Abstract
Nitrogen (N) is an important macronutrient for plants and insects alike, and the availability of this critical element may considerably modify bottom-up effects in tritrophic systems. By using hydroponically cultured Glycine max, we investigated the impact of N deficiency on plant growth, photosynthetic efficiency, primary metabolism, and herbivore-induced volatile (VOC) emission. Cascading effects of N deficiency on higher trophic levels were assessed by measuring the performances of the herbivore Spodoptera frugiperda and its parasitoid Cotesia marginiventris. In addition, we studied the volatile-guided foraging behavior of C. marginiventris to explore whether nutrient stress affects the plant’s indirect defense. Our results show that photosynthetic efficiency, leaf N, and soluble protein content were significantly reduced in N deficient plants whereas root biomass was increased. Nitrogen starved plants emitted the same range of herbivore-induced VOCs as control plants, but quantitative changes occurred in the release of the main compound and two other volatiles. Herbivore growth and the performance of parasitoids developing inside the affected hosts were attenuated when caterpillars fed on N deficient plants. The behavioral response of C. marginiventris to induced VOCs from N deficient hosts, however, remained unaffected. In summary, N stress had strong bottom-up effects over three trophic levels, but the plant’s indirect defense remained intact.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In terrestrial ecosystems, nitrogen (N) is an important macronutrient for plants. Due to soil properties, N availability can be patchy and may vary even on a small scale (Keddy 2007). Plants show plastic responses to N deficiency by profoundly reprogramming N and carbon (C) metabolism (Lou and Baldwin 2004; Scheible et al. 2004). In an effort to acquire the missing nutrients more efficiently, plants resort to altered biomass allocation between shoot and root and enhanced root branching. Deficiency also leads to sugar and starch accumulation in leaves, and exerts negative feedback on photosynthesis. Eventually, impeded uptake will lead to reduced leaf N content and a higher C/N ratio (Hermans et al. 2006).
Ecologically, a shift towards more C and less N can cause significant changes in bottom-up interactions between primary producers and subsequent trophic levels. Plant N content may affect either herbivore development directly (Scriber 1977; Fischer and Fiedler 2000; Berner et al. 2005; Coley et al. 2006), or deficiency effects can cascade up to higher trophic levels, thus altering top-down influences. Soil conditions may indirectly alter the abundance or performance of parasitoids and predators. For instance, ladybird beetles (Aiolocaria hexaspilota) feeding on willow leaf beetles (Plagiodera versicolora) had a higher adult mass and shorter developmental time when their prey was reared on leaves with high N content (Kagata et al. 2005).
A plant’s nutritional quality is determined not only by the amounts of primary compounds such as proteins or carbohydrates but also by the levels of secondary metabolites. Both factors are intertwined as N availability may affect the synthesis of constitutive and induced defensive secondary compounds. Depending on the metabolites and plant species in question, N availability may lead to changing levels of secondary compounds (e.g., Dudt and Shure 1994; Stout et al. 1998; Hemming and Lindroth 1999; Cipollini et al. 2002; Lou and Baldwin 2004; Chen et al. 2008a).
As a response to feeding or egg deposition by herbivores, plants release volatile organic compounds (VOC), which comprise mainly fatty acid derivatives, terpenoids, phenyl propanoids, and benzenoids. Within the ecosystem, these metabolites can have multiple functions, but primarily they are known as signals that guide natural enemies to their herbivorous prey or host (Holopainen 2004; Heil 2008; Dicke 2009). The production and release of VOCs may vary considerably depending on the plant’s nutritional status. Maize seedlings, for example, show decreased emissions of induced plant VOCs when N-P-K fertilization was reduced (Gouinguené and Turlings 2002). Manipulating N availability alone, however, resulted in enhanced levels of induced VOCs in maize (Schmelz et al. 2003) and cotton (Chen et al. 2008a) but not in Nicotiana attenuata at low levels of N (Lou and Baldwin 2004). Concentrations of the phytohormone jasmonic acid (JA) correlated negatively with N availability and positively with VOC induction, thus, it was suggested that changes in JA provide a mechanism to regulate the magnitude of plant defense responses (Chen et al. 2008a).
A blend of VOCs that varies in the composition or quantity of its components due to abiotic factors may constitute a signal with altered information content and may potentially modify the host finding behavior of natural enemies (Turlings and Wäckers 2004; Rostás and Turlings 2008). Several studies have explored the effects of abiotic factors, such as light (Maeda et al. 2000; Gouinguené and Turlings 2002), humidity (Gouinguené and Turlings 2002), carbon dioxide (Vuorinen et al. 2004b), UV radiation (Winter and Rostás 2008; Blande et al. 2009), ozone (Vuorinen et al. 2004a), or nutrient supply (Gouinguené and Turlings 2002) on VOC induction. Only some of these also have tested whether altered VOC blends affect parasitoid or predator attraction (Maeda et al. 2000; Vuorinen et al. 2004a, b; Winter and Rostás 2008; Blande et al. 2009). However, behavioral experiments are necessary to understand whether a given abiotic factor has the potential to disrupt the facultative mutualism between plants and natural enemies. Changes in the release rate of certain compounds do not automatically translate into differential host searching behavior (Rostás et al. 2006), while stronger or weaker attraction may not always be reflected by detectable changes in the measured VOCs of an induced plant (Gouinguené et al. 2005; Rostás and Turlings 2008; D’Alessandro et al. 2009).
