Abstract
Many polyphagous insect species are important economic pests on one or more of their crop hosts. For most important insect pests, the common crop hosts are well-known, but knowledge of weedy and unmanaged hosts is limited. Furthermore, the relative contribution of different hosts to local and regional populations has rarely been ascertained because this requires having some way to determine which plant hosts are the source of the adult moths observed ovipositing in a crop field at a given place and time. One way of determining the larval host of polyphagous pest species is to analyze for several plant-derived chemicals that are each specific to a different small set of related plant species and are preserved in detectable amounts in adult moths. In this paper, we describe novel methods for analyzing adults of the polyphagous lepidopteran, the tobacco budworm (TBW) Heliothis virescens (F.), for plant secondary metabolites, specifically cotinine and gossypol, which are diagnostic for larval feeding on tobacco and cotton, respectively. Cotinine was extracted from individual TBW moths with acetic acid and methanol, then concentrated and analyzed directly by gas chromatography/mass spectrometry (GC/MS). The same moths then were analyzed for bound gossypol by creating a Schiff’s base that used aniline, and the resulting dianilino–gossypol complex was quantified using high pressure chromatography coupled with a triple quadrupole mass spectrometer (MS) as the detector. Based on analysis of standards, the detection limit for the cotinine was less than 1.5 ppb by dry weight. Comparable standards were not available for the gossypol derivative so a quantitative limit of detection could not be calculated. When TBW moths reared on known hosts were analyzed for gossypol and/or cotinine, all of the moths reared on tobacco or cotton were correctly identified, although some false positives were recorded with the gossypol method. Analysis of TBW moths of various ages and at various lengths of time after death determined that a significant gossypol signal was detectable in all moths reared on cotton. TBW moths collected from the vicinity of cotton fields in July and August in North Carolina also were analyzed. A much larger portion of the moths were derived from tobacco (6.7–46.4%) than from cotton (0–3.6%) in both months. Thus, these methods can be reliably used to estimate the proportion of TBW derived from noncotton host plants in populations trapped around Bt cotton fields, thereby providing insight into the risk of TBW evolving resistance to Bt cotton.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Many insect species are highly polyphagous, and many are important economic pests on one or more crop hosts. Understanding patterns of host use is a prerequisite to understanding population dynamics, which, in turn, is necessary for designing appropriate management programs for these pest species. Knowing which host plant species are most responsible for sustaining pest populations and causing pest outbreaks, and when these hosts are used, enables pest monitoring and control programs to be targeted appropriately.
For most important insect pests, the common crop hosts are well-known precisely because that is where these pests cause the most economic impact, but knowledge of weedy and unmanaged hosts is more limited. Furthermore, even where the host range of a pest has been carefully documented, the relative contribution of different hosts to regional metapopulations has rarely been determined because of significant technical barriers. In particular, although larval pest populations are relatively easy to estimate, these populations are highly variable over space and time even on a given host plant species, and thus it is difficult to determine how larval populations on different hosts translate into regional populations.
Armyworms (Spodoptera spp.) and heliothines (particularly Helicoverpa spp. and Heliothis spp.) are important larval pests of cotton, corn, sorghum, and numerous other monocotyledonous and dicotyledonous crops in the Old and New World (Fitt 1989). Adults of these lepidopteran species feed only on nectar and other sugar and moisture sources. To understand the population dynamics of these species, one must determine which plant hosts are the source of the adult moths observed ovipositing in a crop field at a given place and time.
Stable isotope analysis has been used successfully to qualitatively (Gould et al. 2002) and quantitatively (Head et al., unpublished data) infer host use by the important corn, cotton, and vegetable pest Helicoverpa zea Boddie. This analysis is based on the principle that the isotopic composition of the wings of adult moths is determined by the physiology of the larval host plant. However, the stable carbon isotope analysis used in these studies could only distinguish whether adults came from larvae that had fed on C3-type hosts (broad-leaved plant species such as cotton, soybean, peanuts, or tobacco) or C4-type hosts (grass-like plant species such as corn, sorghum, or weedy, warm-season grasses). With highly polyphagous pest species like these noctuids, more specific methods are needed to determine the larval host plants from which individual moths originated.
Conceptually, it should be possible to design analyses comparable to stable isotope approaches but focused on plant-derived chemicals that are specific to one or a small set of related plant species. If these analyses are to provide accurate and useful data, the plant-derived analytes need to be preserved in significant (detectable) amounts in adult moths, and they should be unique to the plant species of interest or cause a unique change in the biochemical makeup of the adult moth. Plant secondary metabolites, particularly those involved in plant defense against herbivores, are a promising target for such analyses because many are relatively limited in their taxonomic distribution, and insect herbivores tend to sequester such compounds because of their toxicity.
An example of such a compound is gossypol, which is uniquely related to the lysigenous glands of cotton (Gossypium spp.) and closely related species. Work by Rojas et al. (1992) demonstrated that adults of the important cotton pest species, the tobacco budworm (TBW) Heliothis virescens (F.), contained gossypol from cotton consumed as larvae. The gossypol was in a bound form; no free gossypol was found. However, the method used pooled samples, and the mass analyzed ranged from hundreds of milligrams to grams.
