m9), n-tetracosane (a11), 11-methyltetracosane (m12), 5-methyltetracosane (m14), 4/2-methyltetracosane (m16), 9-pentacosene (e17), pentacosadiene (n18), n-pentacosane (a19), 11 + 13-methyl pentacosane (m21), 7,9-pentacosadiene (n25), 4/2-methylpentacosane (m26), and 3-methylpentacosane (m29). The areas under these 18 peaks were integrated by using Galaxie v.1.8.508.1 (Varian), and the relative proportions of each peak were calculated as described by Bagnères et al. (1990). Discriminant analyses were performed by using Rgui v.2.10.1. to determine whether pre-defined groups (i.e., the 3 localities and the 9 populations) could be discriminated on the basis of their chemical profiles. This confirmed that the groups corresponded to the classification of the collection points. The same software was used to carry out principal component analysis (PCA) to determine the chemical relationship among the collection points.
The dissimilarity of hydrocarbon profiles among workers from different collection points was quantified by modifying Nei’s standard genetic distance (Nei, 1987) as previously described (Queller, 1993; Dronnet et al., 2006) and by using Euclidean distances. The Nei and Euclidean distances were based on the relative amounts of chemical compounds. For each distance, dissimilarity matrices were constructed for all possible pairs of collection points from the mean relative areas of the CHC peaks at different levels: within each population, among populations within each locality (France, Florida, and Louisiana) and among each pair of localities (France/Florida, France/Louisiana, and Florida/Louisiana). Nei and Euclidean distances vary between 0 and 1; 0 indicates that the chemical profiles are identical, whereas 1 indicates that there are no shared compounds. Non-parametric Kruskal-Wallis tests were used for multiple independent comparisons of populations, localities, and groups of localities. Dunn’s multiple comparison tests were carried out to define the specific difference between cuticular compound variations at each level of comparison by using XLSTAT v.2009.3.1.
Analyses of Soldier Defensive Secretions
Defensive compounds were extracted from 50 soldiers (13 from France, 17 from Florida, and 20 from Louisiana). Because SDSs are volatile compounds, a special method was used for extraction and transport. Each extraction was performed by plunging one soldier into 20 μl of solvent (pentane or hexane) in a conical glass insert for 2 min. Each extract then was transferred to a Transferpettor (BRAND GMBH + CO KG, Wertheim, Germany) cap (4 × 0.2 cm), one end of which was previously sealed. The other end of the glass cap was rapidly flame-sealed to avoid the evaporation of SDSs during transport to the laboratory. Two internal standards were used to check the extraction: one was added to the empty cap before extraction (n-octadecane) and the second was added just before injection (humulene). Two μl of each extract were analyzed by GC-MS with the instrumentation described above. The temperature program ran from 40°C to 200°C at 5°C/min and then increased at a rate of 8°C/min to 320°C with helium as carrier gas at a linear flow rate of 1.5 cm/sec. The chemotype of each sample then was determined according to the presence or absence of peaks on the GC traces. When present, determination of compounds was confirmed by comparing their retention times and mass spectra with data from previous studies on U.S. and French R. flavipes soldiers (Zalkow et al., 1981; Bagnères et al., 1990; Nelson et al., 2001).
Results
Proportions of Cuticular Hydrocarbons in French and American Populations
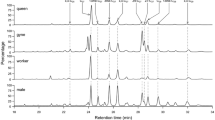
The relative proportion of each cuticular hydrocarbon was determined for the 71 collection points. No qualitative difference was apparent in the CHC components of workers. All profiles had the same 18 hydrocarbons that had previously been found in R. flavipes/R. santonensis (Bagnères et al., 1990) (Fig. 2). However, there were quantitative variations among the collection points.
