Abstract
Larvae of the bollworm Helicoverpa zea (Boddie) show some tolerance to Bacillus thuringiensis (Bt) Cry1Ac, and can survive on Cry1Ac-expressing Bt cotton, which should increase resistance development concerns. However, field-evolved resistance has not yet been observed. In a previous study, a population of H. zea was selected for stable resistance to Cry1Ac toxin. In the present study, we determined in laboratory bioassays if larvae of the Cry1Ac toxin-resistant H. zea population show higher survival rates on field-cultivated Bt cotton squares (= flower buds) collected prebloom—bloom than susceptible H. zea. Our results show that Cry1Ac toxin-resistant H. zea cannot complete larval development on Cry1Ac-expressing Bt cotton, despite being more than 150-fold resistant to Cry1Ac toxin and able to survive until pupation on Cry1Ac toxin concentrations greater than present in Bt cotton squares. Since mortality observed for Cry1Ac-resistant H. zea on Bt cotton was higher than expected, we investigated whether Cry1Ac interacts with gossypol and or other compounds offered with cotton powder in artificial diet. Diet incorporation bioassays were conducted with Cry1Ac toxin alone, and with gossypol and 4% cotton powder in the presence and absence of Cry1Ac. Cry1Ac toxin was significantly more lethal to susceptible H. zea than to resistant H. zea, but no difference in susceptibility to gossypol was observed between strains. However, combinations of Cry1Ac with gossypol or cotton powder were synergistic against resistant, but not against susceptible H. zea. Gossypol concentrations in individual larvae showed no significant differences between insect strains, or between larvae fed gossypol alone vs. those fed gossypol plus Cry1Ac. These results may help explain the inability of Cry1Ac-resistant H. zea to complete development on Bt cotton, and the absence of field-evolved resistance to Bt cotton by this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bollworm, Helicoverpa zea (Boddie), has a naturally high tolerance to the Bacillus thuringiensis (Bt)-derived Cry1Ac protein compared to other target pests of Bt cotton in the US such as tobacco budworm, Heliothis virescens (F.), and pink bollworm, Pectinophora gossypiella (Saunders) (MacIntosh et al. 1990b; Sivasupramaniam et al. 2008). This relatively high tolerance, coupled with preferential feeding on tissues that express lower levels of Bt protein (Brickle et al. 2001), and toxin attenuation due to abiotic stress and plant phenology (e.g., postbloom), can result in complete larval development on Cry1Ac cotton, especially when insect populations are large (Jackson et al. 2004a). Additionally, H. zea is exposed to the Bt Cry1Ab protein in Cry1Ab-expressing corn, which is similar in structure and function to Cry1Ac (Crickmore et al. 1998). This relatively high selection pressure increases the likelihood of resistance evolution (Gould 1998), and it was thus not surprising that early mathematical models predicted resistance development to Bt cotton in H. zea within 3–7 yr (Harris 1991; Roush 1997). Furthermore, the capacity for H. zea to develop resistance to Cry1Ac has been demonstrated in laboratory-selected strains (Luttrell et al. 1999; Jackson et al. 2004b; Anilkumar et al. 2008a). However, contrary to these results and predictions, field-evolved resistance has not occurred in H. zea even after 12 yr of intensive commercial use of Bt cotton in the USA (Ali et al. 2008; Moar et al. 2008).
Although numerous Cry1Ac-resistant strains of H. virescens (Gould et al. 1992, 1995), P. gossypiella (Liu et al. 1999; Tabashnik et al. 2000), and Helicoverpa armigera (Hubner) (Akhurst et al. 2003) have been developed in the laboratory, very few strains have been able to pupate and produce fertile adults on Bt cotton (Tabashnik et al. 2003; Bird and Akhurst 2004). Of those lepidopteran strains that can develop to fertile adults on Bt cotton, there is little correlation between the relative susceptibility of these strains to Cry1Ac, the level of Cry1Ac resistance in these resistant strains, and the relative survivorship of these resistant strains on Bt cotton (Liu et al. 1999; Akhurst et al. 2003; Tabashnik et al. 2003; Bird and Akhurst 2004). Possible explanations for this low correlation include interactions between Bt proteins and secondary plant metabolites (Carrière et al. 2004), use of a form of Bt protein for resistance selection not exclusively found in Bt cotton (Liu et al. 1999; Tabashnik et al. 2000; Akhurst et al. 2003; Henneberry and Jech 2007; Anilkumar et al. 2008a), increased consumption of plant tissues compared to consumption of artificial diet (Woods 1999), loss of genes or a reduction in expression of compounds necessary to survive on cotton during laboratory rearing and Bt resistance selection, or fitness costs associated with Bt resistance (Tabashnik et al. 2003; Gassmann et al. 2009).
Anilkumar et al. (2008a) reported a population of H. zea that was selected for stable and moderately high levels of resistance to the Cry1Ac toxin in the laboratory. Because H. zea is relatively tolerant to Cry1Ac, and arguably the most polyphagous of all target lepidopteran pests of Bt cotton that express Cry1Ac in the US, research was conducted to determine if larvae of the Cry1Ac toxin-resistant H. zea population show higher survival rates or can at least survive to a greater instar than susceptible H. zea on field-cultivated Bt cotton squares (= flower buds) collected during prebloom—bloom stage. Furthermore, this study explored the interaction of Cry1Ac with gossypol and cotton powder in artificial diet to help explain the higher than expected mortality observed for Cry1Ac-resistant H. zea on Bt cotton.
Methods and Materials
Insect Strains
A laboratory susceptible colony of Helicoverpa zea (SC) was established in September 2004 from a laboratory colony from Monsanto (Union City, TN, USA). A resistant strain (AR) was the product of selecting SC for resistance by exposing individual neonates to an artificial diet containing up to 500 μg Bt Cry1Ac toxin/g diet for 25 generations (Anilkumar et al. 2008a). Seven d after exposure to the Cry1Ac toxin, surviving molted larvae were transferred to untreated diet and reared until pupation (Ali et al. 2006; Anilkumar et al. 2008a; Sivasupramaniam et al. 2008). Resistance to Cry1Ac toxin was assessed at selected generations (Anilkumar et al. 2008a), and the AR strain was >150-fold resistant (based on artificial diet bioassays) when survivorship bioassays on Bt and non-Bt cotton squares were initiated.