Soybean plants (Glycine max) demand high amounts of N, and a large proportion of it is acquired from N-fixing rhizobacteria. This makes soybean generally less dependent on soil N, but nevertheless, deficiency may occur in patches where appropriate symbionts are lacking or whenever plants and bacteria fail to establish good root nodulation. Unfavorable environmental conditions or fungicide application has been shown to reduce strongly root nodulation and thus nitrogen acquisition (Roth 2009; Zilli et al. 2009).
Here, we addressed the question, whether N deficiency would result in significant bottom-up effects in a tritrophic system consisting of soybean, the herbivore Spodoptera frugiperda, and its larval parasitoid Cotesia marginiventris. In addition to direct effects on the growth, development, survival, and longevity of plants and insects, we focused on the impact of low N availability on the quantity and quality of herbivore induced plant VOC and the attractiveness of the blends for host searching parasitoids.
Methods and Materials
Plant and Insect Material
Soybean seeds (Glycine max (L) Merr. cv. London) were obtained from Saatbau Linz (Leonding, Austria). Seedlings were grown in plastic trays (30 × 20 × 4.5 cm, Wiesauplast, Wiesau, Germany) containing silica sand for 14–16 d and then subjected to N treatments. Further rearing conditions of plants and insects are described in Winter and Rostás (2008).
Nitrogen Treatments
After 14–16 d, plants (V 1 stage, McWilliams et al. 1999) were removed carefully from the sand, and roots were rinsed with deionized water. All seedlings then were transferred to black plastic containers (30.5 × 20.3 × 13.3 cm, Rotilabo® Drehstapelwanne, Carl Roth, Karlsruhe, Germany) containing 5.6 l of hydroponic solution, aerated with a membrane pump. Fifteen plants were grown in each container with a distance of 7 cm between each individual. The hydroponic solution was exchanged every 3–4 d. To maintain appropriate salt concentrations and pH of the solution, electric conductivity (EC) and pH were regularly controlled with a combined pH/EC tester (Combo 2, Carl Roth, Karlsruhe, Germany). EC was adjusted to 2.2 mS, pH was kept at 5.9–6.1.
Plants were exposed for 5 d to a modified Hoagland solution (Hoagland and Arnon 1938). For plants growing in nitrogen deficient solution (–N treatment), KNO3 was replaced with K2SO4 (Carl Roth, Karlsruhe, Germany), and Ca(NO3)2 with CaCl2 (AppliChem, Darmstadt, Germany) in equivalent concentrations.
Effects of Nitrogen Deficiency on Plant Growth and Physiology
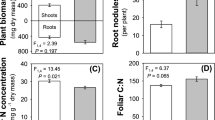
The effect of N deficiency on growth and physiology of soybean was assessed by exposing plants to the respective N treatments for 5 d. Then, shoots and roots of six plants per treatment were freeze-dried for 48 h and weighed to calculate shoot-to-root-ratios. Eight plants per treatment grown in the same cohort were used to determine C/N-ratios and concentrations of soluble proteins in the leaves. For this, leaf discs were cut out with a cork borer (diam 17.8 mm) from unifoliate leaves, freeze-dried, weighed, and ground. Half of the material was used to analyze total C and N content by quantitative decomposition of substances by oxidative combustion (CHN-O-Rapid, Heraeus, Hanau, Germany). The other half was extracted 3 × with 500 µl deionized water. Extracts were combined, and soluble protein content was determined with Bradford reagent (Sigma-Aldrich, Seelze, Germany) using bovine serum albumin (1.4 mg ml−1 in water) as standard. Samples were arranged in a 96 well plate, and absorbance was measured with a photometer (Multiskan EX, Thermo Labsystems, Vantaa, Finland) at 595 nm.
To assess the effects of N limitation on the photosynthetic efficiency of soybean, the adaxial leaf side of the first trifoliate leaf of 5 plants per treatment was examined with a PAM-2000 fluorometer (Walz Mess–u. Regeltechnik, Effeltrich, Germany). Maximum photochemical yield of photosystem II (PSII) was measured in dark-adapted leaves as the ratio of variable (Fv) to maximal (F M) chlorophyll fluorescence at room temperature with Fv/F M = (F M - F 0)/F M (Schreiber et al. 1986). Minimum fluorescence (F 0) was excited at 655 nm and 600 Hz modulation frequency, and maximum fluorescence (F M) was measured with 100 kHz modulation frequency. The FM was elicited by saturating pulses of 0.8 s duration from a built-in halogen lamp.
Plant-Mediated Effects of Nitrogen Deficiency on Herbivore Growth, Developmental Time, Survival and Feeding Behavior
An herbivore performance test was conducted to assess the effects of plant N limitation. Fifty neonate larvae of Spodoptera frugiperda were weighed and kept individually in Petri dishes (diam. 8.5 cm) with moistened filter paper in a climate chamber as described above. They were fed ad libitum with fresh cut leaflets of the first trifoliate soybean leaves from plants kept for 5 d in +N or –N solution. Each plant was harvested only once. Weight increases of larvae were measured between day 5 and 6 (L2) and day 10 and 11 (L3). In addition, pupal and adult weights, as well as developmental times were recorded.
Two feeding trials were performed to see whether larvae (L2) compensated for potentially lower food quality due to N limitation by ingesting larger quantities of leaf tissue (no-choice assay), or by choosing food with potentially higher quality (choice assay).