Discriminant analysis revealed that the relative proportions of a large number of cuticular hydrocarbons discriminated both populations and localities. No single component could be used to separate the nine populations and the three localities, as nearly the whole of the chemical signature (72–83% of the peaks) was needed. The first discriminant analysis performed on relative amounts of the 18 peaks of each collection point discriminated the nine populations significantly. Of these, the relative proportions of 15 compounds (i.e., e2, a3, m4, m7, e8, m9, a11, m12, m14, n18, a19, m21, n25, m26, and m29) varied significantly among populations (Wilks’ λ < 0.05, F = 12.25, df = 120, 354, P < 0.001). The two first principal axes accounted for 76.89% of the overall variance between groups (the first axis accounted for 46.14% and the second for 30.74%). A large percentage (97.18%) of the collection points were classified correctly in the original groups, with only two collection points of the Oléron population assigned to the Olonnes population. The second discriminant analysis performed on the 3 localities distinguished France, Florida, and Louisiana significantly (Fig. 3). Discriminant analysis selected 13 peaks (i.e.,. e1, e2, a3, e8, m9, a11, m12, m14, e17, n18, a19, n25, and m29), grouping all collection points within the assigned localities (Wilks’ λ < 0.05, F = 37.77, df = 24, 114, P < 0.001). The two first axes accounted for 100% of the chemical variation between groups, with 70.27% of the variation explained by the first axis and 29.73% by the second axis. A large percentage (98.59%) of the collection points was correctly assigned to the original locality group, with only one collection point in Louisiana being grouped with the French colonies.
Chemical differentiation of relative proportions of cuticular hydrocarbons of Reticulitermes flavipes among three localities (France, Florida, and Louisiana) on the two first axes of the discriminant analysis. Axes I and II account for 100% (70.27% and 29.73% respectively) of the total variation among localities
Principal component analysis based on cuticular hydrocarbon profiles of worker samples revealed that the first two principal components accounted for 41% of the total chemical variation (Fig. 4). The first axis accounted for most of the variation (25%) among Florida and Louisiana populations. The second axis accounted for 16% of the variation, distinguishing the Florida populations from the others. Principal component analysis showed that proportions of CHCs in French populations appeared closer to the three Louisiana populations than the three Florida populations. Chemical variations within and between populations in France appeared lower than in Florida and Louisiana. Although the French populations in St Trojan and Olonnes covered a greater geographical area than the American populations, their chemical profiles were less variable.
Chemical Dissimilarity Distances
The chemical dissimilarity distances (Euclidean and Nei) within populations, between populations within localities, and between pairs of localities were calculated (Table 1). Non-parametric tests used to compare the chemical dissimilarity distances showed similar results for the Euclidean and Nei distances. The chemical distance within each population (Table 1) was similar for all the populations except for the Olonnes population which had a low variability in the CHC composition (Kruskal-Wallis test, P < 0.001; Dunn’s procedures for Olonnes-Oléron, P < 0.001; Olonnes-Osceola, P < 0.001; Olonnes-Black River, P < 0.05; Olonnes-Wakulla, P < 0.05; Olonnes-New Orleans, P < 0.001; Olonnes-Lafitte, P < 0.001), although it was similar to the chemical distances within the Tours and Baton Rouge populations. The chemical dissimilarity distances, both Euclidean and Nei distances, among populations within localities were significant (Table 1). The chemical distances between French populations were significantly less than those observed between populations in Florida and Louisiana (Kruskal-Wallis test, P < 0.001; Dunn’s procedures for France-Florida, P < 0.001, France-Louisiana, P < 0.001). Similarly, the chemical distances among the Louisiana populations were smaller than those observed among the Florida populations (Kruskal-Wallis test, P < 0.001; Dunn’s procedure, P < 0.001). The chemical distance between localities (Table 1) showed that the distance between French and Louisiana was not significantly different from that between the French and Florida localities (Kruskal-Wallis test, P < 0.001, Dunn’s procedure, P > 0.05). The chemical distance between the Florida and Louisiana localities was significantly greater than those between the French and Florida localities and the French and Louisiana localities (Kruskal-Wallis test, P < 0.001, Dunn’s procedures for Florida/Louisiana vs. France/Florida, P < 0.001; Florida/Louisiana vs. France/Louisiana, P < 0.001).