For an analytical method to be useful for population dynamic and host use studies, it must be suitable for use with large numbers of individual moths, and its selectivity needs to be demonstrated. In addition, having appropriate analyses for several different analytes, each largely or wholly specific to one particular important host plant species, would be particularly useful for gaining an understanding of host use patterns by polyphagous pest species. In the case of H. virescens, this would mean having analytical techniques for a cotton-specific compound such as gossypol to determine cotton-derived moths and for a comparable tobacco-specific compound such as nicotine because tobacco (Nicotiana spp.) also is a major crop host of this pest.
This paper describes and characterizes novel methods for the analysis of adult moths for both gossypol and cotinine, a metabolite of nicotine. The methods are applied to TBW moths reared on known host plant species and to a set of field-collected TBW moths.
Methods and Materials
Moths
For development and characterization of the cotinine and gossypol analyses, TBW larvae were reared individually on leaves of cotton (Gossypium hirsutum), tobacco (Nicotiana spp.), peanut (Arachis hypogea), soybeans (Glycine max), and the weedy host, velvetleaf (Abutilon theophrasti). Larvae were allowed to pupate, and the resulting adults were freeze-dried for 48 hr. Wings were removed, and the thorax and abdomen of the adults were weighed and frozen for further analysis. The dry weight of the moth bodies ranged from 0.020 to 0.040 g.
Adult male TBW also were collected for cotinine and gossypol analyses adjacent to cotton fields in two North Carolina counties (Lenoir and Wilson) in July and August of 2004. These moths were collected in pheromone traps baited with Zealure (Hercon® Luretape® Insect Attractant Dispenser, Hercon Environmental Company, Emigsville, PA, USA), and pheromone was replaced on a weekly basis. The collected moths were processed as described for laboratory-reared moths.
Chemicals for Cotinine Analysis
Chemicals were obtained from the following sources: glacial acetic acid (J.T. Baker ,VWR International, West Chester, PA, USA); ACS-grade methanol (EMD, VWR International); deionized water (Milli-Q Gradient A10 system, Millipore, Billerica, MA, USA); anhydrous diethyl ether (J.T. Baker, VWR International); ethanol (AAPER Alcohol and Chemical Co); 50% solution of sodium hydroxide (J.T. Baker, VWR International). The internal standard d4 cotinine (98.2% D) was from CDN isotopes (C/D/N ISOTOPES INC., Pointe-Claire, Quebec, Canada), and the cotinine standard from (Sigma-Aldrich, St. Louis, MO, USA).
Chemicals for Gossypol Analysis
Chemicals were obtained from the following sources: glacial acetic acid (J.T. Baker,VWR International); gossypol acetic acid standard (95% pure) (Sigma-Aldrich); ACS-grade methanol (EMD, VWR International); HPLC methanol (Burdick & Jackson, Sigma-Aldrich); deionized water (Milli-Q Gradient A10 system (Millipore); ACS Grade dimethylformamide (DMF) (EMD, VWR International); aniline (99.5% pure) (Sigma-Aldrich); d 5-aniline (98% D) (Sigma-Aldrich); high purity acetonitrile (Burdick & Jackson,WR International); acetone for sample processing (Fisher Certified ACS, Fisher Scientific, Houston, TX, USA); HPLC acetone (Burdick & Jackson, VWR International); ammonium hydroxide (28–30%) (J.T. Baker, VWR International).
Equipment
Grinding of moths and extractions were carried out in 96-well polypropylene plates (2.0 ml) (Microliter Analytical Supplies, Suwanee, GA, USA). For grinding, 1- to 6-mm glass balls were used (VWR International). Divinyl benzene (DVB) solid-phase microextraction was carried out by using 96-well SPE Plates (50 or 100 mg DVB, 2 ml capacity), (Supelco [Sigma-Aldrich] and P. J. Cobert, St. Louis, MO, USA). For standards and individual samples, 100-mg DVB 3-ml capacity columns were used (Alltech Associates, Deerfield, IL, USA). The HPLC column was an Agilent Eclipse XDB-C8 (2.1 × 12.5, 5 μ) guard column. Autosampler 96-well plates (0.5 ml) (Agilent Technologies) were capped with Agilent Technologies 96-well Plate Piercible Cap Mats (P. J. Cobert Associates). The gas chromatograph was an Agilent 6890 (Agilent Technologies, Santa Clara, CA, USA) equipped with a Leap autosampler (Leap Technologies, Carrboro, NC, USA). The detector was a Leco® Pegasus III time of flight mass spectrometer (Leco Corporation, St. Joseph, MI, USA). The capillary column was a 15-m DB5 column with an inside diameter of 0.250 mm and a phase thickness of 0.25 μm (P. J. Cobert Associates).
Analytical Determination of Cotinine
To analyze for cotinine and gossypol in the same adult moth requires two different but complementary methods. The extraction of cotinine is carried out first because the gossypol analysis involves a derivatization method. The cotinine determination is accomplished by using d4 cotinine as an internal standard. The selectivity is achieved by combining a solid-phase extraction (SPE) cleanup procedure with gas chromatography coupled with a mass spectrometer (GC/MS) as the detector . To obtain the requisite selectivity for gossypol, a SPE procedure, followed by electrospray ionization with tandem mass spectrometry, is used. The use of the tandem mass spectrometer gives a high degree of selectivity and minimizes the need for high-resolution, high-pressure liquid chromatography techniques (HPLC). The methods are adapted to a 96-well format, allowing several hundred samples to be processed per day.
