Abstract
We investigated the chemical composition and phytotoxicity of the essential oil extracted from leaves of Artemisia scoparia Waldst. et Kit. (red stem wormwood, Asteraceae). GC/GC-MS analyses revealed 33 chemical constituents representing 99.83% of the oil. The oil, in general, was rich in monoterpenes that constitute 71.6%, with β-myrcene (29.27%) as the major constituent followed by (+)-limonene (13.3%), (Z)-β-ocimene (13.37%), and γ-terpinene (9.51%). The oil and β-myrcene were evaluated in a dose–response bioassay under laboratory conditions for phytotoxicity against three weeds—Avena fatua, Cyperus rotundus, and Phalaris minor. A significant reduction in germination, seedling growth, and dry matter accumulation was observed in the test weeds. At the lowest treatment of 0.07 mg/ml Artemisia oil, germination was reduced by 39%, 19%, and 10.6% in C. rotundus, P. minor, and A. fatua, respectively. However, the inhibitory effect of β-myrcene was less. In general, a dose-dependent effect was observed and the growth declined with increasing concentration. Among the three weeds, the inhibitory effect was greatest on C. rotundus, so it was selected for further studies. We explored the explanation for observed growth inhibition in terms of reactive oxygen species (ROS: lipid peroxidation, membrane integrity, and amounts of conjugated dienes and hydrogen peroxide)-induced oxidative stress. Exposure of C. rotundus to Artemisia oil or β-myrcene enhanced solute leakage, indicating membrane disintegration. There were increased levels of malondialdehyde and hydrogen peroxide, indicating lipid peroxidation and induction of oxidative stress. We conclude that Artemisia oil inhibits plant root growth through generation of ROS-induced oxidative damage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic plants and their volatile oils have been in use since antiquity in flavor and fragrances, in medicines, as antimicrobial and insecticidal agents, and to repel insects and stored product pests (Bakkali et al. 2008; Batish et al. 2008). However, due to fumigant and insecticidal activity, the interest in volatile oils has increased tremendously during the last decade or so. Volatile terpenes act as pollinator attractants, provide an important defense strategy against herbivores and pathogenic fungi, play a significant role in plant–plant interactions, and depict an evolutionary relationship with their functional roles (Langenheim 1994; Batish et al. 2008). Of late, volatile oils and their constituents are being explored for weed and pest management and viewed as an important source of lead molecules (Romagni et al. 2000; Batish et al. 2004; Singh et al. 2005; Bakkali et al. 2008). It is, thus, pertinent to explore and characterize the phytotoxic properties of aromatic plants and their volatile oils.
The genus Artemisia (commonly wormwoods; family Asteraceae) consists of a diverse group of around 200 herb and shrub species distributed throughout the world (Anonymous 1993). The plants are rich in volatile oils that exhibit a wide spectrum of biological activity and find extensive use in medicine (Anonymous 1993). Additionally, the volatile oil of certain Artemisia species (Artemisia tridentata, Artemisia princeps, and Artemisia vulgaris) inhibits emergence and growth of nearby plants (Weaver and Klarich 1977; Yun et al. 1993; Barney et al. 2005). The characteristic vegetation patterning around Artemisia californica thickets that is due to volatile terpenes is one of the classical examples of allelopathy (Muller et al. 1964). Artemisia scoparia (red stem wormwood) is an annual aromatic species growing wild in India. It forms monospecific strands along canals, agricultural fields, roadsides, and wastelands (Anonymous 1993). However, the role of its volatile oil in suppressing the emergence and growth of other plants in its vicinity remains largely unknown. We, therefore, extracted and characterized oil from leaves of A. scoparia, and assessed the phytotoxicity of both the oil and its major constituent. We selected Avena fatua and Phalaris minor (weeds of wheat crop), and Cyperus rotundus (a weed of rice crop) as bioassay plants with a view to test Artemisia oil as a weed suppressant and its potential use as a novel bioherbicide. We further explored the possible mechanism of phytotoxicity of Artemisia oil in terms of reactive oxygen species (ROS: lipid peroxidation, membrane integrity, and amounts of conjugated dienes and hydrogen peroxide)-induced oxidative stress.