Soldier Defensive Secretions
Six isoprenoid compounds were identified from the SDS extracts: α-pinene, β-pinene, limonene, γ-cadinene, cadinene aldehyde, and geranyl linalool. These compounds were the same as those found previously by Zalkow et al. (1981), Bagnères et al. (1990) and Nelson et al. (2001). Qualitative analyses of the SDSs on the basis of the presence/absence of peaks revealed 6 chemotypes, three of which were different from those found by Bagnères et al. (1990) (Table 2, chemotypes a, e, and f). γ-Cadinene, cadinene aldehyde, and geranyl linalool were present or absent in the various chemotypes, but the monoterpenes α-pinene, β-pinene, and limonene always were present. Two of the chemotypes were found in the French populations, one of which had already been reported (Bagnères et al., 1990; Quintana et al., 2003) with the monoterpenes and the geranyl linalool (Table 2 chemotype b), and the second, the most common in our samples, not previously reported, was composed only of monoterpenes (Table 2 chemotype a). Two chemotypes were observed in Florida and four in Louisiana. One chemotype (chemotype a) was common to France and Louisiana, but none was common to France and Florida.
Discussion
This study showed that CHCs provide a useful marker for discriminating termite populations, since the results made it possible to discriminate localities and populations within R. flavipes. CHC proportions appear to be useful markers for distinguishing the various populations by determining the similarity within each geographical scale.
One major finding was the unexpected chemical homogeneity observed within introduced populations relative to the chemical variation within native populations. Even though chemical distances within populations were not significantly lower in France than in the U.S., the PCA revealed less divergence among the profiles for each collection point for the introduced populations than for the populations in Florida and Louisiana. This result is all the more significant as the comparison was drawn by using a similar geographical scale within populations, except for two French populations, which had a wider area. The use of two different solvents (i.e., hexane and pentane) cannot explain this variation, primarily because the polarities of the two solvents are similar if not identical. Furthermore, the dried samples all were dissolved a second time in pentane before GC analysis. Finally, if these solvents would have caused significant quantitative differences, then these should have been detected by the PCA. The U.S. populations would have been differentiated from the French populations, and the differences between Florida and Louisiana would have been less apparent.
The remarkable hydrocarbon homogeneity observed within the introduced populations of R. flavipes compared to the native populations has previously been detected in other introduced social insects. Recent research had revealed a similar change in CHC profiles within introduced ants, L. humile (Brandt et al., 2009) and W. auropunctata (Errard et al., 2005). These two studies showed that the CHC profiles of ants from native populations were diverse, whereas the profiles of ants from various localities in introduced populations were uniform. This suggests that the introduction event into a new environment may be the cause of the reduced chemical variability of introduced populations. It is possible that, similarly to the introduced ant hypotheses, the reduced variability of recognition cues observed in introduced populations of R. flavipes is due to a reduction of genetic diversity through a genetic bottleneck (Tsutsui et al., 2000) or a selective process for the less common alleles of recognition (Giraud et al., 2002).
In all cases, the chemical homogeneity occurring in introduced populations of R. flavipes could explain two particular characteristics of the social organization in its French range. The first characteristic is the absence of aggression between colonies, which has been observed among all French populations of R. flavipes (Clément and Bagnères, 1998). Cuticular hydrocarbons generally are considered to have an important role in conspecific and colony member recognition (Clément and Bagnères, 1998; Blomquist and Bagnères, 2010). Thus, a low variability of chemical signature within introduced populations of R. flavipes could induce the recognition of non-nestmates as nestmates, thereby reducing intraspecific aggression. The second characteristic is the significant level of merging between separate colonies that has been found within one of the introduced French populations of R. flavipes studied (the Oléron population) (Perdereau et al., 2010). One of characteristics that appear essential for colony fusion is the absence of intraspecific aggression among individuals of the two parental colonies. The CHC homogeneity in introduced populations of R. flavipes could explain the lack of intraspecific aggression and, indirectly, the high rate of colony fusion within these introduced populations, as has been recently proposed for the Argentine ant, Linepithema humile (Vasquez et al., 2009).
