Abstract
Unicoloniality emerges as a feature that characterizes successful invasive species. Its underlying mechanism is reduced intraspecific aggression while keeping interspecific competitiveness. To that effect, we present here a comparative behavioural and chemical study of the invasive ant Wasmannia auropunctata in parts of its native and introduced ranges. We tested the hypothesis that introduced populations (New Caledonia archipelago) have reduced intraspecific aggression relative to native populations (e.g., Ilhéus area, Brazil) and that this correlates with reduced variability in cuticular hydrocarbons (CHCs). As predicted, there was high intraspecific aggression in the Brazilian populations, but no intraspecific aggression among the New Caledonian populations. However, New Caledonian worker W. auropunctata remained highly aggressive towards ants of other invasive species. The chemical data corresponded with the behaviour. While CHCs of ants from the regions of Brazil diverged, the profiles of ants from various localities in New Caledonia showed high uniformity. We suggest that in New Caledonia W. auropunctata appears to behave as a single supercolony, whereas in its native range it acts as a multicolonial species. The uniformity of recognition cues in the New Caledonia ants may reflect a process whereby recognition alleles became fixed in the population, but may also be the consequence of a single introduction event and subsequent aggressive invasion of the ecosystem. Chemical uniformity coupled with low intraspecific but high interspecific aggression, lend credence to the latter hypothesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The success of invasive species can be attributed to the lack of biotic and abiotic constraints that generally regulate the species in its native range (Suarez et al. 2001). Furthermore, changes in key biological traits of the invasive population associated with the introduction event might increase their competitiveness and result in their rapid spread (Tsutsui et al. 2000; Giraud et al. 2002; Tsutsui and Suarez 2003; Le Breton et al. 2004). Social organization appears to be a key attribute responsible for the ecological domination of invasive ants (Passera 1994; Holway et al. 1998; Giraud et al. 2002), among which unicoloniality, i.e., having no colony boundaries within populations, is most remarkable. These species, which generally do not exhibit aggression towards workers within the unicolonial society, generate networks of intercommunicating aggregations of workers, brood and fertile queens. Nestmate recognition in ants relies on non-volatile olfactory cues, mainly cuticular hydrocarbons (CHCs) that constitute a “colony odour”, according to the “gestalt model” (reviewed by Lenoir et al. 1999). Cues comprise of endogenous compounds (genotype) that may be further modulated by exogenous, environmental, sources (Vander Meer and Morel 1998; Liang and Silverman 2000). Elucidating the chemical basis of nestmate recognition may therefore shed light on the mechanisms leading to the formation and maintenance of unicolonial populations of invasive ants (Astruc et al. 2001; Suarez et al. 2002). Among these, the little fire ant Wasmannia auropunctata (Myrmicine) is of growing concern in areas with tropical climates, especially in the Pacific (Jourdan et al. 2002; Wetterer and Porter 2003). It has spread worldwide from its Neotropical origin by means of man (Jourdan et al. 2002), and is notable for occupying disturbed habitats, leading to subsequent ecological dominance of undisturbed ecosystems within its invasive range (Majer and Delabie 1999; Armbrecht et al. 2001; Armbrecht and Ulloa-Chacón 2003). Wasmannia auropunctata thus constitutes an excellent model to study questions relevant to successful invasion.
In the present study we examined the intraspecific aggression of this species in both its native (Brazil; J. Delabie, unpublished data) and invasive ranges (New Caledonia) in concordance with CHC profiles. The Brazilian population is not necessarily the origin of the NC population, since W. auropunctata is widely spread in the Neotropics both in natural and disturbed environments, and the specifics of its introduction to New Caledonia are unknown. We tested the hypothesis that reduced intraspecific aggression is due to chemical uniformity in CHCs. To estimate the competitive power of invasive W. auropunctata, we also investigated the interspecific aggression towards two invasive ant species, Tetramorium bicarinatum (Nylander) (Myrmicinae) originating from Southeast Asia with a current range that extends through all the tropical and subtropical areas (Bolton 1977); and Linepithema humile (Mayr) (Dolichoderinae), originating from South America and now spreading worldwide (Suarez et al. 2001).