The procedure for determination of cotinine was adapted from Kim et al. (2005) and is summarized in Fig. 1a. This involved extraction and a cleanup with a DVB SPE column. Stable isotope-labeled cotinine was added at the beginning of the extraction so that matrix effects could be minimized. The extracted cotinine was concentrated and analyzed directly by GC/MS. The calibration curve for cotinine was obtained by running standards that ranged from 20 to 600 ng. The quantification was carried out by measuring the ratio of the area of m/z 176 to the area of 180 m/z where 180 m/z was the d4 cotinine internal standard.
To prepare the extraction solution, 50 ml of acetic acid and 200 ml methanol were added to a 1,000-ml volumetric flask, and the volume was brought up to the mark with deionized water. A 20% by volume methanol ether solution was prepared by mixing 20 ml of methanol with 80 ml of ether. A solution of d4 cotinine was used as an internal standard by dissolving d4 cotinine in the extraction solution to give a concentration of 100 ng/20 μl.
Individual freeze-dried TBW moths were placed into the wells of a 2-ml 96-well plate. A glass bead was added to each well, and the plate was capped and placed onto a mega grinder and shaken at 1,100 rpm for 45 sec to grind up the moths (Deppermann 2005). One milliliter of cotinine extraction solution, followed by 20 μl of deuterated cotinine internal standard solution, was added to each of the wells. Parafilm was placed over the top of the plate, and a cap was pressed onto the plate. The moth mixture was vortexed for 15 min, then centrifuged, and the liquid layer removed and added to an 8-ml vial. A second 1 ml of extract solution was added to the moth residue, and the solution was vortexed again for 5 min, centrifuged, and as much liquid removed as possible and combined with the previous extract. One hundred fifty microliters of 40% NaOH, followed by 4 ml of deionized (DI) water, were added to the 8-ml vial containing the extraction solution. The solution was mixed and put through a solid-phase extraction (SPE) DVB column. The 96-well plate containing the moth residue was dried under dry nitrogen and stored at −20°C until needed for the gossypol analysis. An SPE DVB 100 mg (SPE) was used to remove the cotinine from the extraction solution. The DVB solid-phase extraction was prepared by first washing with 2 ml of ethanol, followed by 2 ml of DI water. The cotinine was removed from the extraction solution by passing it through the column. The column was washed with 1 ml of DI water and allowed to dry for 5 to 30 min by drawing or forcing air through the column. The cotinine was eluted with 3.0 ml of 20% methanol/ether. The methanol/ether was removed by using a stream of dry nitrogen. Methanol (150 μl) was added and the sides were carefully washed. The solution was placed into an autosampler vial and injected into a GC/MS.
Gas Chromatography and Mass Spectrometry Conditions for Cotinine
The gas chromatography column was a DB-5 column with a length of 15 m, an inner diameter of 0.25 mm, and a film thickness of 0.25 μm. The inlet temperature was 285°C with the injection set for a splitless injection. One microliter was injected. The carrier gas was He, with a flow rate of 2.1 ml/min. The column was held at 100°C for 0.1 min, after which it was ramped to 175°C at a rate of 40°C/min, then to 300°C at 30°C/min. The mass spectrometer was a Leco Pegasus III time of flight. The ionization was through electron impact at 70 eV. The solvent delay was 50 sec. The scan range was from 50 to 210 m/z. The number of scans was 15 per sec. The ion source temperature was 200°C.
The quantification was carried out by measuring the ratio of the area of m/z 176 to the area of m/z 180, where 180 was the d4 cotinine internal standard. The retention time varied during the running of sets of samples so the retention time was set using the internal standard as a reference. The results for detected cotinine are reported in nanograms.
Analytical Determination of Gossypol
The overall method for gossypol analysis of H. virescens tissues is summarized in Fig. 1b. The method involved the formation of a Schiff base with aniline, which permits the total amount of gossypol present (bound and unbound) to be determined (Pons et al. 1958; Rojas et al. 1992; Kim et al. 1996). The resulting dianiline complex was further isolated from the moth matrix with divinyl benzene solid-phase extraction, and then was quantified by using high-pressure chromatography coupled with a triple quadrupole mass spectrometer (MS) as the detector. Electrospray ionization (ESI) was used with post addition of ammonia after the LC column. The ESI was operated in negative ionization mode. Because the matrix is complex and the HPLC method was not set for optimum selectivity, the triple quadrupole was operated in reaction monitoring mode. In this mode, the pseudomolecular ion representing the dianiline–gossypol (667 m/z) was selected by the first quadrupole, followed by collision-induced dissociation in the second quadrupole region. The collision resulted in the loss of one aniline (574 m/z) and, to a lesser degree, the loss of a second aniline (481 m/z). These fragments were monitored in the third quadrupole. Deuterated aniline complexed with gossypol was used as an internal standard for the HPLC/MS/MS determination.