For the no-choice assay, 15 single larvae (L2) were placed in Petri dishes (diam. 8.5 cm) and fed with one soybean leaflet (first trifoliate leaf) from plants grown for 5 d in +N or –N solution, respectively. For the choice assay, 12 single larvae (L2) were placed in Petri dishes (diam. 8.5 cm) and allowed to choose between two soybean leaflets of the first trifoliate leaf, one from plants grown for 5 d in +N solution, the other leaflet from a plant grown in –N solution.
After 24 h, leaf consumption was measured by scanning the leaves and calculating the removed areas as described in Rostás et al. (2006).
For C/N analyses, another cohort of 8 neonate larvae was treated as described for the performance test. After 6 and 15 d, respectively, the larvae were starved for 1 hr, frozen, freeze dried, and ground. Total C and N content was analysed as described above.
Herbivore-Mediated Effects of Plant Nitrogen Deficiency on Parasitoid Growth, Developmental Time and Longevity
The developmental time, growth, and longevity of the parasitoid Cotesia marginiventris developing inside S. frugiperda was measured to assess host-mediated effects of plant N limitation. Three-day old S. frugiperda larvae were fed with freshly cut soybean leaves of the respective N treatment for 2 d. The larvae then were offered in six groups of 5 to a 4–6 d old mated C. marginiventris female in a Petri dish (diam. 5.5 cm). After parasitism of 15 larvae per treatment was observed, caterpillars were kept separately in Petri dishes (diam. 8.5 cm) with moist filter paper and fed ad libitum with freshly cut leaves from +N or –N plants. Food was exchanged at least every 2nd day. Emerging parasitoid cocoons were transferred to individual Petri dishes (diam. 5.5 cm) with dry filter paper. Eclosed parasitoids were provided with water only. Developmental time, pupal weight at 24 hr post emergence, and longevity of the adult parasitoids were recorded.
Plant-Mediated Nitrogen Effects on VOC Emission and Parasitoid Behavior
The effects of N limitation on the emission of herbivore-induced volatiles and consequently on the behavior of the parasitoid was investigated in a six-arm-olfactometer (for details see Turlings et al. 2004). Soybean plants exposed for five days were placed individually into the cup of an odor source vessel of the six-arm-olfactometer and provided with approximately 50 ml of the accordant hydroponic solution. The cup was covered with two semicircular polycarbonate plugs that had an opening in the center to hold the plant in an upright position and to prevent larvae from falling into the solution. Twenty-five S. frugiperda larvae (L2) were placed on each plant and were allowed to feed overnight (approx. 16 hr). On the following day, volatile collections and behavioral assays were carried out simultaneously from 9:00 AM till 12:00 AM. Ten fluorescent lamps (PAR inside odor source vessels: 130 µmol photons m−2 sec−1 at 30 cm distance from lamps) were switched on 3 hr before testing the wasps. Odor source vessels containing a plant that had received either +N or –N treatment were placed vis-à-vis in the olfactometer. The other four vessels remained empty as controls. After connecting the vessels to the air delivery and the olfactometer, the air stream was allowed to stabilize for 10 min. The flow rate was 1.2 l min−1 for incoming air and 0.6 l min−1 for air going out to the behavioral arena or the volatile traps, respectively.
Mated 3–5 d old females of C. marginiventris were used in the behavioral assays. Wasps had no oviposition experience prior to the experiment. All wasps were tested in groups of 6, as they do not interfere with each other’s choices (Turlings et al. 2004). After 30 min, the choices made by the parasitoids were recorded, and the group was replaced by a new one. Five groups of wasps were tested on the same day. One day with 5 releases was considered as one replicate. Six replicate days were carried out with a new pair of plants and new wasps each day. Volatiles emanating from soybeans were collected with SuperQ traps as described previously (Rostás and Eggert 2008). After each experimental day, the glass and teflon parts of the olfactometer were cleaned with deionized water and rinsed with ethanol (v/v 70%), acetone, and hexane. After evaporation of the solvents, all glass parts were placed in an oven at 200°C for 1 hr.
Trapped volatiles were eluted with 150 µl methylene chloride. Two internal standards (n-octane and nonyl acetate, Sigma-Aldrich, Taufkirchen, Germany, 400 ng each in 20 µl methylene chloride), were added, and the samples were stored at −80°C. The qualitative and quantitative volatile composition of each sample was analyzed on an Agilent Technologies 6890N Network GC System coupled with a 5973 Network Mass Selective Detector. Three µl of each sample were injected with an automated injection system in pulsed splitless mode. The column was an Agilent 19091-s933 HP-1 capillary column (length 30 m, diam 0.25 mm, film thickness 0.25 µm). The oven was held at 35°C for 3 min and then increased with 8°C min−1 to a final temperature of 230°C that was held for 10 min. Helium (1.5 ml min−1) was used as carrier gas. Compounds were identified using MSD ChemStation (Agilent Technologies) software with the Wiley 275 mass spectrum library and by using the software MassFinder3/Terpenoids library (Hochmuth Scientific Software, Hamburg, Germany). Identities were confirmed further by co-injection of authentic standards (Sigma-Aldrich, Taufkirchen, Germany). Quantification was obtained by comparing the area of the compounds to the areas of the internal standards.
Statistical Analyses
Plant parameters were analyzed with a Mann-Whitney-U-test. Values of plant C/N ratios and soluble protein contents were Bonferroni corrected prior to the analyses. Performance parameters of S. frugiperda and C. marginiventris were compared with ANCOVA using initial weight of S. frugiperda as covariable and measured performance parameters as variables. Herbivore mortality was analyzed with a χ 2-test.