In order to avoid complete loss of the AR strain due to fitness costs associated with Cry1Ac resistance selection and rearing in the laboratory for 26 generations (Anilkumar et al. 2008b), AR was crossed with a new Monsanto susceptible strain (from Union City, TN), SC1, resulting in a strain designated AR1. SC1 had higher LC50 (31.25 µg Cry1Ac toxin/g diet) values than SC (8.89–15 µg Cry1Ac toxin /g diet; Anilkumar et al. 2008a). Although both reciprocal crosses were attempted, only AR[♀] X SC1[♂] yielded a viable F1 population due to mating costs associated with AR males (Anilkumar et al. 2008b). Because Cry1Ac resistance in H. zea is inherited as a co-dominant character (Burd et al. 2003; Anilkumar et al. 2008b), high levels of resistance (resistance ratio >50-fold compared to SC1) were observed in the F1 generation (data not shown) confirming that AR1 was resistant to Cry1Ac toxin. AR1 was selected at the regular selection concentration of Cry1Ac (500 µg Cry1Ac toxin/g diet) for two generations (Anilkumar et al. 2008a).
Cry1Ac Toxin
An E. coli strain expressing Cry1Ac protoxin from B. thuringiensis subsp. kurstaki strain HD-1 (provided by L. Masson, Biotechnology Research Institute, National Research Council, Montreal, Canada) was cultured, and the activated toxin prepared as indicated elsewhere (Pusztai-Carey et al. 1994; Moar et al. 1995b).
Lyophilized Leaf Tissue Powder Studies
Lyophilized cotton (Gossypium hirsutum, C312) leaf tissue powder was supplied by Monsanto (St. Louis, MO, USA).
Cotton Plants
Bt cotton, Gossypium hirsutum (DPL555) and the near isogenic non-Bt cotton (NBt) (DPL491) were planted at the Prattville Agricultural Research Unit, Alabama Agricultural Research Station, Prattville, AL, U.S.A.. Planting dates for Bt- and NBt-cotton were April 23rd and 24th, 2007, respectively. Cotton plants were cultivated as per typical practices. NBt-cotton plants were treated with imidacloprid at 1 oz/A on both July 13 and July 24, 2007 for aphid control, and tissues were used 5 d after treatment for bioassays. Cotton plants were >85 d old (prebloom-bloom) when squares were harvested. Pin-head to midpoint-stage squares (7–14 d old) were collected, transported to the laboratory, and stored at 4–7°C until needed, up to a maximum of 13 d.
Cry1Ac Protein Quantification in Plants
Beginning on the day of field collection, and on days when cotton squares were removed from refrigeration and used in bioassays, a random sample of 10 Bt squares was placed at −80°C. After all bioassays were completed, all −80°C samples were shipped to Monsanto (St. Louis, MO, USA) for Cry1Ac protein quantification. Square tissues were lyophilized, and Cry1Ac expression was determined using ELISA and compared against a positive Bt cotton standard as described in Greenplate (1999) and Sivasupramaniam et al. (2008). Three replicate assays were conducted for each sample.
Bioassay: H. zea Survival and Development on Cotton Squares
Moist cotton balls were immersed in distilled water, squeezed to remove excess water, and placed in petri dishes (35 × 10 mm) to reduce cotton square desiccation. Individual neonates from both H. zea strains were placed on the outside of square bracts on one square in each petri dish, and squares were changed every 3 d (replacing larvae on bracts) until the end of the experiments. For both Bt and NBt-cotton tests, larvae and squares were transferred to 30 ml cups that contained three to five squares when larvae reached 4th instar, and rearing was continued until pupation. Larval mortality and stadia were recorded beginning on the 4th d, and subsequently at 3 d intervals. Larval weights were recorded after 7 d, and tests were continued until survivors reached pupation. Bioassay trays were incubated at 27 ± 1°C, RH 50%, and a photoperiod of 14:10 (L:D) h. Thirty larvae from each strain were tested in each replication. Experiments were repeated three times.
Bioassays: H. zea Survival and Development on Differently Treated Food General
Gossypol and/or Cry1Ac toxin were added when the diet temperature was <60°C, and the diet was mixed thoroughly. Diets were filled into 128-well bioassay trays (CD-International, Pitman, NJ, USA) at about 1 g per well. Neonate larvae of strains AR1 and SC1 were transferred individually into each well and covered with ventilated covers. Bioassay trays were incubated at 27 ± 1°C, RH 50%, and a photoperiod of 14:10 (L:D) h. Larval mortality and stadia were recorded beginning on the 4th d, and subsequently at 3 d intervals, up to 25 d. IC50 values (μg Cry1Ac/g diet) were determined as those inhibitory concentrations at which half of the larvae failed to molt to the 3rd instar after 7 d. Larval weights were recorded after 7 d, and tests were continued until survivors reached pupation. EC50 or EC90 (μg Cry1Ac/g diet) were determined as those effective concentrations at which larval weight was reduced by 50% or 90% compared to larvae fed on an untreated control diet (Jalali et al. 2004). Thirty-two larvae were tested for each treatment. Three replicates were conducted for the diet with Cry1Ac and the diet with Cry1Ac plus gossypol; six replicates were run for the diet with Cry1Ac plus lyophilized cotton powder.
-
(a)
Artficial diet with Cry1Ac. Larvae were exposed to a level of Cry1Ac toxin four to six times greater than detected in fresh Bt cotton squares by ELISA, i.e., 15 μg Cry1Ac toxin/g diet.
-
(b)
Artficial diet with Cry1Ac and gossypol. Larvae were exposed to diet containing 3.75 μg, 7.5 μg, or 15 μg Cry1Ac/g diet, or 0.0375%, 0.075%, or 0.15% gossypol (95% in acetic acid crystals, Sigma, St. Louis, MO, USA), and a 1:1 combination of Cry1Ac and gossypol (at respective dilutions from the maximum concentration used). For control, larvae were kept on a diet without Cry1Ac and gossypol additions. Because gossypol was dissolved in 1.0 % dimethylsulfoxide (DMSO), all diets contained a final concentration of 1.0% DMSO.