The derivatizing reagent was prepared by mixing 1 ml aniline, 5 ml glacial acetic acid, and 44 ml dimethylformamide. This reagent lasted for up to 1 wk if refrigerated at 4°C. The deuterated derivatizing reagent was prepared by mixing 0.1 ml d5-aniline, 0.5 ml acetic acid, and 4.4 ml dimethylformamide. This was used within 24 hr for preparing the deuterated internal standard. The other necessary solutions were, by volume, 20% water/methanol, 10% water/methanol, 5% acetone/methanol, 50% DMF/water, 0.6% NH4OH, wash solvent of 100% methanol, acetonitrile/1% acetic acid, and 2:1 acetonitrile/acetone.
The standard stock solution of gossypol was prepared by weighing approximately 10 mg gossypol-acetic acid into a 100-ml volumetric flask, which was filled to the mark with acetonitrile/1% acetic acid. This solution was stored in an amber glass bottle at 4°C and used within 3 mo. The gossypol-acetic acid standard stock solution was used to prepare a 10 μg/ml standard solution of the dianilino–gossypol by taking 500 μl of the gossypol-acetic acid standard stock solution and removing the solvent under a dry nitrogen stream. To this, 1.0 ml of the aniline derivatizing reagent was added, and the solution was heated at 90°C for 30 min. The excess aniline was removed with a 100 mg DVB SPE column that had been prerinsed with 1.0 ml of acetone, 1.0 ml of methanol, and 1.0 ml of 1:1 water/DMF. The column beds were wetted with the 1:1 water/DMF. One milliliter of DI water was added to the 1.0 ml of dianiline–gossypol solution. This solution was put through the column, which retained the dianiline–gossypol. The column was rinsed with 0.8 ml of 20:80 water/methanol. The gossypol derivative was eluted from the column with 2 ml of acetone into an amber vial, and the acetone was removed with dry nitrogen. This yielded 50 μg of dianiline–gossypol, which was used with acetonitrile to prepare standards ranging from 25 to 75 ng/ml. These standards were prepared on a daily basis because the dianiline–gossypol was not stable once diluted with acetonitrile.
The deuterated dianiline–gossypol internal standard also was prepared on a daily basis. The 1.0 ml of the standard gossypol stock solution was blown dry, and 1.0 ml of the d5-aniline derivatizing reagent solution was added to the dry residue. The solution was capped and heated for 30 min at 90°C. To use this d 10-dianiline-gossypol as an internal standard, it was necessary to remove the excess d5-aniline. This was accomplished by using a 100-mg DVB SPE column, which was prerinsed with 1.0 ml of acetone, 1.0 ml of methanol, and 1.0 ml of 1:1 water/DMF. The column beds were wetted with the 1:1 water/DMF, and 1.0 ml of DI water was added to the 1.0 ml of d 10-dianiline–gossypol solution. This solution was passed through the column, which retained the d 10-dianiline–gossypol. The column was rinsed with 0.5 ml of 20:80 water/methanol, and the d 10-dianiline–gossypol was eluted from the column with 2 ml acetone into an amber vial. The acetone was removed with dry nitrogen, yielding 100 μg of d 10-dianiline–gossypol. This was used to prepare a 5 μg/ml solution by using acetonitrile.
The individual freeze-dried moths were placed into a 2-ml 96-well plate. For routine analysis, five method blanks, two positive control moths reared on cotton, and two negative control moths reared on plants other than cotton were included. These controls were placed at random in the plate by using a pseudorandom number generator. A glass bead was added to each well, and the plate was capped and placed onto a mega grinder and shaken at 1,100 rpm for 45 sec to grind up the moths (Deppermann 2005). If the moths had been through the cotinine determination, the grinding steps had already occurred. The aniline derivatizing solution (1.0 ml) was added to each well. The plate was sealed by placing a sheet of Parafilm over the top, a new cap was added, and the cap was pressed into place. The plate was vortexed at a low setting for 1 min. The Parafilm and cap were removed, and the plate was covered with aluminum foil and placed in a well heater set at 80–90°C. The solution was heated for 1 hr and then allowed to cool. The samples were cleaned up by using a 96-well DVB plate containing 50 mg of DVB and a positive pressure apparatus (SpeedDisk 96 Pressure Processor from Mallinckrodt Baker, Inc., Phillipsburg, NJ, USA). The 50-mg SPE DVB 2-ml 96-well plate was prerinsed with 0.5 ml of acetone, 0.5 ml of methanol, and 0.5 ml of 1:1 water/DMF. Column beds were wetted with 1:1 water/DMF. Water (0.5 ml) was added to each well of the 96-well plate. The plate was resealed with a clean sheet of Parafilm, capped, and the cap was pressed into place. The plate was centrifuged at 2,500 to 3,000 rpm for 5 min. With a multipipetter, the solution was transferred to the DVB 96-well plate, taking care to leave solid residue behind. The residue was washed with 1.0 ml of 1:1 water/DMF, and this was passed through the DVB 96-well plate. The dianiline–gossypol was retained in the DVB. The DVB column was washed with 0.5–0.6 ml of 20:80 water/methanol, followed by 0.45–0.5 ml of 5:95 acetone/methanol. A clean 96-well plate was placed under the DVB 96-well plate, and the dianiline–gossypol was eluted with 0.75 ml of acetone. The acetone was removed under a dry stream of nitrogen. It was found, at this point, that the samples could be stored for 48 hr without loss of signal. For HPLC/MS/MS determination, 200 μl of acetonitrile were added to each well, along with 20 μl of 5 μg/ml d 10-dianiline–gossypol internal standard. The autosampler was equipped for 0.5 ml 96-well plates so it was necessary to transfer the solution to such a plate. The plate was capped and the autosampler was set for less than 18°C.