Methods and Materials
Extraction of the Oil
Volatile oil was extracted from fresh leaves of A. scoparia Waldst. & Kit. (red stem wormwood; hereafter Artemisia) by hydrodistillation using Clevenger’s apparatus. For this, during the second week of June 2007 (summer season), leaves were collected from Artemisia plants growing in wastelands around Chandigarh.
Leaves (250 g) were chopped and mixed with distilled water (1 l) in a round bottom flask (2 l) and boiled for 3 h. The oil was collected from the nozzle of the condenser, dried under sodium sulfate, and stored at 4°C for identification and bioassay. The oil was clear yellow in color with a yield of 0.17% (v/w, fresh weight basis).
Oil Analysis
The extracted oil was analyzed by gas chromatography (GC) and gas chromatography–mass spectroscopy (GC-MS) as per Singh et al. (2009).
GC was done on a Shimadzu GC-17A gas chromatograph equipped with a flame ionization detector (FID) and DB-5 column (60 m × 0.25 mm, i.d., film thickness 0.25 μm). Helium (He) at a split ratio 1:20 and a flow rate 1 ml min−1 was used as carrier gas. The injector and detector temperature were set at 250°C and 280°C, respectively. Initially, the oven temperature was 50°C, held isothermally for 2 min, then increased to 260°C at the rate of 4°C min−1, and finally held at 260°C for 3 min. The relative amount of different constituents was determined by computerized peak area normalization based upon three injections of the oil, without any correction factor. Peaks were compared with data from GC-MS.
GC-MS analysis was performed on a Shimadzu QP 2010 mass spectrophotometer equipped with fused silica (SGE BP 20) capillary column (30 m × 0.25 mm, i.d., 25 µm film thickness). He at a split ratio of 1:50 and a linear velocity of 38.5 cm s−1 was used as carrier gas. The injector and detector temperature were set at 220°C and 250°C, respectively. The temperature was programmed from 70°C (held isothermally for 4 min) to 220°C at the rate of 4°C min−1 and held at 220°C isothermally for 5 min. Mass spectral range was recorded from m/z 40 to 600 amu. The identification of different constituents was based on: (1) the comparison of their retention times with those of pure reference samples from Sigma-Aldrich (St. Louis, MO, USA), Fluka (Buchs, Switzerland), Acros (Geel, Belgium), AlfaAeser (Ward Hill, MA, USA), and TCI (Tokyo, Japan); (2) co-elution with available authentic standards; (3) comparison of retention indices (RI) with reference to a homologous series of n-alkanes (C 7 -C 30 ; Supelco, Bellefonte, PA, USA); and (4) computer matching of mass spectra by using library search system HP-5872 (Hewlett-Packard) and consulting data bases of Wiley 275 and NBS 75K Libraries (McLafferty 1989), NIST 98 (Stein 1990), and compilation by Adams (1995).
Procurement of Materials
For laboratory bioassay, seeds of A. fatua, C. rotundus, and P. minor were collected locally from agricultural fields on the outskirts of Chandigarh. These were surface-sterilized with sodium hypochlorite (1%, w/v) for 2 min, washed under running tap water (for 5 min) followed by distilled water and stored for further use. β-Myrcene (the major component of Artemisia oil) of technical grade (purity 90%) was used in the bioefficacy experiments and purchased from AlfaAesar (Ward Hill, MA, USA). All other chemicals were of analytical grade and purchased from the best available sources (Sisco Research Laboratory, Mumbai, India; Loba-Chemie, Mumbai; Sigma-Aldrich, St. Louis, MO, USA).
Laboratory Bioassay
Artemisia oil and β-myrcene were evaluated for phytotoxicity in a dose–response manner under laboratory conditions. Seeds of test plants were imbibed in distilled water for 16 h. Pre-imbibed seeds (15 of A. fatua or 25 of C. rotundus and P. minor) were placed in 15-cm diameter Petri dishes on Whatman No. 1 filter paper moistened with 7 ml of distilled water. Different amounts (0.5–5.0 µl) of Artemisia oil or β-myrcene were loaded onto the inner side of the lid of the Petri dish to obtain concentrations of 0.07, 0.14, 0.35, and 0.70 mg/ml. After treatment, dishes were sealed with Parafilm®. A set of Petri dishes without oil or β-myrcene served as a parallel control. For each treatment, including control, five Petri dish replicates were maintained in a randomized block design. All dishes were kept in a growth chamber set at 25 ± 2°C temperature, 16/8 h L/D photoperiod with photosynthetic photon flux density of ~225 µmole m−2 s−1 and a relative humidity of nearly 80%. After 1 week, the number of seeds that germinated was counted and the length (from tip of root to tip of shoot) and dry weight (by oven drying at 60°C for 48 h) of emerged seedlings were measured. Among the three weeds, the inhibitory effect was the greatest on C. rotundus, so it was selected for further biochemical studies by exploring the induction of oxidative damage as a possible mechanism of phytotoxicity. Roots of C. rotundus were excised and kept at 4°C prior to further studies/analyses.