Materials and methods
Collection of ants
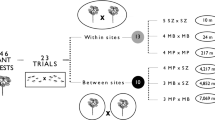
We collected native populations of W. auropunctata from cocoa plantations in the Ilhéus region (Bahia, Brazil; n=32 nests), over between-nests ranges of 0.05 km to 123 km. We collected introduced W. auropunctata (n=40 nests) from eight localities throughout New Caledonia main island over between-nests ranges of 0.02 km to 250 km (list of the sites sampled is available upon request). We collected two colonies of T. bicarinatum from Crasto, Santa Luzia do Itanhi (Sergipe, Brazil) and two colonies of introduced populations of L. humile from Port-Leucate (Aude, France). All colonies were reared in the laboratory in artificial plaster nests (28×28 cm) and maintained under standard conditions (26°C±2°C, 70% RH, 10:14 h D/L). Each colony consisted of several queens, brood and several hundred workers. They were regularly fed with an equal diet, consisting of honey, sugared water, and artificial food cubes (Keller et al. 1989).
Behavioural assays
We quantified the intraspecific aggression between colonies of W. auropuncatata using dyadic encounters according to Holway et al. (1998). For each test, one worker was confronted with either a nestmate or a non-nestmate in a neutral arena (Petri dish: 4.5 cm diameter, 1 cm high). Between-worker aggression was scored during 5-min tests on a scale from 1 to 4 (low to high aggression). We conducted ten replicates using different workers for each test to avoid possible effects of familiarization.
Interspecific aggression was assessed using group encounters (Clément 1986) between W. auropunctata, and L. humile or T. bicarinatum. Ten ants of each species were encountered for 30 min in a glass Petri dish (4.5 cm diameter, 1.7 cm high). Before each test, the ants were allowed to calm down by secluding them in a glass tube for 1 min in the test arena. At the end of the test, the number of dead and injured ants of each species was recorded and an aggression index was calculated as Ag = D +W/2, where D represents the number of dead ants and W the number of injured ants. For each encounter we scored the aggression index of each of the species involved separately. Each interspecific encounter was replicated ten times, using different individuals. For each species, intraspecific encounters (control) were also conducted between ants from different nests.
Chemical analyses
Total body washes of 50-pooled workers of W. auropunctata from each locality were used for identification of CHCs by GC/MS. The extracts were run on a DB5 capillary column, temperature programmed from 120°C to 300°C at 5°C/min. The compounds were identified by their mass fragmentation pattern and retention time comparison. Quantitative analyses were performed using pools of ten workers by GC equipped with a CPSIL 5 capillary column, temperature programmed from 80°C to 180°C at 10°C per minute, and from 180°C to 280°C at 5°C per minute, then kept for 10 min at 280°C. The relative amount of each peak was quantified by peak integration and the various profiles compared thereafter by hierarchical cluster analyses.
Results
In the intraspecific aggression tests, workers of W. auropunctata from native habitats in Brazil showed little aggression between nests within populations, but exhibited high aggression between nests from different populations (Table 1). Among the New Caledonian ants there was no intercolonial aggression irrespective of the population of origin. In contrast, the interspecific encounters involving W. auropunctata from New Caledonia culminated in high aggression. Although we can not tell which species initiated the aggressive acts, the resulting indices indicated that W. auropunctata were highly aggressive towards nests of L. humile (Kruskal-Wallis: H=16.47; p<0.001), considerably more than reciprocally. Towards T. bicarinatum they showed only little aggression (Kruskal-Wallis: H=13.95; p<0.001) that was nevertheless significantly higher than the control (Kruskal-Wallis: H=25.96; p<0.001). This confirms that W. auropunctata forms a unicolonial system in New Caledonia, as suggested by Le Breton et al. (2004).
The chemical analyses revealed clear differences in CHC patterns between New Caledonia (invasive range) and Brazil (native range) W. auropunctata (Fig. 1a, Table 2). Ants from Brazil exhibited greater chemical complexity than those from New Caledonia, mainly in having larger amounts of branched hydrocarbons. A hierarchical cluster analysis based on GC analyses of 34 extracts (15 and 19 nests respectively from native and invasive ranges; Fig. 1b) revealed a significant divergence between the different locations, according to Euclidean distance (F13,225 = 22.35; p<0.001). The first node (172.20) separated Brazilian from New Caledonia populations, while the second node (78.92) divided the Urucuca, Itajuipe and Ilheus Brazilian colonies (BU1-4, BT1-4, BL1-3) from the other Brazilian colonies of Santa Luzia (BS1-4). The third node (60.38) separated the New Caledonian W. auropunctata irrespective of locality.