If only a few samples were to be analyzed, then individual SPE DVB 3-ml columns were used in place of the DVB 96-well plate. The same procedure as for the 96-well DVB plates was followed, except that the volumes were doubled.
HPLC/MS/MS
Solvent A was 10% water/methanol and solvent B was acetonitrile/acetone 2:1. The flow rate through the Agilent Eclipse XDB-C8 (2.1 × 12.5, 5 μ) guard column was set for 0.250 ml/min. The gradient was 0% B for 2.1 min, which then was brought to 60% B in 4 min. Post column, a 0.6% ammonium hydroxide modifier was added at a flow rate of 0.050 ml/min to enhance the negative ion signal. The volume of the sample extract injected was 5 μl. The 0.3 ml/min output of the HPLC system was put directly through the electrospray ionization source of a Micromass Quattro Ultima LC/MS/MS system (Waters, Milford, MA, USA). The electrospray source was operated in negative ion mode. The process forms M–H− pseudomolecular ions (667 m/z), which undergo collision activation to form a rearrangement fragment at 574 m/z reflecting the loss of one aniline. For further confirmation, the loss of a second aniline at 481 m/z also was monitored. The sensitivity of the loss of the second aniline was relatively low so it was used only for confirmation.
Characterization of the Cotinine and Gossypol Analyses
Two methods were used to characterize the accuracy and sensitivity of both analytical methods. First, recovery experiments were conducted with both methods. For the cotinine analysis, cotinine was spiked into samples of TBW adult moths that had not been reared on tobacco. For the gossypol analysis, gossypol was spiked into samples of adult moths that had not been reared on cotton. The spike levels ranged from 5 to 20 ng per moth, and the recovery experiment was replicated over a number of days. The percent recovery was calculated as the ratio of the measured response for the spiked moth to the measured response for the standard. In each case, the response was a ratio of the peak area measured for the analyte to the peak area measured for the internal standard. Because the cotinine method provides an absolute measurement, absolute recoveries could be obtained for the cotinine analysis as compared to relative values for the gossypol analysis.
The second approach for estimating bias was to estimate positive and negative error rates by analyzing adult moths that were reared on known hosts. The gossypol analysis was characterized with a set of 193 TBW moths reared on different known host plants: cotton (118), soybean (7), peanut (5), velvet leaf (30), and an artificial laboratory diet with no cotton content (33). The moths were submitted for analysis with labels that did not indicate on which plant they were reared. Based on the chromatograms for individual moths, Tukey’s honestly significantly different (HSD) multiple comparison procedure was used to conduct all possible pair-wise comparisons of the average response ratio and signal-to-noise ratio for the different larval host plants. The statistical analyses were carried out by using SAS Version 8.2 (SAS 2001). A set of criteria for determining if gossypol were present (whether the test was positive) was determined by using a set of cotton reared moths and noncotton reared moths. The criteria were based on the response ratio and the signal-to-noise ratio for the chromatogram signal.
The characterization of the cotinine analysis was carried out in the same fashion but on a smaller scale so that estimates of positive and negative error rates could not be determined. In addition, to determine if the cotinine method and the gossypol method could be used on the same moths, a set of moths reared on different plants were analyzed for both cotinine and gossypol: soybean (3), peanut (3), tobacco (3), and cotton (3).
An additional experiment was conducted to determine if the gossypol signal in adult TBW reared on cotton is sufficiently stable to be detectable under a reasonable range of field conditions. TBW were reared in cages on cotton or chickpea plants in the field. After larvae pupated and emerged as adults, a total of 73 cotton-reared adult TBW were held alive for various lengths of time (0–11 d), then sacrificed and left exposed to the elements for different lengths of time (0–7 d) before being frozen. These treatments were chosen to cover the range of conditions that might occur in collecting moths in the field. Eleven days represents the upper end of the TBW adult lifespan and, therefore, is as long as an adult would be expected to live before being captured in the field. Similarly, moths might remain in a trap for several days after dying and before collection. The 73 cotton-reared moths, together with 19 TBW moths reared on chickpea, were coded and run through a blind analysis for gossypol. Tukey’s HSD multiple comparison procedure was used to conduct all possible pair-wise comparisons of the average response ratio and signal-to-noise ratio for the different treatment combinations. The statistical analyses were carried out by using SAS Version 8.2 (SAS 2001).
Application of the Cotinine and Gossypol Analyses to Field-collected TBW Moths
To demonstrate the suitability of the cotinine and gossypol analyses for the study of field-collected moths, a small set of moths collected in North Carolina was first analyzed for cotinine and then for gossypol. In total, 30 moths from Lenoir County and 78 moths from Wilson County were analyzed individually as described above.