-
(c)
Artificial diet with Lyophilized cotton powder and Cry1Ac. Larvae were exposed to 4% cotton powder, 15 μg Cry1Ac/g diet, and their 1:1 combinations in two generations.
Quantification of Gossypol Uptake by Larvae
Five larvae from each replication that survived after 7 d in bioassays that contained gossypol (gossypol alone, gossypol plus Cry1Ac) or cotton powder (cotton powder alone, cotton powder plus Cry1Ac) were weighed and placed individually into 30 ml plastic cups that contained no diet for 10–12 h to allow for purging of gut contents. Insects were transferred individually to a microcentrifuge tube and frozen at −80°C. Gossypol content per insect was determined as described by Orth et al. (2007).
Data Analysis
Effect of Storage on Cry1Ac Stability in Plants
Plant age at sampling was considered a fixed classification effect, and duration of storage as a fixed effect covariate nested with age to analyze the effect of storage on Cry1Ac stability. The sole random effect in the model was replicate(age), and Proc Mixed predicted a separate intercept for each age class with the no intercept option (SAS Institute 2003).
Larval Feeding upon Cotton Squares
Larval mortality was arcsine square root transformed and analyzed by repeated measures ANOVA with SAS Proc GLM using the normal distribution function. The effect of diet on larval development (instars 3 and 4) was modeled with SAS Proc GLIMMIX with a binomial distribution function and the logit link function. Strain, treatment, and their interactions were fixed effects and replicate the sole random effect. The degrees of freedom for t-tests and confidence intervals were calculated as the number of group means minus the number of fitted parameters (Schabenberger and Pierce 2004).
Larval Feeding upon Diet with Cry1Ac
Larval mortality was arcsine square root transformed and analyzed by repeated measures ANOVA with SAS Proc GLM using the normal distribution function.
Larval Feeding upon Diet with Gossypol and Cry1Ac
Mortality of larvae that feed upon these diets was modeled by logistic regression with SAS Proc NLmixed using the binomial distribution function. The CONTRAST statement was used to evaluate the statistical significance of toxin differences within strains and ESTIMATE to calculate IC50 values (see above) plus associated 95% confidence intervals, as well as the contrasts between strains. For larval weight data, replicate x strain interaction means in response to toxin rate were modeled with SAS Proc NLmixed by using an exponential decay model with a lower asymptotic limit and a normal distribution function. EC50 and EC90 values were estimated from this analysis (see above). Interactions of effects Cry1Ac and gossypol on larvae were evaluated as described by Salama et al. (1984). Differences in observed mortality and theoretical mortality for the mixture of Cry1Ac and gossypol were analyzed with χ2 tests. Interaction was considered 1) synergistic, if observed mortality was more than expected mortality coupled with significant chi square values, 2) additive, if observed mortality was more than expected mortality coupled with non significant chi square values, 3) antagonistic, if observed mortality was less than expected mortality coupled with significant chi square values.
Larvae Feeding upon Diet with Lyophilized Leaf Tissue Powder and Cry1Ac
Larval mortality was modeled with SAS Proc GLIMMIX with a normal distribution function. The residual variance was modeled by using the group option to account for heterogeneous variances among treatments. Larval weight and gossypol concentration/mg larva were modeled using the same procedure but with lognormal distribution function, which was necessary as residuals under the normal assumption were extremely right-skewed. Treatment, strain, and their interaction were treated as fixed effects. However, random effects were different for each of parameter. For larval weight, generation, replicate (generation), and their interactions with fixed effects. For larval mortality, generation and replicate (generation) were considered to be random effects. Least squares diet × strain interaction means were calculated. The slicediff (for larval weight and mortality), pdiff (gossypol concentration/larvae), and simulation options were employed to assess differences among strains and diets while controlling the Type I error rate. Analysis of synergism between Cry1Ac and cotton powder was evaluated as explained above.
Quantification of Gossypol in Larvae
Since the distribution of gossypol concentration per larva was right skewed, data were analyzed with a generalized linear models framework that utilized SAS Proc GLIMMIX. The lognormal distribution function resulted in a symmetrical distribution of residuals. Strain and toxin were fixed effects class variables, and toxin rate was treated as a fixed effects covariate. Differences among toxin x strain combinations then were predicted at toxin rates 375, 750, and 1,500 using the AT option of the LSmeans statement with the simulation adjustment to control the Type I error rate.
Results
Cry1Ac Expression in Cotton Squares
The concentration of Cry1Ac in our field-collected Bt cotton squares (flower buds) was not significantly different from levels of Cry1Ac found in Monsanto’s positive control, DP50 Bollgard® squares used to determine if commercially grown Bollgard® is producing acceptable levels of Cry1Ac (Fig. 1). Cry1Ac expression was reduced (F 5 = 248.75, P < 0.001) by 45% from 86 d (July 18) to 124 d (August 25) after planting; and storage at 4–7°C did not affect the stability of the Cry1Ac for all samples except the July 18th samples (P = 0.049).
Quantity of Cry1Ac protein (μg Cry1Ac/g lyophilized tissue) expressed in Bt cotton squares measured using ELISA. R1-3 = replicates 1–3. Squares were harvested July 18–Aug 25, and squares were refrigerated at 4–7°C until fed to Helicoverpa zea from July 20–Aug. 29. Means and SD of three replicates are given
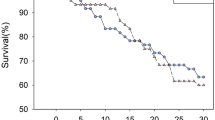
Survivorship of Helicoverpa zea Larvae of AR and SC Strains on Bt Cotton and NBt Cotton
Only tissue type (Bt and NBt) (F 1,8 = 140.02, P < 0.001), but not strains (F 1,8 = 2.65, P = 0.143) or their interactions (F 1,8 = 4.48, P = 0.067) had a significant effect on cumulative mortality at 31 d (Fig. 2a). Mean (± SE) cumulative mortality on Bt cotton for SC larvae at 4 d and 7 d was 60.00 (±8.33) and 76.67(±6.01) percent, respectively, and was significantly (P < 0.05) higher compared to AR larvae (34.45 ± 1.92 and 54.45 ± 1.93% at 4 d and 7 d, respectively). No significant differences were observed in cumulative mortality on 10 d, 13 d, and 16 d between AR (70.00 ± 7.26, 77.78 ± 6.94, and 81.11 ± 7.51%) and SC larvae (86.67 ± 4.41, 88.89 ± 4.19, and 95.56 ± 2.55%) on Bt squares. All SC larvae on Bt squares were dead by 19 d compared to 84.45 ± 6.74% mortality for AR larvae, and these differences were significant (P < 0.05). Both AR and SC larvae survived equally well on NBt cotton; neither duration nor total survivorship varied between strains.