Plant photosynthetic efficiency, larval feeding in the no-choice assay, larval C/N ratios, and differences in VOC compositions were analyzed with Student’s t-tests for independent samples. Only VOCs occurring in at least 50% of all samples were analyzed statistically. Larval feeding in the choice assay was assessed with a t-test for dependent samples. The analyses were conducted using the STATISTICA 7.1 software package (StatSoft, Tulsa, OK, USA).
For the six-arm-olfactometer, the entity computing a repetition in the statistical analysis corresponds to the response of a group of 6 wasps released, which was shown to follow a multinomial distribution (Ricard and Davison 2007). As the data did not conform to simple variance assumptions implied in using the multinomial distribution, we used quasilikelihood functions to compensate for the overdispersion of parasitoids within the olfactometer. The model was fitted by maximum quasi-likelihood estimation in the software package R (http://www.R-project.org), and its adequacy was assessed through likelihood ratio statistics and examination of residuals (Turlings et al. 2004).
Results
Effects of Nitrogen Limitation on Plant Morphology and Physiology
Plants exposed to –N conditions for 5 d showed a significantly decreased shoot-to-root ratio due to increased root biomass (dry weight) compared to +N exposed plants (Table 1). In leaves of –N plants, the amounts of soluble proteins were reduced significantly whereas the C/N ratio was increased due to a lower N content (Table 1). Maximum photochemical efficiency of PS II, measured as F V/F M, was reduced significantly in –N compared to +N treated plants (Table 1).
Plant-Mediated Nitrogen Effects on Herbivore Growth, Developmental Time, Survival, and Behavior
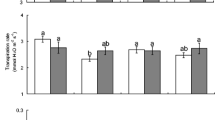
Herbivore larvae fed with leaves from N deficient plants gained significantly less biomass during their development, which resulted in significantly lower pupal and adult weights (Table 2). The C/N ratios in these larvae were reduced compared to larvae fed with +N treated plants. However, developmental time (Table 2), as well as larval mortality (χ 2 test, χ 2 = 0.08, df = 1, P > 0.5) and successful adult emergence (χ 2 test, χ 2 = 0.35, df = 1, P > 0.5), were not influenced by plant N limitation. In choice and no-choice tests, the larvae did not consume significantly more leaf tissue from –N treated than from +N treated plants (Fig. 1).
Leaf consumption by second instar Spodoptera frugiperda larvae measured as leaf area removed in 24 hr. Soybean plants were exposed to either full (+N) or N deficient (–N) Hoagland solution for 5 d. Box-plots show median (line), 25–75% percentiles (box), 10–90% percentiles (whisker) and outliers (dots). a Choice test: Students t-test for dependent samples (t = −2.14 P > 0.05, N = 12). b No-choice test: Students t-test for independent samples (t = 0.00, P > 0.5, N +N = 15, N -N = 14)
Plant-Mediated Nitrogen Effects on Parasitoid Growth, Developmental Time, and Longevity
Individuals of C. marginiventris reared in caterpillars of S. frugiperda, which in turn were fed with leaves from –N treated soybeans, had significantly lower pupal weights. Developmental time and longevity did not differ between wasps reared in herbivore larvae fed with +N or –N treated leaves (Table 3).
Plant-Mediated Nitrogen Effects on VOC Emission and Parasitoid Behavior
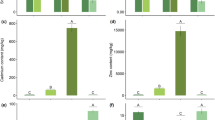
In both N treatments, only trace amounts of (E,E)-α-farnesene could be detected from undamaged soybean plants. In contrast, plants damaged by S. frugiperda larvae released 22 different compounds of which 18 were identified (Fig. 2). The main constituents were (E,E)-α-farnesene (20% and 41% of total emission for +N and –N plants, respectively) and indole (24% and 12% of total emission for +N and –N plants, respectively) when emission was corrected for plant fresh weight. While the same compounds were emitted by +N and –N treated soybean plants, three substances were released in significantly different amounts. The release rates of the sesquiterpenes β-bergamotene and (E,E)-α-farnesene were approx. five times (t = −2.51. df = 10, P < 0.05) and three times (t = −2.64. df = 18, P < 0.05) higher in –N plants than in +N plants. In contrast, a 50% decrease was observed in the emission of (Z)-3-hexenyl-α-methylbutyrate (t = 2.30. df = 10, P < 0.05). All other volatiles were released in similar amounts. The statistical values given here refer to emission rates corrected for plant fresh weight, but the same pattern was obtained for uncorrected values (Student’s-t-test). Differences in the quantities of the three volatiles did not cause a significant shift in overall emission.
Plant VOC emission in response to herbivory and full (+N) or deficient (–N) supply of N for 5 days. Bars indicate mean values, whiskers are ± SE. Asterisks indicate statistically significant differences (*P < 0.05, Student’s t-test, N = 10). Compounds were identified according to their retention times, library mass spectra and by coelution with commercial standards and are arranged by retention times in the Figure. 1) n. i. 2) (Z)-3-Hexenal 3) n. i. 4) n. i. 5) (Z)-3-Hexenol 6) n. i. 7) α-Pinene 9) (Z)-3-Hexenyl acetate 10) (E)-β-Ocimene 11) (Z)-3-Hexenyl propionate 12) Benzeneacetonitrile 13) (Z)-3-Hexenylisobutyrate 14) Methyl salicylate 15) (Z)-3-Hexenyl-α-methylbutyrate 16) n. i. 17) Indole 18) (E)-Caryophyllene 19) α-Humulene 20) Germacrene D 21) β-Bergamotene 22) (E,E)-α-Farnesene. n.i. = Compound not identified
Naïve females of C. marginiventris were highly attracted by the herbivore-induced volatiles of both +N and –N-treated plants when compared to clean air. But wasps had no preference for either one of the offered plant odors (log-linear- model, P > 0.05, N = 6, Fig. 3).