Results
Cotinine Analysis
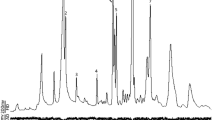
Figure 2 shows the ion chromatogram for cotinine for a moth reared on cotton and a moth reared on tobacco. The signal for cotinine in the tobacco-reared moth represents 115 ng. The cotinine ion chromatogram for the cotton-reared moth (the bottom chromatogram) represents a detection limit of less than 1.5 ppb by dry weight (Fig. 2). Figure 3 shows the calibration curve for cotinine over the range of 200 to 2.6 ng. The method for cotinine also can provide estimates for nicotine.
Ion chromatogram for 176 m/z for adult moths reared on tobacco (top) and cotton (bottom). The bottom figure is the ion chromatogram for 176 m/z for an adult moth with cotton as the host plant. The peak in the top figure at 159 sec is cotinine. The detection limit represented by the lack of a peak at 159 sec in the bottom figure is 1.5 ppb by dry weight
Gossypol Analysis
Figure 4 shows the reaction ion monitoring chromatogram for a gossypol standard (5 ng in 200 μl). The bottom figure shows the pseudomolecular ion of the dianiline–gossypol 667 m/z losing an aniline to give 574 m/z. This represents 125 pg injected. The top portion of Fig. 4 is the corresponding chromatogram for d 10-dianiline–gossypol (677 m/z with loss of one aniline to give 579 m/z). This represented 2.2 ng that was injected. The chromatograms were normalized to 100. The peak intensity is shown in the upper-right-hand corner and was 1.38 × 106 for the internal standard and 1.78 × 104 for the 5-ng standard. The internal standard was for use with the HPLC/MS/MS system only and did not correct for incomplete conversion to the dianiline–gossypol complex or for recovery.
Figure 5 shows the reaction monitoring chromatogram for an adult moth reared on soybeans; the bottom chromatogram is the response for the dianiline–gossypol and the top is the internal d 10-dianiline–gossypol standard as in Fig. 4. The d 10-dianiline–gossypol internal standard is not pure and has a small contribution to the dianiline–gossypol signal but the level of d 10-dianiline–gossypol was selected so that the contribution would be below detection limits.
Figure 6 shows the results for an adult moth that was reared on cotton. The comparison of the adult moth reared on soybean and the cotton-reared adult moth show that the two can be readily distinguished.
Recovery Experiments for Gossypol
The recovery experiments were carried out on 16 different days over the course of a 37-d period. The average over all of runs was 111% (SE = 10%). If one extreme outlier (240%) is excluded, the average recovery was 102% (SE = 5%). The outlier probably resulted from excess aniline remaining in the extraction solution.
Estimation of Positive and Negative Error Rates for Gossypol Method
Table 1 shows the results for the analysis of 193 moths reared on known plant hosts. All of the cotton-reared moths were classified as positive for gossypol, indicating that the method has a low rate of false negatives. However, for the criteria used, 14 of the 75 moths reared on noncotton hosts were incorrectly judged as positive for gossypol, indicating that the method yields some false positive results, at least with the decision criteria used in this case. The rate of false positives tended to be higher for moths reared on artificial diet than for moths reared on noncotton plant hosts (25% vs. 16%). However, all of the false positives had relatively low response ratios and signal-to-noise ratios (see Table 2) indicating that it should be possible to adjust the decision criteria to reduce these error rates. This was accomplished in subsequent testing, in which false positive rates were consistently <10% (data not shown).
Accuracy of Cotinine and Gossypol Analyses When Performed on the Same Insects
Table 2 shows the results of cotinine analysis followed by gossypol analysis for a set of moths reared on known plant hosts. All moths reared on tobacco were correctly identified with no errors. Likewise, the moths that were reared on cotton and had gone through the determination for cotinine followed by the gossypol determination were correctly identified as cotton-reared moths.
Stability and Detection of Gossypol in Older and Deceased Moths
The results of the gossypol analysis on aged moths are presented in Table 3. The blind analysis correctly identified all moths; 100% of the cotton-reared moths were determined to be positive for gossypol, and none of the moths reared on noncotton plants was determined positive for gossypol. Therefore, a significant gossypol signal was detected in all cotton-reared moths regardless of the age of the moth and/or the length of time the moth was held in a trap after death. The strength of the gossypol signal (measured as either signal-to-noise ratio or as relative response) for cotton-reared moths was always greater than the highest signal observed for moths reared on noncotton plants.
Cotinine and Gossypol Analyses of Field-collected TBW Moths
The results of the analyses of the field-collected moths are shown in Table 4. In both counties (Lenoir and Wilson), and in both months (July and August), a higher proportion of the moths was positive for cotinine than for gossypol, indicating that more of the moths developed on tobacco than on cotton. In all cases, the percentage of cotton-derived TBW was less than 4%. The percentage of tobacco-derived moths was higher in the samples from Wilson County than those from Lenoir County, and was higher in the samples from July than in the samples from August.