a Gas Chromatograms of total body wash of 50 workers of Wasmannia auropunctata from Santa Luzia Brazil (top: putative native population) and New Caledonia (bottom: invasive population). Peak numbers correspond to the list in Table 2. Peaks denoted from a–f are of non-hydrocarbons as follows: a palmitic acid; b linoleic acid; c oleic acid; d stearic acid; e squalene; f cholesterol. b Similarity between chemical profiles of the different colonies was assessed by using 54 peaks and comparing 34 extracts (one extract per nest), 16 from Brazil (4 from Santa Luzia: BS1-4, 4 from Uruçuca: BU1- 4, 4 from Itajuipe: BT1-4 and 3 from Ilhéus: BL1-3) and 19 from New Caledonia (5 from Pouembout: NCT1-5, 5 from Ponérihouen: NCH1-5, 5 from Nouméa: NCN1-5 and 4 from Rivière Bleue: NCR1-4)
Discussion
The tendency of invasive species to create large supercolonies, often over the entire invasive range, was confirmed here for W. auropunctata. In New Caledonia, W. auropunctata workers showed no intraspecific aggression, even between nests several hundred kilometers apart, indicating that they behave as a single supercolony, as suggested by Le Breton et al. (2004). Similar supercolonies over wide geographic scales have been described for the Argentine ant in southern California (Tsutsui et al. 2000) and in southern Europe (Giraud et al. 2002). In contrast, in Brazil, as expected from native populations, W. auropunctata expressed a normal multicolonial system, as attested by the high intercolonial aggression exhibited by the ants. These results are similar to those obtained in studies of South American populations of L. humile. This is consistent with the hypothesis that unicoloniality is associated with invasion of new habitats.
The changes in aggressive behaviour were also correlated with CHC profiles exhibited by workers W. auropunctata. These are more diverse in workers coming from the native range as compared to the introduced range, including qualitative differences in CHC composition. A similar negative relationship between CHC-profile similarity and intercolonial aggression was also reported for Argentine ant (Suarez et al. 2002).
If bypassing the nestmate recognition process is a key factor in the success of invasive ant species, W. auropunctata in New Caledonia provides a new compelling case. Its loss of intraspecific but not interspecific aggression contributes to its successful displacement of other ant species. A similar pattern was also described for the Argentine ant, whose aggressive behaviour certainly contributes to its competitive success against several native ants species (Human and Gordon 1999; Holway et al. 1998). Whether this loss of aggression is due to the loss of recognition ability remains elusive. The fact that in New Caledonia, but not in Brazil, the ants exhibited similar CHC profiles, lends credence to the loss of recognition ability. However, it is possible that this CHC similarity reflects a single-nest introduction-event of W. auropunctata, which, due to high competitiveness, has grown to become a large unicolonial species in its new habitat.
References
Armbrecht I, Jimenez E, Alvarez G, Ulloa-Chacón P, Armbrecht H (2001) An ant mosaic in the Colombian rain forest of Choco (Hymenoptera: Formicidae). Sociobiology 37:491–509
Armbrecht I, Ulloa-Chacón P (2003) The Little Fire ant Wasmannia auropunctata (Roger) (Hymenoptera: Formicidae) as a diversity indicator of ants in tropical dry forest fragments of Colombia. Environ Entomol 32(3):542–547
Astruc C, Malosse C, Errard C (2001) Lack of intraspecific aggression in the ant Tetramorium bicarinatum: a chemical hypothesis. J Chem Ecol 27:1229–1248
Bolton B. (1977) The ant tribe Tetramoriini (Hymenoptera: Formicidae). The genus Tetramorium Mayr in the Oriental and Indo-Australian regions, and in Australia. Bull Br Mus (Nat. Hist.) Entomol 36:67–151
Clément J-L (1986) Open and closed societies in Reticulitermes termites (Isoptera, Rhinotermidae). Geographic and seasonal variations. Sociobiology 11:311–323
Giraud T, Pedersen JS, Keller L (2002) Evolution of supercolonies: the Argentine ants of southern Europe. Proc Natl Acad Sci USA 99:6075–6079