Larval weight after 7 d was different when comparing their food (Bt and NBt cotton tissues (P < 0.001), but not when comparing AR and SC strains (P = 0.406): Neither was the interaction of food tissue and strain significant (P = 0.125) (Table 1). When considering larval mortalitiy, tissue (P < 0.001) and its interaction (P = 0.028) with strain, but not the strain alone (P = 0.099) had an effect on the number of larvae reaching 3rd instar. The proportion of larvae reaching 3rd instar differed significantly between AR and SC on Bt, but not on non-Bt tissues, and only AR-larvae reached 4th instar on Bt cotton squares.
Effect of Cry1Ac in Artificial Diet
All factors such as strains (F 1,8 = 22.32, P = 0.002), type of diet (Cry1Ac treated or untreated) (F 1,8 = 80.20, P < 0.001), and their interactions (F 1,8 = 40.56, P < 0.001) had effects on cumulative mortality after 25 d (Fig. 2b). For within subject effects, only duration of exposure (F 7,2 = 49.92, P = 0.020) and its interaction with type of diet (F 7,2 = 34.57, P = 0.028) had an effect on mortality over time. However, no differences were observed for interaction of duration of exposure with strains (F 7,2 = 5.12, P = 0.173) nor for their combined interaction with type of diet (F 7,2 = 11.18, P = 0.085). Larvae of SC1 and AR1 strains performed similarly on untreated diet resulting in 10–17% mortality after 25 d. SC1-larvae feeding on 15 μg Cry1Ac toxin/g diet had 92% mortality after 25 d, whereas AR1-larvae had only 45% mortality feeding on 15 μg Cry1Ac toxin/g diet after 25 d. After 25 d, only 8.33% SC1-larvae reached pupation as compared to 55% pupation in AR1. For both strains, the largest increase in mortality occurred between 16–19 d. At this time mortality of AR1-larvae feeding upon Cry1Ac toxin increased significantly compared to the untreated control.
Effect of Cry1Ac and Gossypol in Artificial Diet
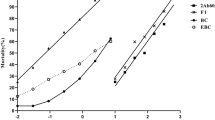
IC50 values (i.e., Cry1Ac toxin concentration at which 50% of larvae fail to molt to third instar) were significantly different between AR1 (17.56) and SC1 (7.07) larvae. With the addition of gossypol to Cry1Ac toxin, however, the IC50 value for AR1-larvae (10.04) decreased significantly and was not significantly different (P = 0.05) from SC1-larvae on Cry1Ac alone (Table 2). The IC50 value for SC1-larvae with the Cry1Ac plus gossypol combination did not significantly decrease (P = 0.05) compared to Cry1Ac alone (Table 2). EC50 values (i.e., Cry1Ac toxin concentration at which larval weight was reduced by 50%) for AR1 (1,172) and SC1-larvae (1,204) were similar for gossypol, and were significantly higher than the values for Cry1Ac toxin (AR1 = 0.99, SC1 = 0.97) and their mixtures (AR1 = 1.35, SC1 = 0.94) (Table 3). Gossypol concentration in larvae did not differ significantly between strains at any concentration (375 µg/g diet, 750 µg/g diet and 1,500 µg/g diet) of gossypol in the presence or absence of Cry1Ac after 7 d (Fig. 3a). However, irrespective of strain, larvae feeding on 0.15% gossypol in the presence of 15 µg Cry1Ac toxin/g diet had significantly less gossypol/mg body weight.
Concentration of gossypol in Cry1Ac-resistant (AR1) and susceptible (SC1) Helicoverpa zea. When fed on a different concentrations of gossypol (375 µg/g, 750 µg/g & 1,500 µg/g) in the diet alone and in 1:1combination with Cry1Ac (3.75 µg/g, 7.5 µg/g, 15 µg/g). CPG = Cry1Ac + Gossypol; Gos = Gossypol. b 4% cotton powder alone or in combination with 15 µg Cry1Ac/g diet. The data represent the mean of three replications and standard errors are back-transformed values from logarithmic scale
Effect of Cry1Ac and Cotton Powder
Strain (F 1 = 32.48, P < 0.001), treatment (F 3 = 602.14, P < 0.001), and the strain X treatment interaction (F 3 = 71.38, P < 0.001) had a significant effect on the percentage of larvae molting to third instar (Table 4). Differences between strains were observed with Cry1Ac toxin (P < 0.001). However, no differences were observed between strains on untreated diet (P = 0.933), nor on 4% cotton powder in the presence (P = 0.051) or absence (P = 0.262) of Cry1Ac. Gossypol concentration in larvae did not differ between strains (F 1 = 0.0, P = 0.985), and there was no significant interaction with treatment (F 2 = 0.70, P = 0.501). However, larvae feeding on different treatments had significantly (F 2 = 243.84, P < 0.001) different levels of gossypol (Fig. 3b). Treatments (F 3 = 103.91, P < 0.001), strains (F 1 = 6.24, P = 0.018), and their interactions (F 3 = 12.35, P < 0.001) influenced larval weight significantly (Fig. 4). AR1 and SC1 larval weights differed on Cry1Ac toxin alone (P < 0.001), but not in other treatments.