Response of naïve Cotesia marginiventris to herbivore induced volatiles of soybean. Plants were reared in either full (+N) or nitrogen deficient (–N) Hoagland solution. Control was clean air; total numbers of wasps choosing one of the four control arms were divided by four. Bars represent mean (+SE) numbers of wasps making a choice in the olfactometer. Different letters indicate statistically significant differences (Log-linear model fitted to quasipoisson distribution; N = 6 independent experiments with 30 wasps each). Pie chart shows mean percentage of wasps making a choice
Discussion
Deficiency in N severely affected the morphology and physiology of soybeans. In our study, plants lacking N increased their root biomass while shoot biomass remained unchanged (Table 1). Shifting the root-shoot-ratio is a well known response to low N availability and is considered to be an adaptation to suboptimal substrates which allows plants to more efficiently absorb nitrate from the soil (Schopfer and Brennicke 2005; Hill et al. 2006). It has been proposed that changes in phytohormonal balances in conjunction with sugar signals orchestrate cell division and differentiation, thus leading to optimized root morphology (Hermans et al. 2006).
We found that shortage in N supply negatively affected photosynthesis as indicated by the reduced maximum photochemical efficiency of PSII (Table 1). These results corroborate several studies that have used chlorophyll fluorescence to monitor the responses to N deprivation (Lu and Zhang 2000; Kumagai et al. 2007). Deficiency downregulates open PS II reaction centers and leads to increased light induced non-photochemical quenching and enhanced susceptibility to photoinhibition (Lu and Zhang 2000). A lack in chlorophyll content and ribulose-1,5-bisphosphate-carboxylase/-oxygenase (RuBisCo), the most abundant protein in plants, is likely to also have contributed to a decline in photosynthesis. Chlorophyll and RuBisCo were not measured specifically, but leaves of soybeans exposed to N stress had lower leaf N levels, contained less protein (Table 1), and were lighter in color.
Negative effects on the leaf’s N and protein content correlated with poor growth of S. frugiperda larvae. Caterpillars fed with –N leaves gained less weight during their development and had reduced pupal and adult weights compared to larvae reared on +N leaves (Table 2). Similar results were reported from Spodoptera exigua developing on N deficient cotton (Chen et al. 2008b) or larvae of other Lepidoptera feeding on several host plants (Coley et al. 2006). However, studies exist where the correlation between food N content and herbivore growth is less consistent, implying that N is a limiting nutrient for larval development, but not the only one (Tabashnik 1982). Clancy (1992) hypothesized that host plant N determines the amount of food ingested and thus affects the amount of other nutrients incorporated, resulting in an altered growth rate and survival rates of the herbivore caused by a deficiency of other nutrients than N.
Interestingly, despite poorer growth on –N leaves, S. frugiperda larvae did not prefer the superior food when both types of leaves were offered in a dual-choice assay, nor did they show any compensational feeding in a no-choice setup (Fig. 1). This contrasts with findings reported by Chen et al.(2008b) and Merkx-Jacques et al.(2008) who found that S. exigua larvae opted for leaves of high N content or a protein-biased diet, respectively. The incongruent feeding behavior of the two species may reflect their different feeding preferences. Larvae of S. exigua, for instance, are known to prefer leaves while S. frugiperda may often change to fruiting structures when available.
The impact of N deficiency on the first trophic level was transmitted indirectly to the third trophic level as the growth of C. marginiventris also was affected. Parasitoids had significantly lower pupal and adult weights when their hosts had fed on N-deprived soybean leaves (Table 3). Pupal weight often is correlated with fecundity and consequently with fitness (Bourchier 1991). Thus, females of C. marginiventris that hatch from poor hosts may be expected to produce fewer eggs and have fewer offspring. Plant quality is crucial for the performance and fertility of parasitoids in many cases (Campan and Benrey 2004; Setamou et al. 2005; Caron et al. 2008; Sarfraz et al. 2008). Cotesia flavipes had a higher mean progeny size on its host Chilo partellus, when this was reared on cultivated compared to wild gramineous plants (Setamou et al. 2005). Eventually, the effect of poor plant quality on parasitoid fitness depends on the species’ life history. Gregarious, koinobiont parasitoids, for instance, should be affected more strongly than solitary parasitoids when choosing poor hosts. They spend a considerable amount of time in examining their hosts and will lay their eggs in one or relatively few individuals (Brodeur and Boivin 2004). In contrast, females of the generalist, solitary endoparasitoid C. marginiventris allocate their offspring to many caterpillars by feeding on different host plants and thus reducing the fitness costs imposed by parasitizing an inferior host. Nevertheless, fitness costs could be substantial if a larger patch is affected by N deficiency and if wasps forage exclusively within this patch.