Determination of Cell Survivability
Cell survivability was determined spectrophotometrically by using 2,3,5-triphenyl tetrazolium chloride (TTC) as per Singh et al. (2002). It provides an indirect method of measurement of cell respiration since TTC absorbs electrons from the respiratory chain (Batish et al. 2007a). Absorbance was read at 530 nm and the values expressed with respect to control.
Effect on Membrane Integrity
Effect of Artemisia oil or β-myrcene was studied on membrane integrity (an indicator of cellular damage) in terms of ion leakage from the roots of C. rotundus by measuring conductivity of the bathing medium (Duke and Kenyon 1993). Roots (200 mg) were incubated in 5 ml of 1 mM MES buffer (2-[N-morpholino] ethanesulphonic acid sodium salt, pH 6.5) containing 2% sucrose with Artemisia oil or β-myrcene (0.14 and 0.35 mg/ml; selected on the basis of the laboratory bioassay) dissolved in Tween-80. A parallel set up without Artemisia oil or β-myrcene, but containing MES buffer and Tween-80 only, was maintained as control. The conductivity of the bathing medium containing C. rotundus roots with or without treatment was measured with a conductivity meter (ECOSCAN CON5, Eutech Instuments Pte. Ltd., Singapore) at regular intervals in dark (0, 1, 2, 4, 8, 12, 16, 18, and 20 h) followed by exposure to light for a further 10 h (i.e., at 22, 24, 26, 28, and 30 h). The maximum electrolyte leakage from root tissue was determined by boiling the roots for 15 min. The experiment was repeated twice with five replicates and the data are presented as means.
Lipid Peroxidation
Lipid peroxidation was measured in terms of malondialdehyde (MDA) and conjugated dienes content. MDA, a major thiobarbituric acid reactive species, was determined as per Heath and Packer (1968). Roots (100 mg) of C. rotundus were homogenized in 5 ml of trichloroacetic acid (TCA, 0.1%, w/v) in a pre-chilled pestle and mortar and centrifuged at 10,000×g for 10 min. One milliliter of the supernatant was added to 4 ml of thiobarbituric acid (0.5%, w/v, in 20% TCA). The mixture was heated at 95°C for 30 min, cooled over ice, and then centrifuged at 10,000×g for 10 min. The absorbance of the supernatant was read at 532 nm and corrected for non-specific absorbance at 600 nm. MDA content was determined by using the extinction coefficient (ε) of 155 mM cm−1 and expressed as nmol g−1 FW (fresh weight).
Conjugated dienes content was determined by homogenizing roots (100 mg) of C. rotundus in 5 ml of 95% (v/v) ethyl alcohol (Singh et al. 2007). The mixture was centrifuged at 10,000×g for 10 min, and the absorbance was read at 234 nm. The content of conjugated dienes was determined by using an extinction coefficient of 26.5 mM cm−1, and expressed as µmol g−1FW.
Determination of Hydrogen Peroxide (H2O2) Content
Roots (100 mg) of C. rotundus were homogenized with 5 ml of 0.1% TCA (w/v) in a pre-chilled pestle and mortar and centrifuged at 12,000×g for 15 min (Singh et al. 2007). To 0.5 ml of supernatant, 0.5 ml of phosphate buffer (pH 7), and 1 ml of 1 M potassium iodide were added. The absorbance of reaction mixture was measured at 390 nm. H2O2 content was determined by using an extinction coefficient (ε = 0.28 µM cm−1) and expressed as nmol g−1FW.