This study also revealed that CHC profiles differed significantly among U.S. populations, whereas the three French populations exhibited similar CHC profiles. One possible explanation for this general pattern is that the three French populations all derived from a single original source population. This hypothesis is supported by several phylogeographic studies performed on Reticulitermes species, which showed that discrimination based on CHC profiles often is consistent with discrimination based on DNA markers (Jenkins et al., 2000; Clément et al., 2001; Copren et al., 2005; Austin et al., 2007). Another explanation that cannot be excluded is that the new habitat in France may have more consistent ecological factors, which may be the reason for the homogenous CHC profiles in all French populations of R. flavipes. Although studies have revealed that food, temperature, and social environment can affect the composition of CHC profiles (Florane et al., 2004; Dronnet et al., 2006), this second hypothesis is unlikely to explain the results obtained over this large geographic scale. Phylogeographic studies are needed to determine whether the French populations were founded from one or a few North American source population(s).
Concerning the source of the three introduced populations, results based on both CHC profiles and SDS chemotypes suggest that the French populations analyzed may have originated from Louisiana rather than from Florida. Principal component analysis showed that the CHC profiles of the three populations of Louisiana were closer to the chemical profiles of the three French populations than those in Florida. The analyses of SDSs showed, for the first time, a similar chemotype in the native and introduced ranges. This chemotype, not previously reported, is composed only of monoterpenes and is found in both France and Louisiana. The hypothesis that French populations came from Louisiana is also plausible from a historical point of view. During the 17th and 18th centuries, Louisiana was part of “New France” and New Orleans was the main trading port. Populations of R. flavipes may have been arrived in France on boats carrying agricultural and forestry products. In support of this, the first invasion of termites into France was reported in two major ports (Rochefort and La Rochelle) that were involved in international trade (Bobe-Moreau, 1843; Quatrefages, 1853).
The absence of γ-cadinene and cadinene aldehyde compounds in the SDS chemotypes in France raises questions about the caste differentiation system within introduced populations. In French populations, all colonies exhibited an unexplained high proportion of active secondary reproductives in comparison to colonies from the U.S. (Dronnet et al., 2005; Perdereau et al., 2010). Little is known about the caste regulation process in termites, and this is an area of intensive research (Hanus et al., 2010; Schwander et al., 2010). Juvenile hormone III (JH III) could play a role in regulating the soldier and reproductive castes, with a high concentration of JH III inducing the differentiation of workers into secondary reproductives and a higher concentration inducing differentiation into soldiers (Scharf et al., 2003; Park and Raina, 2004; Elliott and Stay, 2007; Leniaud, 2008). Recent research also has revealed that γ-cadinene and the cadinene aldehyde, two isopreonid SDS components in R. flavipes, act in synergy with juvenile hormone in inducing differentiation of workers in soldiers (Tarver et al., 2009). It also has been suggested that soldiers may intervene in worker differentiation in other castes (Henderson, 1998). Thus, it is possible that the absence of γ-cadinene and cadinene aldehyde from populations introduced into France may be one of the reasons for the larger proportion of secondary reproductives: without γ-cadinene and cadinene aldehyde, the concentration of juvenile hormone would be too low to induce workers to develop into soldiers and they would differentiate into secondary reproductives instead.
This study illustrates that hydrocarbon analysis is effective at discriminating populations of a Reticulitermes species. The CHC profiles reveal the possible origin of R. flavipes populations introduced into France and the history and routes of invasion. The homogeneity of the cuticular hydrocarbon profiles observed within introduced populations seems to be related to particular biological characteristics of introduced populations. Further studies should be carried out into the relationship between CHC variations and intraspecific aggression.
References
Austin, J. W., Szalanski, A. L., Uva, P., Bagnères, A. -G., and Kence, A. 2002. A comparative genetic analysis of the subterranean termite genus Reticulitermes (Isoptera: Rhinotermitidae). Ann. Entomol. Soc. Am. 95:753–760.
Austin, J. W., Szalanski, A. L., Scheffrahn, R. H., Messenger, M. T., Dronnet, S., and Bagnères, A. -G. 2005. Genetic evidence for the synonymy of two Reticulitermes species: Reticulitermes flavipes and Reticulitermes santonensis. Ann. Entomol. Soc. Am. 98:395–401.