The host foraging behavior of parasitoid wasps is influenced by herbivore-induced plant VOCs, which are used as signals to locate those particular plants that are infested by herbivores. Soybeans grown in N deficient hydroponic solution emitted the same spectrum of herbivore-induced VOCs as fertilized plants. However, quantitative changes were found for some VOCs. Low N availability led to more than three times higher emission rates of the main compound (E,E)-α-farnesene, and significantly increased the release of the sesquiterpene β-bergamotene. Emission of the green leaf volatile (Z)-3-hexenyl-α-methylbutyrate, however, was significantly reduced (Fig. 2). Despite these changes, total amounts of VOCs were not affected by N deficiency.
Increased levels of constitutive mono- and sesquiterpenes have been found in N limited Heterotheca subaxillaris (Mihaliak and Lincoln 1985). Looking at induced VOCs, higher levels were found also in N deficient, hydroponically cultivated maize, and in soil-grown cotton. In both plants, the accumulation of the phytohormone jasmonic acid (JA), an important factor in the signaling cascade leading to volatile biosynthesis, correlated negatively with N (Schmelz et al. 2003; Chen et al. 2008a). It has been argued that N starved plants produce greater induced defense responses because N deficiency commonly leads to higher levels of leaf sugars and starch. This larger pool of nonstructural carbohydrates may be used for enhanced VOC biosynthesis (Schmelz et al. 2003).
Females of Cotesia spp. have been reported to respond in a dose-dependent manner to the total blend of herbivore-induced VOCs (NgiSong et al. 1996; Turlings et al. 2004). With the main compound (E,E)-α-farnesene being emitted in higher amounts by N deficient soybeans, a preference for this odor could have been expected. Naïve C. marginiventris females, however, did not differentiate between the blends of stressed and fertilized plants (Fig. 3). Our results thus confirm observations by Chen et al. (2008a) from cage experiments with cotton plants. The authors found that C. marginiventris parasitized the same numbers of caterpillars irrespective of the plants’ N levels and concluded that the parasitoids did not differentiate between the herbivore induced VOC blends.
Several reasons for this behavior are conceivable. First, the VOCs that were emitted in higher quantities were not the relevant key compounds that induce wasp attraction. This notion is supported by an elegant experiment using transgenic Arabidopsis thaliana that overexpressed the maize terpene synthase gene TPS10. In this study, Schnee et al. (2006) demonstrated that several sesquiterpenes, among them (E)-β-farnesene or (E)-α-bergamotene, were not attractive to naïve C. marginiventris. Moreover, the wasps responded only if they had experienced these compounds during oviposition. Further evidence comes from studies that tested specific fractions of the induced odor blend. D’Alessandro and Turlings (2005) confirmed that a blend lacking most sesquiterpenes was as attractive to naïve and experienced C. marginiventris as the full mix. The same holds true for a blend that lacked VOCs from the shikimic acid pathway, while on the other hand, wasps responded strongly to unknown compounds in quantities that were too low to be detected (D’Alessandro et al. 2006, 2009; Rostás and Turlings 2008). In fact, our knowledge on which VOCs innately trigger attraction in parasitoids is fairly rudimentary and needs further testing. Moreover, in nature, such cues are rarely isolated but always occur within the context of background odor, that may mask or enhance the odors of the target plant (Mumm and Hilker 2005; Schroeder and Hilker 2008).
In summary, N deprivation had a strong negative impact on the whole tritrophic system, attenuating the performance of soybean plants, herbivorous caterpillars, and the parasitoid larvae that developed inside S. frugiperda. Despite such adverse effects on the plant’s physiology and some alterations in VOC emission, the signal that indicates the presence of potential hosts obviously remained unchanged as the wasps’ host searching behavior was not affected. These results suggest that the induced indirect defense against herbivores remains stable and effective even under low N conditions. On the parasitoid’s side, reduced fitness due to low N availability cannot be ruled out, but might be negligible if only few plants within a patch are affected. From the plant’s perspective and from a biocontrol point of view, this should be beneficial as abiotic stress in this case does not promote higher susceptibility to another biotic stress factor.
References
Berner, D., Blanckenhorn, W. U., and Körner, C. 2005. Grasshoppers cope with low host plant quality by compensatory feeding and food selection: N limitation challenged. Oikos 111:525–533.
Blande, J. D., Turunen, K., and Holopainen, J. K. 2009. Pine weevil feeding on Norway spruce bark has a stronger impact on needle VOC emissions than enhanced ultraviolet-B radiation. Environ. Pollut. 157:174–180.
Bourchier, R. S. 1991. Growth and development of Compsilura concinnata (Meigan) (Diptera, Tachinidae) parasitizing gypsy-moth larvae feeding on tannin diets. Can. Entomol. 123:1047–1055.
Brodeur, J. and Boivin, G. 2004. Functional ecology of immature parasitoids. Annu. Rev. Entomol. 49:27–49.
Campan, E. and Benrey, B. 2004. Behavior and performance of a specialist and a generalist parasitoid of bruchids on wild and cultivated beans. Biol. Control 30:220–228.
Caron, V., Myers, J. H., and Gillespie, D. R. 2008. Fitness-related traits in a parasitoid fly are mediated by effects of plants on its host. J. Appl. Entomol. 132:663–667.
Chen, Y., Schmelz, E. A., Wäckers, F., and Ruberson, J. R. 2008a. Cotton Plant, Gossypium hirsutum L., defense in response to nitrogen fertilization. J. Chem. Ecol. 34:1553–1564
Chen, Y. G., Ruberson, J. R., and Olson, D. M. 2008b. Nitrogen fertilization rate affects feeding, larval performance, and oviposition preference of the beet armyworm, Spodoptera exigua, on cotton. Entomol. Exp. Appl. 126:244–255.