Statistical Analyses
The relative amount of different constituents of Artemisia oil determined by GC/GC-MS analyses was based upon three injections of the oil. The dose–response laboratory bioassay was conducted in a randomized block design with five replications, each comprised of a single Petri dish. Five independent tissue samples were used as replicates for each analysis. The data are presented as mean ± SE and analyzed by one-way ANOVA followed by the comparison of mean values using post-hoc Tukey’s test at P ≤ 0.05.
Results and Discussion
Composition of Essential Oil from Young Leaves
GC and GC-MS analyses of Artemisia oil revealed it to be a mixture of monoterpenoids, sesquiterpenes, aromatic compounds, aliphatic esters, ketones, and alcohols, which eluted between 4.16 to 40.29 min. A total of 33 chemical constituents representing 99.83% of the essential oil, were identified (Table 1). Of these, β-myrcene (29.27%) was the main constituent, followed by (+)-limonene (13.3%), (Z)-β-ocimene (13.37), γ-terpinene (9.51%), and acenaphthene (17.8%). These together constituted ~86% of the oil (Table 1). The oil, in general, was rich in monoterpenes (71.6%), while the other compounds constituted 28.23%. The composition of the characterized oil was in sharp contrast to earlier reports. Earlier, 1-phenyl-penta-2,4-diyne, β-pinene, limonene, and (E)-β-ocimene (Safaei-Ghomi et al. 2005), or methyl eugenol (Basher et al. 1997), or thujone, camphor, and 1,8-cineole (Mirjalili et al. 2007) were identified as the main constituent of the oil from aerial parts of A. scoparia. These components were either absent or present in small amounts in the present study. Nevertheless, our observations are parallel to earlier studies that reported β-myrcene as the main constituent in the oil extracted from leaves and residues of A. scoparia (Singh et al. 2008, 2009). The observed variations in oil composition among those reported in literature are apparently largely due to differences in growth stages, plant parts, harvesting time, variations in edaphic and climatic factors, and geographical region (Batish et al. 2006b, 2008).
Effect on Germination and Early Growth
A significant reduction in germination of test weeds was observed in response to Artemisia oil and β-myrcene (Table 2). At the lowest treatment of 0.07 mg/ml Artemisia oil, germination was reduced by 39%, 19%, and 10.6% in C. rotundus, P. minor, and A. fatua (Table 2). The inhibitory effect of β-myrcene was less. Inhibitory effects were greatest on C. rotundus, followed by P. minor and then A. fatua. In general, a gradual decline in germination with increasing concentration was observed, thus indicating a dose-dependent effect.
Artemisia oil and β-myrcene significantly reduced radicle growth and seedling dry weight of test weeds (Tables 3 and 4). Radicle elongation of A. fatua was reduced by ~47% and 26% in response to 0.14 mg/ml of Artemisia oil and β-myrcene, respectively. At 0.35 mg/ml concentration of Artemisia oil and β-myrcene, radicle growth was reduced in the range of 37–94% and 12–63%, respectively (Table 3). Upon exposure to 0.35 mg/ml Artemisia oil, ~94% reduction in root length was observed. Parallel to root growth, a significant reduction in dry weight of the emerged seedlings also was observed in response to both Artemisia oil and β-myrcene (Table 4).
Reduction in germination and radicle growth by volatile terpenes from A. scoparia is not surprising. Earlier, the volatile oils from A. tridentata, A. princeps, A. vulgaris, and A. scoparia were reported to inhibit emergence and growth of associated vegetation (Weaver and Klarich 1977; Yun et al. 1993; Barney et al. 2005; Singh et al. 2008). Several studies have documented that the oil from aromatic plants and volatile terpenes are potent inhibitor of seed germination and root elongation. For example, Muller et al. (1964) demonstrated that volatile oils from Salvia leucophylla and Artemisia californica reduced the growth of associated plants, thus resulting in characteristic vegetational patterning. Batish et al. (2004, 2006a, b) reported a growth inhibitory effect of volatile oil from Eucalyptus citriodora on the emergence, radicle, and early seedling growth of several plants.