Austin, J. W., Bagnères, A. -G., Szalanski, A. L., Scheffrahn, R. H., Heintschel, B. P., Messenger, M. T., Clément, J. -L., and Gold, R. E. 2007. Reticulitermes malletei (Isoptera : Rhinotermitidae): a valid nearctic subterranean termite from eastern North America. Zootaxa (1554):1–26.
Bagnères, A. -G. 2006. Recent data on termite invasion and infestation in Western Europe, p. 80, in Proceedings of National Conference on Urban Entomology, May 14–21, 2006, Raleigh, NC, USA.
Bagnères, A. -G. and Wicker-thomas, C. 2010. Chemical taxonomy with hydrocarbons, pp. 121–162, in G. J. Blomquist and A.-G. Bagnères (eds.). Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology. Cambridge University Press, Cambridge, UK.
Bagnères, A. -G., Clément, J. -C., Blum, M. S., Severson, R. F., Joulie, C. and Lange, C. 1990. Cuticular hydrocarbons and defensive compounds of Reticulitermes flavipes (Kollar) and R. santonensis (Feytaud): polymorphism and chemotaxonomy. J. Chem. Ecol. 16:3213–3244.
Blomquist, G. J. and Bagnères, A.-G. 2010. Insect Hydrocarbons: Biology, Biochemistry, and Chemical Ecology. Cambridge University Press, Cambridge, UK, p. 528.
Bobe-moreau, J. 1843. Mémoire sur les Termites observés à Rochefort et dans divers autres lieux du département de la Charente-Inférieure. Saintes.
Brandt, M., Van Wilgenburg, E., and Tsutsui, N. D. 2009. Global-scale analyses of chemical ecology and population genetics in the invasive Argentine ant. Mol. Ecol. 18:997–1005.
Brown, W. V., Watson, J. A. L., Carter, F. L., Lacey, M. J., Barett, R. A., and Mcdaniel, C. A. 1990. Preliminary examination of cuticular hydrocarbons of worker termites as chemotaxonomic characters for some Australian species of Coptotermes (Isoptera: Rhinotermitidae). Sociobiol. 16:305–328.
Carlin, N. F. and Hölldobler, B. 1986. The kin recognition system of carpenter ants (Camponotus spp.) I. Hierarchical cues in small colonies. Behav. Ecol. Sociobiol. 19:123–134.
Clément, J. -L. and Bagnères, A. -G. 1998. Nestmate recognition in termites, pp. 126–155, in R. K. Vander Meer, M. D. Breed, K. E. Espelie, and M. L. Winston (eds.). Pheromone Communication in Social Insects. Ants, Wasps, Bees, and Termites: Westview Press, Boulder, CO, US.
Clément, J. -L., Bagnères, A. -G., Uva, P., Wilfert, L., Quintana, A., Reinhard, J., and Dronnet, S. 2001. Biosystematics of Reticulitermes termites in Europe: morphological, chemical and molecular data. Insectes Soc. 48:202–215.
Copren, K. A., Nelson, L. J., Vargo, E. L., and Haverty, M. I. 2005. Phylogenetic analyses of mtDNA sequences corroborate taxonomic designations based on cuticular hydrocarbons in subterranean termites. Mol. Phylogenet. Evol. 35:689–700.
Dronnet, S., Chapuisat, M., Vargo, E. L., Lohou, C., and Bagnères, A. -G. 2005. Genetic analysis of the breeding system of an invasive subterranean termite, Reticulitermes santonensis, in urban and natural habitats. Mol. Ecol. 14:1311–1320.
Dronnet, S., Lohou, C., Christidès, J. -P., and Bagnères, A. -G. 2006. Cuticular hydrocarbon composition reflects genetic relationship among colonies of the introduced termite Reticulitermes santonensis Feytaud. J. Chem. Ecol. 32:1027–1042.
Elliott, K. L. and Stay, B. 2007. Juvenile hormone synthesis as related to egg development in neotenic reproductives of the termite Reticulitermes flavipes, with observations on urates in the fat body. Gen. Comp. Endocrinol. 152:102–110.