Discussion
Characteristics and Limitations of the Analyses
The prerequisites for the gossypol and cotinine analyses included high selectivity, low detection limits, and the capability to analyze hundreds of samples (insects) per day. The selectivity and high throughput for the gossypol analyses were accomplished by using a combination of analyte isolation followed by the use of tandem mass spectrometry. The gossypol extraction involves the formation of a Schiff base with aniline. The formation of an amine Schiff base is a common approach to determining the total amount of gossypol in a variety of different matrices including cotton (Smith 1967; Stipanovic et al. 1985; Benson et al. 2001), ovine tissues (Kim et al. 1996), blood plasma (Dabrowski et al. 2001), and oils. Bound gossypol is believed to form a Schiff base with proteins (Dabrowski et al. 2001). The addition of excess aniline removes the gossypol from the protein forming an extractable dianilino–gossypol Schiff base.
The sample preparation before determination of gossypol by the HPLC ESI/MS/MS was complicated. When performed by using a single SPE column, no cross-contamination of samples was observed. To accommodate 200 or more analyses per day, the sample processing was adapted to a 96-well plate format. The lyophilized moths were ground with glass beads that could remain in the well and not interfere with the extraction solution. Metal ball bearings were tried initially but interfered with the extraction. Attempts to remove the metal ball bearings caused cross-contamination between wells. Cross-contamination between wells was believed to be the main source of false positives in this approach. Attempts to minimize this included the use of Parafilm between the 96-well cap and the plate to provide a tight seal during the extraction step. The routine use of blanks and other negative and positive controls were necessary because of the potential for cross-contamination of wells. The blanks were method blanks with no moth present and were placed at random throughout the plate by using a pseudorandom number generator.
As shown in Figs. 4, 5, 6, the dianilino–gossypol detection was complete after 2.5 min. The top of the peak occurred at approximately 1 min. The C8 guard column used in the introduction of the sample to the ESI source helped remove material that diminished the electrospray ionization process, but did not provide the selectivity of a high-resolution analytical column. The best conditions for separation were at low pH. However, these conditions provide poor ionization. The best ionization was observed under neutral or basic conditions. Several other columns were examined with the best separation occurring with a polydivinyl column (PRP-1). However, the solvents used for this column were not conducive to the ESI process. In addition, the time for each analysis was over 12 min. This limited the number of samples that could be analyzed per day. In Figs. 4, 5, 6, the time period after 2.5 min was used for washing the guard column. The total cycle time for each sample injection was 5 min. Thus, over a 20-hr period, 240 samples could be processed through the HPLC ESI/MS/MS system.
The internal standard for the ESI/MS/MS had two main purposes: first, to verify that injection had occurred, and second, to correct for matrix effects that occurred because of the electrospray ionization process. Variation in the mass of the moth caused variation in the electrospray ionization process, leading to similar spike levels producing different peak areas. The lower peak areas appeared to occur with larger moths. The use of the d10-dianiline–gossypol corrected for this variation because the ratio of dianiline–gossypol to d10-dianiline–gossypol remained constant throughout.
The ideal internal standard would be a stable isotope of gossypol. Because this was not available at the time, deuterated aniline was used to generate a stable isotope, which led to some methodological challenges. First, if excess aniline or deuterated aniline remained in solution with the dianiline–gossypol and the d10 dianiline, this would lead to exchange of the complexed aniline with the free aniline. If excess aniline were present in the extraction solution, this would have exchanged with the deuterium-labeled aniline of the internal standard, giving rise to aniline-d5 anilino gossypol and dianiline gossypol, which would have been seen as false positives. If there is no desire to estimate the amount of gossypol present in a sample, the d10-aniline-gossypol could be eliminated and an internal standard for injection such as butylanthraquinone could be used (Benson et al. 2001). This would not correct for matrix effects on the ionization but would provide confirmation of sample injection.
The cotinine determination has all of the components for a highly selective and accurate method. The method uses a stable isotope internal d4 cotinine standard. The d4 cotinine is added at the beginning of the extraction procedure for cotinine compensating for recovery losses through all of the steps of the procedure. The high selectivity of the method yields detection limits that are less than 0.037 ng per moth or for a 30-mg moth, a detection limit of less than 1.5 ppb by dry weight. This detection limit is based on a signal-to-noise in the cotinine response of three to one. Therefore, recovery is assumed to be 100% for this level of detection. A nicotine stable isotope can be used in this analysis. However, environmental contamination apparently led to low levels of nicotine being detected in moths reared on host plants other than tobacco. The levels were low compared to moths on which the host was tobacco (data not shown). The likely source of the nicotine contamination was handling of the moths by people using tobacco products.
Applications of the Method
One way to slow the rate at which insects evolve resistance to insecticides is to ensure the presence of a refuge where susceptible pest insects are not exposed to the insecticide. This strategy has been widely used in managing transgenic corn and cotton containing insecticidal proteins derived from the bacterium Bacillus thuringiensis (so-called Bt crops). The refuge acts as a source of susceptible pest insects that are available to mate with any resistant insects emerging from the Bt crop fields, thereby “diluting” any resistance present and increasing the durability of the Bt crop.