Synergistic Interactions
Significant synergistic interactions of Cry1Ac toxin with cotton powder and gossypol were observed for AR1-larvae, but not for SC1-larvae when assessing percent failure to molt to third instar (Table 5). However, there was an additive interaction only at lower levels of Cry1Ac and gossypol for both insect strains.
Discussion
When reared on NBt cotton, Helicoverpa zea larvae of AR and SC strains did not differ in survivorship nor in time required for pupation. In contrast, Cry1Ac-resistant H. virescens (Tabashnik et al. 2003), H. armigera (Bird and Akhurst 2004), and P. gossypiella (Liu et al. 1999) showed significantly slower larval development on NBt cotton than susceptible individuals (Bird and Akhurst 2004). These results suggest that the H. zea AR-strain differs from the H. zea SC-strain primarily by the ability to survive higher concentrations of Cry1Ac toxin.
Bt cotton squares used for this study contained commercially acceptable concentrations of Cry1Ac toxin as measured by ELISA. ELISA results are particularly important for this study since cotton Cry1Ac toxin levels may change due to drought or treatment of samples after collection. Indeed, mean Cry1Ac expression levels were reduced by 45% from 86 d to 124 d after planting during a very hot and dry season. Greenplate (1999) found a similar reduction of Cry1Ac expression with the increase in the age of cotton plants. In contrast, refrigeration of harvested squares at 4–7°C for up to 13 d did not result in a significant reduction in Cry1Ac levels. These results may help future investigations in which Bt cotton squares will need to be refrigerated for extended periods prior to use.
Cry1Ac toxin was tested at 15 μg/g diet, the minimum concentration observed in lyophilized Bt cotton squares by using ELISA. However, fresh Bt cotton tissue contains about six to eight fold less Cry1Ac toxin compared to lyophilized tissue (SS unpublished data). Therefore, even at a concentration four to six fold higher than found in fresh Bt cotton squares (typically 2–4 μg/g; SS unpublished data), 55% of AR1-larvae could develop to pupation thus suggesting that AR-larvae should be able to develop to pupation on Bt cotton especially if larvae feed selectively on tissues that express lower Cry1Ac concentrations (such as bracts) than squares (KJA and WJM unpublished data; Sivasupramaniam et al. 2008). However, no AR-larvae reached pupation on Bt cotton even though the AR strain showed significant decreases in mortality compared to SC at 4 d and 7 d. Similar results were found by Tabashnik et al. (2008). Our results suggest that testing the survivability of putative Cry1Ac-resistant insects on tissues that express Cry1Ac for a time that is not sufficient for pupation may overestimate the capability for field-evolved resistance.
Because the nutritional value of plant material is significantly lower than nutritionally-rich artificial diet, larvae can consume up to 6–8-fold more plant material compared to artificial diet (Naeem et al. 1992; Woods 1999). Therefore, both susceptible and resistant H. zea would be expected to consume much higher levels of Cry1Ac toxin when feeding on Bt cotton squares than with similar toxin concentrations incorporated into diet, thus resulting in much higher than expected mortality (which was observed). This is primarily the reason why a six to eight fold increase in Cry1Ac toxin concentration (15 μg/g diet) was chosen as the highest concentration representing Bt cotton squares. When the AR1 strain was reared on 100 μg Cry1Ac toxin/g diet (about 16–42 times the level of Cry1Ac toxin found in Bt cotton squares) from 3rd instar to 5th instar after being selected on 500 μg Cry1Ac toxin/gram diet, many larvae died before pupation, and those larvae not dead were severely stunted (WJM, unpublished data). Furthermore, increased consumption of plant secondary compounds such as gossypol (that have been shown to synergize Cry1Ac toxin), in addition to Cry1Ac toxin, could also help explain the increased mortality of AR-larvae on cotton squares as compared to artificial diet. Thus, consideration of the actual amount of insecticidal compounds consumed is critical when comparing toxicities from different diets.
Results from Cry1Ac mixtures with gossypol and cotton powder showed synergistic interactions at the highest concentration tested for AR1, but not for SC1. The highest concentration of gossypol tested (0.15%) was the mean of the two concentrations of gossypol tested by Carrière et al. (2004), and it is representative of the gossypol concentration in cottonseed in Pima and Upland cotton varieties (as discussed in Carrière et al. 2004). In addition, 4% cotton powder was synergistic in its activity with Cry1Ac toxin against AR1 even though it contained only 6 µg gossypol/g (data not shown). Other than gossypol, cotton plants produce many other insecticidal secondary metabolites such as heliocides H1 and H2, and hemigossypolone (Hedin et al. 1991). These compounds also could potentially interact to reduce AR survivorship on Bt cotton. Sachs et al. (1996) showed that cotton plants that express high levels of terpenoids (e.g., gossypol) along with Cry1Ab had reduced survivorship of tobacco budworm, H. virescens, compared to cotton plants that expressed low to conventional levels of terpenoids. Interestingly, the use of corn powder instead of cotton powder in combination with Cry1Ac also resulted in a synergistic response with Cry1Ac-resistant, but not with susceptible larvae (data not shown), suggesting that a more generalized plant effect (not limited to cotton) is interacting with Cry1Ac against resistant insects. Because Bt toxin is known to have antifeedant activity (Whalon and Wingerd 2003), AR-larvae would be expected to consume more diet that contains either Cry1Ac or Cry1Ac + gossypol than SC-larvae, thus agreeing with the observation that synergism between Cry1Ac toxin and gossypol or cotton powder should occur only for AR, However, because no differences in effects of different gossypol concentrations on AR and SC-larvae were observed, the synergism of Cry1Ac toxin and gossypol or cotton powder in AR-larvae should be related to the increased consumption of Cry1Ac toxin, not gossypol.