Errard, C., Delabie, J., Jourdan, H., and Hefetz, A. 2005. Intercontinental chemical variation in the invasive ant Wasmannia auropunctata (Roger) (Hymenoptera Formicidae): a key to the invasive success of a tramp species. Naturwissenschaften 92:319–323.
Feytaud, D. J. 1924. Le termite de Saintonge. Compte-Rendus de l’Académie des Sciences 171:203–205.
Florane, C. B., Bland, J. M., Husseneder, C., and Raina, A. K. 2004. Diet-mediated inter-colonial aggression in the Formosan subterranean termite Coptotermes formosanus. J. Chem. Ecol. 30:2559–2574.
Giraud, T., Pedersen, J. S., and Keller, L. 2002. Evolution of supercolonies: The Argentine ants of southern Europe. Proc. Natl. Acad. Sci. USA. 99:6075–6079.
Hanus, R., Vrkoslav, V., Hrdy, I., Cvacka, J., and Sobotnik, J. 2010. Beyond cuticular hydrocarbons: evidence of proteinaceous secretion specific to termite kings and queens. Proc. Royal Soc. B Biol. Sci. 277: 995–1002.
Haverty, M. I., Nelson, L. J., and Page, M. 1990. Cuticular hydrocarbons of fourpopulations of Coptotermes formosanus Shiraki (Isoptera, Rhinotermitidae) in the United States—Similarities and origins of introductions. J. Chem. Ecol. 16:1635–1647.
Haverty, M. I., Nelson, L. J., and Page, M. 1991. Preliminary investigations of the cuticular hydrocarbons from North American Reticulitermes and Tropical and Subtropical Coptotermes (Isoptera: Rhinotermitidae) for chemotaxonomic studies. Sociobiology 19:51–76.
Haverty, M. I., Grace, J. K., Nelson, L. J., and Yamamoto, R. T. 1996. Intercaste, intercolony, and temporal variation in cuticular hydrocarbons of Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). J. Chem. Ecol. 22:1813–1834.
Haverty, M. I., Collins, M. S., Nelson, L. J., and Thorne, B. L. 1997. Cuticular hydrocarbons of termites of the British Virgin Islands. J. Chem. Ecol. 23:927–964.
Haverty, M. I., Woodrow, R. J., Nelson, L. J., and Grace, J. K. 2000. Cuticular hydrocarbons of termites of the Hawaiian Islands. J. Chem. Ecol. 26:1167–1191.
Henderson, G. 1998. Primer pheromones and possible soldier caste influence on the evolution of sociality in lower termites, pp. 314–330, in R. K. Vander Meer, M. D. Breed, M. L. Winston, and K. E. Espelie (eds.). Pheromone Communication in Social Insects: Ants, Wasps, Bees and Termites. Westview Press, Boulder, CO, US.
Howard, R. W. 1993. Cuticular hydrocarbons and chemical communication, pp. 179–226, in D. W. Stanley-Samuelson and D. R. Nelson (eds.). Insect Lipids: Chemistry, Biochemistry and Biology. University of Nebraska Press, Lincoln, NE, US.
Howard, R. W. and Blomquist, G. J. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 50:371–393.
Howard, K. J., Mcdaniel, C. A., and Blomquist, G. J. 1978. Cuticular hydrocarbons of the eastern subterranean termite, Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae). J. Chem. Ecol. 4:233–245.
Howard, R. W., Mcdaniels, C. A., Nelson, D. R., Blomquist, G. J., Gelbaum, L. T., and Zalkow, L. H. 1982. Cuticular hydrocarbons of Reticulitermes virginicus (Banks) and their role as potential species and caste recognition cues. J. Chem. Ecol. 8:1227–1239.
Jenkins, T. M., Haverty, M. I., Basten, C. J., Nelson, L. J., Page, M., and Forschler, B. T. 2000. Correlation of mitochondrial haplotypes with cuticular hydrocarbon phenotypes of sympatric Reticulitermes species from the southeastern United States. J. Chem. Ecol. 26:1525–1542.