Cipollini, M. L., Paulk, E., and Cipollini, D. F. 2002. Effect of nitrogen and water treatment on leaf chemistry in horsenettle (Solanum carolinense), and relationship to herbivory by flea beetles (Epitrix spp.) and tobacco hornworm (Manduca sexta). J. Chem. Ecol. 28:2377–2398.
Clancy, K. M. 1992. Response of western spruce budworm (Lepidoptera, Tortricidae) to increased nitrogen in artificial diets. Environ. Entomol. 21:331–344.
Coley, P. D., Bateman, M. L., and Kursar, T. A. 2006. The effects of plant quality on caterpillar growth and defense against natural enemies. Oikos 115:219–228.
D’alessandro, M. and Turlings, T. C. J. 2005. In situ modification of herbivore-induced plant odours: a novel approach to study the attractiveness of volatile organic compounds to parasitoids. Chem. Senses 30:739–753.
D’alessandro, M., Held, M., Triponez, Y., and Turlings, T. C. J. 2006. The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J. Chem. Ecol. 32:2733–2748.
D’alessandro, M., Brunner, V., Von Merey, G., and Turlings, T. C. J. 2009. Strong attraction of the parasitoid Cotesia marginiventris towards minor volatile compounds of maize. J. Chem. Ecol. 35:999–1008.
Dicke, M. 2009. Behavioural and community ecology of plants that cry for help. Plant Cell Environ. 32:654–665.
Dudt, J. and Shure, D. 1994. The influence of light and nutrients on foliar phenolics and insect herbivory. Ecology 75:86–98.
Fischer, K. and Fiedler, K. 2000. Response of the copper butterfly Lycaena tityrus to increased leaf nitrogen in natural food plants: evidence against the nitrogen limitation hypothesis. Oecologia 124:235–241.
Gouinguené, S. P. and Turlings, T. C. J. 2002. The effects of abiotic factors on induced volatile emissions in corn plants. Plant Physiol. 129:1296–1307.
Gouinguené, S., Pickett, J. A., Wadhams, L. J., Birkett, M. A., and Turlings, T. C. J. 2005. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol. 31:1023–1038.
Heil, M. 2008. Indirect defence via tritrophic interactions. New Phytol. 178:41–61.
Hemming, J. D. C. and Lindroth, R. L. 1999. Effects of light and nutrient availability on aspen: Growth, phytochemistry, and insect performance. J. Chem. Ecol. 25:1687–1714.
Hermans, C., Hammond, J. P., White, P. J., and Verbruggen, N. 2006. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 11:610–617.
Hill, J. O., Simpson, R. J., Moore, A. D., and Chapman, D. F. 2006. Morphology and response of roots of pasture species to phosphorus and nitrogen nutrition. Plant Soil 286:7–19.
Hoagland, D. R. and Arnon, D. I. 1938. The water-culture method for growing plants without soil. University of California, California Agricultural Experiment Station. Berkeley, Circular 347:1–39.
Holopainen, J. K. 2004. Multiple functions of inducible plant volatiles. Trends Plant Sci. 9:529–533.
Kagata, H., Nakamura, M., and Ohgushi, T. 2005. Bottom-up cascade in a tri-trophic system: different impacts of host-plant regeneration on performance of a willow leaf beetle and its natural enemy. Ecol. Entomol. 30:58–62.
Keddy, P. A. 2007. Plants and Vegetation: Origins, Processes, Consequences. P. 683, Cambridge University Press, UK.
Kumagai, E., Apaki, T., and Kubota, F. 2007. Effects of nitrogen supply restriction on gas exchange and photosystem II function in flag leaves of a traditional low-yield cultivar and a recently improved high-yield cultivar of rice (Oryza sativa L.). Photosynthetica 45:489–495.
Lou, Y. G. and Baldwin, I. T. 2004. Nitrogen supply influences herbivore-induced direct and indirect defenses and transcriptional responses to Nicotiana attenuata. Plant Physiol. 135:496–506.
Lu, C. M. and Zhang, J. H. 2000. Photosynthetic CO2 assimilation, chlorophyll fluorescence and photoinhibition as affected by nitrogen deficiency in maize plants. Plant Sci. 151:135–143.
Maeda, T., Takabayashi, J., Yano, S., and Takafuji, A. 2000. Effects of light on the tritrophic interaction between kidney bean plants, two-spotted spider mites and predatory mites, Amblyseius womersleyi (Acari : Phytoseiidae). Exp. Appl. Acarol. 24:415–425.
Mcwilliams, D. A., Berglund, D. R., and Endres, G. J. 1999. Soybean growth and management quick guide. North Dakota State University, A-1174. http://www.ag.ndsu.edu/pubs/plantsci/rowcrops/a1174/a1174w.htm
Merkx-Jacques, M., Despland, E. and Bede, J. C. 2008. Nutrient utilization by caterpillars of the generalist beet armyworm, Spodoptera exigua. Physiol. Entomol. 33:51–61.
Mihaliak, C. A. and Lincoln, D. E. 1985. Growth-pattern and carbon allocation to volatile leaf terpenes under nitrogen-limiting conditions in Heterotheca subaxillaris (Asteraceae). Oecologia 66:423–426.
Mumm, R. and Hilker, M. 2005. The significance of background odour for an egg parasitoid to detect plants with host eggs. Chem. Senses 30:337–343.