Holway DA, Suarez AV, Case TJ (1998) Loss of intraspecific aggression in the success of a widespread invasive social insect. Science 282:949–952
Human KG, Gordon DM (1999) Behavioral interactions of the invasive Argentine ant with native ant species. Insectes Soc 46:159–163
Jourdan H, Sadlier RA, Bauer AM (2001) The impact of the little fire ant invasion (Wasmannia auropunctata (Roger) on the New Caledonian herpetofauna: results of a study in sclerophyll forest habitat. Sociobiology 38:283–301
Jourdan H, Bonnet de Larbogne L, Chazeau J (2002) The recent introduction of the neotropical ant Wasmannia auropunctata (Roger) into Vanuatu archipelago (Southwest Pacific). Sociobiology 40(3):483–509
Keller L, Cherix D, Ulloa-Chacón P (1989) Description of a new artificial diet for rearing ant colonies as Iridomyrmex humilis, Monomorium pharaonis and Wasmannia auropunctata (Hymenoptera; Formicidae). Insectes Soc 36:348–352
Le Breton J, Chazeau J, Jourdan H (2003) Immediate impacts of invasion by Wasmannia auropunctata (Hymenoptera: Formicidae) on native litter ant fauna in a New Caledonian rainforest. Aust Ecol 28:204–209
Le Breton J, Delabie J, Chazeau J, Dejean A, Jourdan H (2004) Experimental evidence of large-scale unicoloniality in the tramp ant Wasmannia auropunctata (Roger). J Insect Behav 17(2):263–271
Lenoir A, Fresneau D, Errard C, Hefetz A (1999) Individuality and colonial identity in ants: the emergence of the social representation concept. In: Detrain C, Deneubourg J-L, Pasteels J-M (eds) Information Processing in Social Insects. Birkhäuser Verlag, Basel, pp 219–237
Liang D, Silverman J (2000) “You are what you eat”: diet modifies cuticular hydrocarbons and nestmates recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87:412–416
Majer JD, Delabie JHC (1999) Impact of tree isolation on arboreal and ground ant communities in cleared pasture in the Atlantic rain forest region of Bahia, Brazil. Insectes Soc 46:281–290
Passera L (1994) Characteristics of tramp species. In: Williams DF (ed) Exotic ants: Biology, Impact, and control of introduced species. Westview Press, Boulder, pp 23–43
Suarez AV, Holway DA, Case TJ (2001) Patterns of spread in biological invasions dominated by long-distance jump dispersal: insights from Argentine ants. Proc Natl Acad Sci USA 98:1095–1100
Suarez AV et al (2002) Spatiotemporal patterns of intraspecific aggression in the invasive Argentine ant. Anim Behav 64:697–708
Tsutsui ND, Suarez AV, Holway DA, Case TJ (2000) Reduced genetic variation and the success of an invasive species. Proc Nat Acad Sci USA 97:5948–5953
Tsutsui ND, Suarez AV (2003) The colony structure and population biology of invasive ants. Cons Biol 17:48–58
Tsutui ND, Suarez AV, Grosberg RK (2003) Genetic diversity, asymmetrical aggression, and cooperation in a widespread invasive species. Proc Natl Acad Sci USA 100:1078–1083
Vander Meer RK, Morel L (1998) Nestmate recognition in ants. In: Vander Meer RK, Breed M, Winston M, Espelie KE (eds) Pheromone communication in social insects. Westview Press, Boulder, pp 79–103
Wetterer JK, Porter SD (2003) The little fire ant, Wasmannia auropunctata: distribution, impact, and control. Sociobiology 42:1–41
Acknowledgements
We thank the Brazilian Cocoa Research Centre (CEPEC/CEPLAC) and its technician Jose Raimundo Maia dos Santos for field assistance. JHCD acknowledges the Brazilian Council of Research (CNPq) for his research grants (process 550701/02-8). We thank Pr. Luc Passera for providing Linepithema humile colonies and four anonymous referees for helpful comments. Thanks to Raymond Jegat and Celine Glaude for assistance in the laboratory. Thanks to Naomi Paz for editing the English
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Errard, C., Delabie, J., Jourdan, H. et al. Intercontinental chemical variation in the invasive ant Wasmannia auropunctata (Roger) (Hymenoptera Formicidae): a key to the invasive success of a tramp species. Naturwissenschaften 92, 319–323 (2005). https://doi.org/10.1007/s00114-005-0628-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00114-005-0628-y