Jenkins, T. M., Dean, R. E., Verkerk, R., and Forschler, B. 2001. Phylogenetic analyses of two mitochondrial genes and one nuclear intron region illuminate European subterranean termite (Isoptera: Rhinotermitidae) gene flow, taxonomy, and introduction dynamics. Mol. Phylogenet. Evol. 20:286–293.
Kaib, M., Brandl, R., and Bagine, R. K. N. 1991. Cuticular hydrocarbon profiles: a valuable tool in termite taxonomy. Naturwissenschaften 78:176–179.
Leniaud, L. 2008. Potentialités ontogéniques, différenciation des castes et conséquences sur la structure génétique des termites du genre Reticulitermes. Ph.D. Dissertation. Université François Rabelais, Tours, p 193.
Nei, M. 1987. Molecular evolutionary genetics. Columbia University Press, New York.
Nelson, L. J., Cool, L. G., Forschler, B. T., and Haverty, M. I. 2001. Correspondence of soldier defense secretion mixtures with cuticular hydrocarbon phenotypes for chemotaxonomy of the termite genus Reticulitermes in North America. J. Chem. Ecol. 27:1449–1479.
Nelson, L. J., Cool, L. G., Solek, C. W., and Haverty, M. I. 2008. Cuticular Hydrocarbons and soldier defense secretions of Reticulitermes in Southern California: A critical analysis of the taxonomy of the genus in North America. J. Chem. Ecol. 34:1452–1475.
Nowbahari, E., Lenoir, A., Clément, J. -L., Lange, C., Bagnères, A. -G., and Joulie, C. 1990. Individual, geographical and experimental variation of cuticular hydrocarbons of the ant Cataglyphis cursor (Hymenoptera, Formicidae) - Their use in nest and subspecies recognition. Biochem. Syst. Ecol. 18:63–73.
Page, M., Nelson, L. J., Forschler, B. T., and Haverty, M. I. 2002. Cuticular hydrocarbons suggest three lineages in Reticulitermes (Isoptera: Rhinotermitidae) from North America. Comp. Biochem. Physiol. B 131:305–324.
Park, Y. I. and Raina, A. K. 2004. Juvenile hormone III titers and regulation of soldier caste in Coptotermes formosanus (Isoptera: Rhinotermitidae). J. Insect Physiol. 50:561–566.
Perdereau, E. 2010. Biologie de l’invasion d’un termite americain en France: Evolution de l’organisation sociale et conséquences sur le succès invasif. Ph.D. Dissertation, Université François Rabelais, Tours, p. 274.
Perdereau, E., Bagnères, A. -G., Dupont, S., and Dedeine, F. 2010. High occurrence of colony fusion in a European population of the American termite Reticulitermes flavipes. Insectes Soc. doi:10.1007/s00040-010-0096-z.
Piskorski, R., Hanus, R., Vasickova, S., Cvacka, J., Sobotnik, J., Svatos, A., and Valterova, I. 2007. Nitroalkenes and sesquiterpene hydrocarbons from the frontal gland of three Prorhinotermes termite species. J. Chem. Ecol. 33:1787–1794.
Piskorski, R., Hanus, R., Kalinova, B., Valterova, I., Krecek, J., Bourguignon, T., Roisin, Y., and Sobotnik, J. 2009. Temporal and geographic variations in the morphology and chemical composition of the frontal gland in imagoes of Prorhinotermes species (Isoptera: Rhinotermitidae). Biol. J. Linn. Soc. 98:384–392.
Quatrefages, A. D. 1853. Note sur les termites de La Rochelle. Annales de la Société Zoologique 30:16.
Queller, D. C. 1993. Genetic relatedness and its components in polygynous colonies of social insects, pp. 132–151, in L. Keller (ed.). Queen Number and Sociality in Insects. Oxford University Press, Oxford, UK.
Quintana, A., Reinhard, J., Faure, R., Uva, P., Bagnères, A. -G., Massiot, G., and Clément, J. L. 2003. Interspecific variation in terpenoid composition of defensive secretions of European Reticulitermes termites. J. Chem. Ecol. 29:639–652.