This is the first report of plant compounds other than protein inhibitors that synergize the activity of Bt proteins, especially against Cry1Ac-resistant insects. To date, most compounds that are synergistic with Bt Cry proteins are other Bacillus spp. or Bt products such as spores or spore crystal mixtures (Liu et al. 1998; Moar et al. 1989, 1995a), zwittermicin A (Broderick et al. 2000), β-exotoxin (Moar et al. 1986), CytA (Wirth et al. 1997), and a peptide expressed in E. coli that contains a corresponding Bt binding sequence (Chen et al. 2007). However, plant protease inhibitors and several chemical insecticides also have been reported to synergize Bt proteins (Herfs 1965; MacIntosh et al. 1990a). Gossypol occurs naturally in an enantiomeric mixture of both (+)-gossypol and (−)-gossypol, and the ratio varies among commercial cotton cultivars. Both of these forms reduced the survivorship of H. zea, and a racemic mixture of 1:1 had a synergistic effect at 0.16% (Stipanovic et al. 2006). The gossypol obtained from Sigma used in the current study was extracted from cotton seeds, and the ratio of enantiomeric forms of gossypol was not provided. Therefore, further studies are warranted to quantify the ratios of enantiomers and to evaluate their interactions with Cry1Ac.
The synergistic interaction of gossypol and cotton powder with Cry1Ac observed in AR1-larvae may help explain the inability of AR-larvae to survive and produce fertile adults on Bt cotton. Carrière et al. (2004) suggested that increased susceptibility of Cry1Ac-resistant P. gossypiella to gossypol was linked to the cadherin mutation resistance mechanism (Morin et al. 2003; Carrière et al. 2006). However, AR-larvae have no observable differences in Cry1Ac binding (essentially eliminating a cadherin mutation as a potential resistance mechanism), and appear to have altered proteolysis as a resistance mechanism (Anilkumar et al. 2008a; WJM and KJA unpublished data). Additionally, the AR1 strain was not differentially susceptible to gossypol alone compared to the SC1 strain, nor did AR1-larvae contain more gossypol than SC1-larvae, as has been suggested for Cry1Ac-resistant P. gossypiella (Carrière et al. 2004). One possible explanation for synergistic effects of gossypol and Cry1Ac only on AR-larvae is that gossypol is degraded by upregulation of a cytochrome P450 in Helicoverpa armigera (Mao et al. 2007). Typically, overexpression of detoxification enzymes results in fitness costs (Matsumura 1985). Assuming that Cry1Ac-resistance in AR-larvae is due to proteolysis, (most likely overexpression of a digestive protease), overexpression of two enzymes may result in increased fitness costs, which were observed in AR-larvae (Anilkumar et al. 2008b). A change of midgut protease activity also could affect nutritional protein digestion which could also influence larval growth, as was observed for AR1 (Gassmann et al. 2009). Because synergism was determined only for AR1-larvae based on their failure to molt to third instar, differences in observed development also could be influenced by changes in hormone [such as juvenile hormone (JH) 1] concentrations caused by competition with gossypol for JH1 binding sites, as has been suggested by Carrière et al. (2004). Therefore, future studies are warranted to determine how resistance mechanisms not associated with binding (or fitness costs involved with Cry1Ac selection) are affected by the presence of gossypol and other plant compounds.
The prediction of field-evolved Bt resistance on the basis of laboratory bioassays has always been difficult. Field-evolved resistance has not occurred in H. zea even after 12 y of commercial use of Bt cotton in the USA (Ali et al. 2006, 2008; Moar and Anilkumar 2007; Moar et al. 2008) even though laboratory experiments have shown that H. zea does have the capability to become resistant to the Bt protein (Cry1Ac) in Bt cotton (Luttrell et al. 1999; Jackson et al. 2004b; Anilkumar et al. 2008a). Results presented in this study help to illustrate that the actual hurdles that H. zea must overcome to become resistant to Bt cotton in the field are complex, and they help to explain the absence of field-evolved resistance in this pest.
References
Akhurst, R.J., James, W., Bird, L. J., and Beard, C. 2003. Resistance to the Cry1Ac delta-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 96:1290–1299.
Ali, M.I., Luttrell, R. G., and Young, S. Y. 2006. Susceptibilities of Helicoverpa zea and Heliothis virescens (Lepidoptera: Noctuidae) populations to Cry1Ac insecticidal protein. J. Econ. Entomol. 99:164–175.
Ali, I., Abel, C., Bradley, J. R., Head, H., Jackson, R., Kurtz, R., Leonard, B. R., Lopez, J., Luttrell, R. G., Moar, W. J., Mullins, W., Ruberson, J., Sivasupramaniam, S., and Storer, N. P. 2008. Monitoring Helicoverpa zea susceptibilities to Bt toxins: Results of 2007 studies. pp.1020–1034. in Proc. Beltwide Cotton Conf., National Cotton Council, Memphis, TN.
Anilkumar, K. J., Rodrigo-Simon, A., Ferre, J., Pusztai-Carey, M., Sivasupramaniam, S., and Moar, W. J. 2008a. Production and characterization of Bacillus thuringiensis Cry1Ac-resistant cotton bollworm Helicoverpa zea (Boddie). Appl. Environ. Microbiol. 74: 462–469.
Anilkumar, K.J., Pusztai-Carey, M., and Moar, W. J. 2008b. Fitness costs associated with Cry1Ac-resistant Helicoverpa zea (Lepidoptera: Noctuidae): A factor countering selection for resistance to Bt cotton? J. Econ. Entomol. 101(4):1421–1431.
Bird, L.J., and Akhurst, R. J. 2004. Relative fitness of Cry1A-resistant and -susceptible Helicoverpa armigera (Lepidoptera: Noctuidae) on conventional and transgenic cotton. J. Econ. Entomol. 97:1699–709.
Brickle, D.S., Turnipseed, S.G., and Sullivan, M. J. 2001. Efficacy of insecticides of different chemistries against Helicoverpa zea (Lepidoptera: Noctuidae) in transgenic Bacillus thuringiensis and conventional cotton. J. Econ. Entomol. 94:86–92.
Broderick, N.A., Goodman, R. M. Raffa, K. F., and Handelsman, J. 2000. Synergy between zwittermicin A and Bacillus thuringiensis subsp. kurstaki against gypsy moth (Lepidoptera: Lymantriidae). Environ. Entomol. 29:101–107.