Ngisong, A. J., Overholt, W. A., Njagi, P. G. N., Dicke, M., Ayertey, J. N., and Lwande, W. 1996. Volatile infochemicals used in host and host habitat location by Cotesia flavipes (Cameron) and Cotesia sesamiae (Cameron) (Hymenoptera: Braconidae), larval parasitoids of stemborers on graminae. J. Chem. Ecol. 22:307–323.
Ricard, I. and Davison, A. C. 2007. Statistical inference for olfactometer data. J. R. Stat. Soc. Ser. C-Appl. Stat. 56:479–492.
Rostás, M. and Eggert, K. 2008. Ontogenetic and spatio-temporal patterns of induced volatiles in Glycine max in the light of the optimal defence hypothesis. Chemoecology 18:29–38.
Rostás, M. and Turlings, T. C. J. 2008. Induction of systemic acquired resistance in Zea mays also enhances the plant’s attractiveness to parasitoids. Biol. Control 46:178–186.
Rostás, M., Ton, J., Mauch-Mani, B., and Turlings, T. C. J. 2006. Fungal infection reduces herbivore-induced plant volatiles of maize but does not affect naive parasitoids. J. Chem. Ecol. 32:1897–1909.
Roth, G. 2009. Soybean nodulation issues. Field Crop News 9:21. http://fcn.agronomy.psu.edu/
Sarfraz, M., Dosdall, L. M., and Keddie, B. A. 2008. Host plant genotype of the herbivore Plutella xylostella (Lepidoptera : Plutellidae) affects the performance of its parasitoid Diadegma insulare (Hymenoptera : Ichneumonidae). Biol. Control 44:42–51.
Scheible, W. R., Morcuende, R., Czechowski, T., Fritz, C., Osuna, D., Palacios-Rojas, N., Schindelasch, D., Thimm, O., Udvardi, M. K., and Stitt, M. 2004. Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 136:2483–2499.
Schmelz, E. A., Alborn, H. T., Engelberth, J., and Tumlinson, J. H. 2003. Nitrogen deficiency increases volicitin-induced volatile emission, jasmonic acid accumulation, and ethylene sensitivity in maize. Plant Physiol. 133:295–306.
Schnee, C., Kollner, T. G., Held, M., Turlings, T. C. J., Gershenzon, J., and Degenhardt, J. 2006. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. Natl. Acad. Sci. U. S. A. 103:1129–1134.
Schopfer, P. and Brennicke, A. 2005. Pflanzenphysiologie. P 702, Spektrum Akademischer Verlag, Heidelberg.
Schreiber, U., Schliwa, U. and Bilger, W. 1986. Continuous recording of photochemical and nonphotochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10:51–62.
Schroeder, R. and Hilker, M. 2008. The relevance of background odor in resource location by insects: A behavioral approach. Bioscience 58:308–316.
Scriber, J. M. 1977. Limiting effects of low leaf-water content on the nitrogen-utilization, energy budget, and larval growth of Hyalophora cecropia (Lepidoptera, Saturniidae). Oecologia 28:269–287.
Setamou, M., Jiang, N. Q., and Schulthess, F. 2005. Effect of the host plant on the survivorship of parasitized Chilo partellus Swinhoe (Lepidoptera : Crambidae) larvae and performance of its larval parasitoid Cotesia flavipes Cameron (Hymenoptera : Braconidae). Biol. Control 32:183–190.
Stout, M. J., Brovont, R. A., and Duffey, S. S. 1998. Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. J. Chem. Ecol. 24:945–963.
Tabashnik, B. E. 1982. Responses of pest and non-pest Colias butterfly larvae to intraspecific variation in leaf nitrogen and water-content. Oecologia 55:389–394.
Turlings, T. C. J. and Wäckers, F. 2004. Recruitment of predators and parasitoids by herbivore-injured plants, pp. 21–75, in R. T. Cardé, and J. Millar (eds.). Advances in Insect Chemical Ecology. Cambridge University Press, Cambridge, UK.
Turlings, T. C. J., Davison, A. C., and Tamò, C. 2004. A six-arm olfactometer permitting simultaneous observation of insect attraction and odour trapping. Physiol. Entomol. 29:45–55.
Vuorinen, T., Nerg, A. M., and Holopainen, J. K. 2004a. Ozone exposure triggers the emission of herbivore-induced plant volatiles, but does not disturb tritrophic signalling. Environ. Pollut. 131:305–311.
Vuorinen, T., Nerg, A. M., Ibrahim, M. A., Reddy, G. V. P., and Holopainen, J. K. 2004b. Emission of Plutella xylostella-induced compounds from cabbages grown at elevated CO2 and orientation behavior of the natural enemies. Plant Physiol. 135:1984–1992.
Winter, T. R. and Rostás, M. 2008. Ambient ultraviolet radiation induces protective responses in soybean but does not attenuate indirect defense. Environ. Pollut. 155:290–297.
Zilli, J. E., Ribeiro, K. G., Campo, R. J., and Hungria, M. 2009. Influence of fungicide seed treatment on soybean nodulation and grain yield. Rev. Bras. Cienc. Solo 33:917–923.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winter, T.R., Rostás, M. Nitrogen Deficiency Affects Bottom-Up Cascade Without Disrupting Indirect Plant Defense. J Chem Ecol 36, 642–651 (2010). https://doi.org/10.1007/s10886-010-9797-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9797-z