Scharf, M. E., Ratliff, C. R., Hoteling, J. T., Pittendrigh, B. R., and Bennett, G. W. 2003. Caste differentiation responses of two sympatric Reticulitermes termite species to juvenile hormone homologs and synthetic juvenoids in two laboratory assays. Insectes Soc. 50:346–354.
Schwander, T., Lo, N., Beekman, M., Oldroyd, B. P., and Keller, L. 2010. Nature versus nurture in social insect caste differentiation. Trends Ecol. Evol. 25: 275–282
Su, N. Y., Ye, W. M., Ripa, R., Scheffrahn, R. H., and Giblin-davis, R. M. 2006. Identification of Chilean Reticulitermes (Isoptera : Rhinotermitidae) inferred from three mitochondrial gene DNA sequences and soldier morphology. Ann. Entomol. Soc. Am. 99:352–363.
Takematsu, Y. and Yamaoka, R. 1999. Cuticular hydrocarbons of Reticulitermes (Isoptera: Rhinotermitidae) in Japan and neighboring countries as chemotaxonomic characters. Appl. Entomol. Zool. 34:179–188.
Tarver, M. R., Schmelz, E. A., Rocca, J. R., and Scharf, M. E. 2009. Effects of soldier-derived terpenes on soldier caste differentiation in the termite Reticulitermes flavipes. J. Chem. Ecol. 35:256–264.
Tsutsui, N. D., Suarez, A. V., Holway, D. A., and Case, T. J. 2000. Reduced genetic variation in the success of an invasive species. Proc. Nat. Acad. Sci. USA. 97:5948–5953.
Uva, P., Clément, J. -L., and Bagnères, A. -G. 2004. Colonial and geographical variations in agonistic behaviour, cuticular hydrocarbons and mtDNA of Italian populations of Reticulitermes lucifugus (Isoptera, Rhinotermitidae). Insectes Soc. 51:163–170.
Vasquez, G. M., Schal, C., and Silverman, J. 2009. Colony fusion in Argentine ants is guided by worker and queen cuticular hydrocarbon profile similarity. J. Chem. Ecol. 35:922–932
Watson, J. A., Brown, W. V., Miller, L. R., Carter, F. L., and Lacey, M. J. 1989. Taxonomy of Heterotermes (Isoptera: Rhinotermitidae) in south-eastern Australia: cuticular hydrocarbons of workers, and soldier and alate morphology. Syst. Entomol. 14:299–325.
Ye, W., Lee, C. -Y., Scheffrahn, R. H., Aleong, J. M., Su, N. -Y., Bennett, G. W., and Scharf, M. E. 2004. Phylogenetic relationships of nearctic Reticulitermes species (Isoptera: Rhinotermitidae) with particular reference to Reticulitermes arenincola Goellner. Mol. Phylogenet. Evol. 30:815–822.
Zalkow, L. H., Howard, R. W., Gelbaum, L. T., Gordon, D. M., Deutsch, H. M., and Blum, M. S. 1981. Chemical ecology of Reticulitermes flavipes (Kollar) and R. virginicus (Banks) (Rhinotermitidae): Chemistry of the soldier cephalic secretions. J. Chem. Ecol. 7:717–731.
Acknowledgments
We thank Simon Dupont for help in collecting samples of R. flavipes in France, Ed Vargo, and Claudia Husseneder for help in collecting samples in Louisiana (U.S.), Michael Scharf and Nan-Yao Su’s team for collecting samples in Florida (U.S.), and Tony Tebby for editing the manuscript. We thank Sylvain Guyot for his help in Rgui analyses. This work is part of the Ph.D. thesis of E. Perdereau.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Perdereau, E., Dedeine, F., Christidès, JP. et al. Variations in Worker Cuticular Hydrocarbons and Soldier Isoprenoid Defensive Secretions Within and Among Introduced and Native Populations of the Subterranean Termite, Reticulitermes flavipes . J Chem Ecol 36, 1189–1198 (2010). https://doi.org/10.1007/s10886-010-9860-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9860-9