Burd, A.D., Gould, F., Bradley, J. R., Van Duyn, J. W., and Moar, W. J. 2003. Estimated frequency of nonrecessive Bt resistance genes in bollworm, Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in eastern North Carolina. J. Econ. Entomol. 96:137–142.
Carrière Y, Ellers-Kirk, C., Biggs, R., Higginson, D. M., Dennehy, T. J., and. Tabashnik, B. E. 2004. Effects of gossypol on fitness costs associated with resistance to Bt cotton in pink bollworm. J. Econ. Entomol. 97:1710–1718.
Carrière Y, Ellers-Kirk, C., Biggs, R. W., Nyboer, M. E., Unnithan GC, Dennehy, T. J., and Tabashnik, B. E. 2006. Cadherin-based resistance to Bacillus thuringiensis cotton in hybrid strains of pink bollworm: fitness costs and incomplete resistance. J. Econ. Entomol. 99:1925–1935.
Chen, J., Hua, G., Jurat-Fuentes, J. L., Abdullah, M. A., and Adang, M. J. 2007. Synergism of Bacillus thuringiensis toxins by a fragment of a toxin-binding cadherin. Proc. Natl. Acad. Sci. USA 104:13901–13906.
Crickmore, N., Zeigler, D. R., Feitelson, J., Schnepf, E., Van Rie, J., Lereclus, D., Baum, J., and Dean, D. H. 1998. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:807–813.
Gassmann, A. J., Carriere, Y., and Tabashnik, B. E. 2009. Fitness costs of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2009. 54:147–163.
Gould, F. 1998. Sustainability of transgenic insecticidal cultivars: Integrating pest genetics and ecology. Annu. Rev. Entomol. 43:701–726.
Gould, F., Martinez-Ramirez, A., Anderson, A., Ferre, J., F. Silva, F. J., and Moar, W. J. 1992. Broad-spectrum resistance to Bacillus thuringiensis toxins in Heliothis virescens. Proc. Natl. Acad. Sci. USA. 89:7986–7990.
Gould, F., Anderson, A., Reynolds, A., Bumgarner, L., and Moar, W. 1995. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J. Econ. Entomol. 88: 1545-1559.
Greenplate, J.T. 1999. Quantification of Bacillus thuringiensis insect control protein Cry1Ac over time in Bollgard cotton fruit and terminals. J. Econ. Entomol. 92:1377–1383.
Harris, M.K. 1991. Bacillus thuringiensis and pest control. Science. 253:1075.
Hedin, P.A., Parrott, W. L., and Jenkins, J. N. 1991. Effects of cotton plant allelochemicals and nutrients on behavior and development of tobacco budworm. J. Chem. Ecol. 17:1107–1121.
Henneberry, T.J. and Jech, L. J. 2007. Effects of pink bollworm resistance to transgenic cotton on moth mating, oviposition and larval progeny development. Arthropod Manag. Tests 32, Report No. M3.
Herfs, W. 1965. Die vertraeglichkeit von Bacillus thuringiensis Praeparaten mit chemishen Pflanzenschutzmittlen und mit Beistoffen. Z. Pflanzenkra. Pflanzenschutz. 72:584–599.
Jackson, R.E., Bradley JR, J. R., Van Duyn, J. W., and Gould, F. 2004a. Comparative production of Helicoverpa zea (Lepidoptera: Noctuidae) from transgenic cotton expressing either one or two Bacillus thuringiensis proteins with and without insecticide oversprays. J. Econ. Entomol. 97:1719–1725.
Jackson, R.E., Bradley JR, J. R., and Van Duyn, J. W. 2004b. Performance of feral and Cry1Ac-selected Helicoverpa zea (Lepidoptera: Noctuidae) strains on transgenic cottons expressing one or two Bacillus thuringiensis spp. kurstaki proteins under greenhouse conditions. J. Entomol. Sci. 39:46–55.
Jalali, S. K., Mohan, K. S., Singh, S. P., Manjunath, T. M., and Lalitha, Y. 2004. Baseline-susceptibility of the old-world bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) populations from India to Bacillus thuringiensis Cry1Ac insecticidal protein. Crop Prot. 23:53–59.
Liu, Y.B., Tabashnik, B. E., Moar, W. J., and Smith, R. A. 1998. Synergism between Bacillus thuringiensis spores and toxins against resistant and susceptible diamondback moths (Plutella xylostella). Appl. Environ. Microbiol. 64:1385–1389.
Liu, Y.B., Tabashnik, B. E., Dennehy, T. J., Patin, A. L., and Bartlett, A. C. 1999. Development time and resistance to Bt crops. Nature 400: 519.
Luttrell, R.G., Wan, L., and Knighten, K. 1999. Variation in susceptibility of noctuid (Lepidoptera) larvae attacking cotton and soybean to purified endotoxin proteins and commercial formulations of Bacillus thuringiensis. J. Econ. Entomol. 92:21–32.
Macintosh, S. C., Kishore, G. M.,, Perlak, F. J., Marrone, P. G., Stone, T. B., Stevens, S. R., and Fuchs, R. L. 1990a. Potentiation of Bacillus thuringiensis insecticidal activity by serine protease inhibitors. J. Agric. Food Chem. 38:1145–1152.
Macintosh, S. C., Stone, T. B., Sims, S. R., Hunst, P. L., Greenplate, J. T., Marrone, P. G., Perlak, F. J., Fischhoff, D. A., and Fuchs, R. L. 1990b. Specificity and efficacy of purified Bacillus thuringiensis proteins against agronomically important insects. J. Invertebr. Pathol. 56:258–66.
Mao, Y.-B., Cai, W.-J., Wang, J.-W., Hong, G.-J., Tao, X.-Y., Wang, L.-J., Huang, Y.-P., and Chen, X.-Y. 2007. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nature Biotechnol. 25:1307–1313.
Matsumura, F. 1985. Toxicology of Insecticides, 2nd Edition. Plenum. New York and London. 598 pp.
Moar, W. J. and Anilkumar, K. J. 2007. The power of the pyramid. Science. 318: 1561–1562.
Moar, W.J., Osbrink, W. L. A., and Trumble, J. T. 1986. Potentiation of Bacillus thuringiensis var. kurstaki with Thuringiensin on beet armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol., 79:1443–1446.