The refuge may consist of portions of the crop of interest that are untreated, like structured conventional cotton refuges for transgenic Bt cotton. The refuge also may include other suitable crop and weedy hosts of the insect pest (natural or alternative host refuge). Evaluating the refuge available for an insect that is capable of developing on multiple host plant species requires some means of evaluating the portion of the insect population that exists on the different potential hosts. For example, studies of larval host use by the important cotton pest, Helicoverpa armigera, in India (Ravi et al. 2005) and China (Wu et al. 2002) demonstrated that many commonly planted crops supported larger larval populations than cotton at the same time that this pest is found feeding on cotton. However, larval surveys of this sort are labor intensive and provide only inferential information on the composition of adult populations. Trapping adults and using suitable diagnostic tools to deduce the host on which a given insect has completed larval development can provide more accurate and comprehensive information about the relative contribution of different plant hosts to regional pest population dynamics.
The method described in this paper has been used for this purpose; the proportion of TBW moths derived from alternative noncotton hosts (the natural refuge) was estimated for populations trapped around Bt cotton fields using the gossypol assay on thousands of individual moths collected in pheromone traps (Head et al., unpublished data). When these assays were run at a large scale, no false negatives were observed with the positive controls, and a rate of <5% of false positives was observed with the negative controls (moths reared on noncotton hosts and method blanks). Therefore, analytical methods that detect the presence of host plant-specific chemicals such as those described in this paper have the ability to quantify accurately host use patterns in polyphagous agricultural pests.
References
Benson, C. G., Wyllie, S. G., Leach, D. N., and Fitt, G. P. 2001. Improved method for rapid determination of terpenoid aldehydes in cotton. J. Agric. Food Chem. 49:2181–2184.
Dabrowski, K., Lee, K. J., Rinchard, J., Ciereszko, A., Blom, J. H., and Ottobre, J. S. 2001. Gossypol isomers bind specifically to blood plasma proteins and spermatozoa of rainbow trout fed diets containing cottonseed meal. Biochim. Biophys. Acta 1525:37–42.
Deppermann, K. L. 2005. U.S. Patent number 6,880,771.
Fitt, G. P. 1989. The ecology of Heliothis species in relation to agroecosystems. Annu. Rev. Entomol. 34:17–52.
Gould, F., Blair, N., Reid, M., Rennie, T. L., Lopez, J., and Micinski, S. 2002. Bacillus thuringiensis-toxin resistance management: Stable isotope assessment of alternate host use by Helicoverpa zea. Proc. Natl. Acad. Sci. U S A 99:16581–16586.
Kim, H. L., Calhoun, M. C., and Stipanovic, R. D. 1996. Accumulation of gossypol enantiomers in ovine tissues. Comp. Biochem. Physiol. 113B:417–420.
Kim, I., Darwin, W. D., and Huestis, M. A. 2005. Simultaneous determination of nicotine, cotinine, norcotinine, and trans-3′-hydroxcotinine in human oral fluid using solid phase extraction and gas chromatography-mass spectrometry. J. Chromatography B 814:233–240.
Pons, W. A. Jr., Pittman, R. A., and Hoffpauir, C. L. 1958. 3 Amino-1-propanol as a complexing agent in the determination of total gossypol. J. Am. Oil Chem. Soc. 35:93–97.
Ravi, K. C., Mohan, K. S., Manjunath, T. M., Head, G., Patil, B. V., Greba, D. P. A., Premalatha, K., Peter, J., and Rao, N. G. V. 2005. Relative abundance of Helicoverpa armigera (Lepidoptera: Noctuidae) on different host crops in India and the role of these crops as natural refuge for Bacillus thuringiensis cotton. Environ. Entomol. 34:59–69.
Rojas, M. G., Stipanovic, R. D., Williams, H. J., and Vinson, S. B. 1992. Metabolism of gossypol by Heliothis virescens (F.) (Lepidotera: Noctuidae). Environ. Entomol. 21:518–526.
SAS Institute. 2001. SAS, Version 8.2. SAS Institute, Cary, NC.
Smith, F. N. 1967. Determination of gossypol in leaves and flower buds of Gossypium. J. Am. Oil Chem. Soc. 44:267–269.
Stipanovic, R. D., Williams, H. J., Smith, L. A., and Vinson, S. B. 1985. The hormetic effect of gossypol on the growth of Heliothis virescens larvae, pp. 392–393, in J. M. Brown and T. C. Nelson (eds.). Proceedings—Beltwide Cotton Conferences. NCC, Memphis, TN.
Wu, K., Guo, Y., and Gao, S. 2002. Evaluation of the natural refuge function for Helicoverpa armigera (Lepidoptera: Noctuidae) within Bacillus thuringiensis transgenic cotton growing areas in North China. J. Econ. Entomol. 95:832–837.
Acknowledgments
We thank Ryan Jackson (USDA-ARS, Stoneville, MS) for providing the TBW moths reared on known hosts and for coding samples for the blind experiment on gossypol signal stability. James Jennings, Melinda McCann, and Eric Sachs (Monsanto Company) provided helpful comments on the manuscript. Sharon Alexander and Mary Anderson (Monsanto Company) contributed tireless technical assistance with the gossypol assays.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orth, R.G., Head, G. & Mierkowski, M. Determining Larval Host Plant Use by a Polyphagous Lepidopteran Through Analysis of Adult Moths for Plant Secondary Metabolites. J Chem Ecol 33, 1131–1148 (2007). https://doi.org/10.1007/s10886-007-9284-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9284-3