Moar, W.J., Trumble, J. T., and Federici, B. A. 1989. Comparative toxicity of spores and crystals from the NRD-12 and HD-1 strains of Bacillus thuringiensis subsp. kurstaki to neonate beet armyworm (Lepidoptera: Noctuidae). J. Econ. Entomol. 82:1593–1603.
Moar, W. J., Pusztai-Carey, M., and Mack, T. P. 1995a. Toxicity of purified proteins and the HD-1 strain from Bacillus thuringiensis against lesser cornstalk borer (Lepidoptera: Pyralidae). J. Econ. Entomol. 88:606–609.
Moar, W.J., Pusztai-Carey, M., Faassen, H. V., Bosch, D., Frutos, R., Rang, C., Luo, K., and Adang, M. J. 1995b. Development of Bacillus thuringiensis CryIC resistance by Spodoptera exigua (Hubner) (Lepidoptera: Noctuidae). Appl. Environ. Microbiol. 61:2086–2092.
Morin, S., Biggs, R. W., Sisterson, M. S., Shriver, L., Ellers-Kirk, C., Higginson, D., Holley, D., Gahan, L. J., Heckel, D. G., Carriere, Y., Dennehy, T. J., Brown, J. K., and Tabashnik, B. E. 2003. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Natl. Acad. Sci. USA 100:5004–5009.
Moar W., Roush, R., Shelton, A. Ferre, J., Macintosh, S., Leonard, B. R., and Abel, C. 2008. Field evolved resistance to Bt toxins. Nat. Biotech. 26:172–174
Naeem, M., G. P. Waldbauer, and S. Friedman. 1992. Heliothis zea larvae respond to diluted diets by increased searching behavior as well as by increased feeding. Entomol. Exp. Appl. 65: 95–98.
Orth, R.G., Head, G., and Mierkowski, M. 2007. Determining larval host plant use by a polyphagous lepidopteran through analysis of adult moths for plant secondary metabolites. J. Chem. Ecol. 33: 1131–1148.
Pusztai-Carey, M., Carey, P., Lessard, T., and Yaguchi, M. 1994. USA patent # 5356788.
Roush, R.T. 1997. Bt-transgenic crops: just another pretty insecticide or a chance for a new start in resistance management? Pestic. Sci. 51:328–334.
SAS Institute. 2003. SAS software, release 9.1. SAS Institute, Cary, NC.
Sachs, E. S., Benedict, J. H., Taylor, J. F., Stelly, D. M., Davis, S. K., and Altman, D. W. 1996. Pyramiding Cry1A(b) insecticidal protein and terpenoids in cotton to resist tobacco budworm (Lepiodptera: Nocutidae) Environ. Entomol. 25:1257–1266
Salama, H.S., Foda, M. S., Zaki, F. N., and Moawad, S. 1984. Potency of combinations of Bacillus thuringiensis and chemical insecticides on Spodoptera littoralis (Lepidoptera: Noctuidae). J. Econ. Entomol. 77:885–890.
Schabenberger, O. and Pierce, F. J. 2004. Contemporary Statistical Models for the Plant and Soil Sciences. CRC, Boca Raton, FL, USA.
Sivasupramaniam S., Moar, W. J., Ruschke, L. G., Osborn, J. A., Jiang, C., Sebaugh, J. L., Brown, G. R., Shappley, Z. W., Oppenhuizen, M. E., Mullins, J. W., and Greenplate, J. T. 2008. Toxicity and characterization of cotton expressing Bacillus thuringiensis Cry1Ac and Cry2Ab2 proteins for control of lepidopteran pests. J. Econ. Entomol. 101:546–554.
Stipanovic, R.D., Lopez, J., Dowd, M. K., Puckhaber, L. S., and Duke, S. E 2006. Effect of racemic and (+)- and (−)-gossypol on the survival and development of Helicoverpa zea larvae. J. Chem. Ecol. 32:959–968.
Tabashnik, B. E., Patin, A. L, Dennehy, T. J., Liu, Y., Carriere, Y., Sims, M. A., and Antilla, L. 2000. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc. Natl. Acad. Sci. USA 97: 12980–12984.
Tabashnik B.E., Carrière, Y., Dennehy, T. J., Morin, S., Sisterson, M. S., Roush, R. T., Shelton, A. M., and Zhao, J. Z. 2003. Insect Resistance to Transgenic Bt Crops: Lessons from the laboratory and field. J. Econ. Entomol. 96:1031–1038.
Tabashnik, B. E., Gassman, A. J., Crowder, D. W., and Carriere, Y. 2008. Field evolved resistance to Bt toxins. Nat. Biotech. 26(10): 174–176
Whalon, M.E. and Wingerd, B.A. 2003. Bt: mode of action and use. Arch. Insect Biochem. Physiol. 54: 200–211.
Wirth, M.C., Georghiou, G. P., and Federici, B. A. 1997. CytA enables CryIV endotoxins of Bacillus thuringiensis to overcome high levels of CryIV resistance in the mosquito, Culex quinquefaciatus. Proc. Natl. Acad. Sci. USA 94:10536–10540.
Woods, H. A. 1999. Patterns and mechanisms of growth of fifth-instar Manduca sexta caterpillars following exposure to low-or high-protein food during early instars. Physiol. Biochem. Zool. 72:445–454.
Acknowledgements
The authors thank Nancy Adams, Monsanto Co., Union City, TN for providing H. zea; Arthur Appel, Dept of Entomology, Auburn University for providing statistical guidance; and Marianne Carey, Case Western Reserve University, for providing purified Cry1Ac toxin. This research was partially supported by USDA, and Cotton Incorporated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anilkumar, K.J., Sivasupramaniam, S., Head, G. et al. Synergistic Interactions Between Cry1Ac and Natural Cotton Defenses Limit Survival of Cry1Ac-resistant Helicoverpa Zea (Lepidoptera: Noctuidae) on Bt Cotton. J Chem Ecol 35, 785–795 (2009). https://doi.org/10.1007/s10886-009-9665-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9665-x