Phytotoxicity of volatile oils and terpenes also has been implicated as one of the possible reasons for successful colonization by invasive weeds (Kong et al. 1999; Barney et al. 2005; Ens et al. 2008). Kong et al. (1999) characterized a number of allelopathic constituents from the essential oil of Ageratum conyzoides that possibly are responsible for its invasive nature. Similarly, Barney et al. (2005) identified a number of volatile allelochemicals from the fresh leaves of invasive weed mugwort (A. vulgaris). These workers showed that nine of the identified monoterpenes act synergistically, impart phytotoxicity to the weed, and thus help in successful colonization, and habitat invasion (Barney et al. 2005). Recently, volatile sesquiterpenes (β-maaliene, α-isocomene, β-isocomene, δ-cadinene, 5-hydroxycalamenene, and 5-methoxycalamenene) that emanate from roots of the invasive plant bitou bush (Chrysanthemoides monilifera spp. rotundata) were shown to inhibit the seedling growth of associated native vegetation, and thus possibly help in successful invasion in the introduced sites (Ens et al. 2008). Additionally, several constituent terpenes are potent inhibitors of seed germination and seedling growth. These include: 1,4-and 1,8-cineole (Romagni et al. 2000), citronellal, citronellol, linalool (Singh et al. 2002, 2006b), α-pinene (Abrahim et al. 2000; Singh et al. 2006a), and limonene (Abrahim et al. 2000).
Inhibition of germination and root growth by oil from foliage of A. scoparia suggests that under natural conditions these volatile terpenes may emanate from the plant, enter the soil, and may be involved in suppression of associated vegetation, thus resulting in the formation of its monospecific strands. The soil from underneath the A. scoparia plants contains a mixture of mono-and sesquiterpenes. The major ones include limonene, eugenol, 1,8-cineole, ocimene, and caryophyllene oxide (data not presented), and all these are potent germination inhibitors (Singh et al. 2003).
Although the mode of inhibitory action of the oils remains somewhat unclear, volatile oils and monoterpenes inhibit cell division and induce structural breakdown and decomposition in roots (Romagni et al. 2000; Nishida et al. 2005; Singh et al. 2006a, b). Vaughn (1991) reported that essential oil from Cinnamomum zeylanicum and Thymus vulgaris inhibit the sprout growth in potato by killing meristematic cells. Batish et al. (2007b) reported that volatile oil from E. citriodora inhibited root growth by suppressing mitotic activity. Earlier, Scrivanti et al. (2003) reported that essential oil from Tagetes minuta and ocimene (a constituent monoterpene) induce severe lipid peroxidation and membrane disintegration in maize. Recently, it was demonstrated that α-pinene, a volatile monoterpene, inhibits root growth of Cassia occidentalis by inducing oxidative stress measured in terms of increased lipid peroxidation, H2O2 accumulation, and membrane disintegration (Singh et al. 2006a).
Effect on Cell Survivability
Artemisia oil caused a significant reduction in cellular survivability (measured as % TTC reduction) in roots of test weeds (Table 5). The cellular survivability decreased 12–24% and 6−22% in response to 0.07 mg/ml of Artemisia oil and β-myrcene, respectively. It declined further with increasing concentrations. In general, inhibition in cellular survivability was the greatest in C. rotundus followed by A. fatua, and P. minor, respectively (Table 5).
Cell viability provides an indirect measurement of cell respiration (Batish et al. 2007a). In viable (respiring) tissue, TTC absorbs electrons from the mitochondrial transport chain, get reduced, and thus correlates positively with respiratory activity (Batish et al. 2007a). The decrease in cellular survivability (thus respiration) upon exposure to Artemisia oil or β-myrcene implies an interference with energy metabolism involved in synthesis of macromolecules, thus resulting in reduced growth. These observations are in agreement with earlier studies (Abrahim et al. 2000; Singh et al. 2005). Abrahim et al. (2000) demonstrated that monoterpenes, due to their high lipophilicity, act as uncouplers of oxidative phosphorylation, and imbalance the cellular energy levels.
Effect on Membrane Integrity
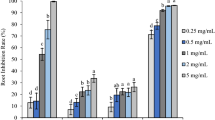
Membrane disruption by monoterpenoids has been suggested as one of the mechanisms for fungicidal and bactericidal activity (Singh et al. 2006a); we, therefore, studied the effect of Artemisia oil and β-myrcene on membrane integrity. Artemisia oil and β-myrcene caused a significant ion leakage from roots of C. rotundus as indicated by increased electrical conductivity of the bathing medium (MES buffer). The ion leakage increased with time up to 20 h in the dark and then for another 8 h in light (i.e., up to 28 h). After 28 h, conductivity of the bathing medium in response to 0.14 and 0.35 mg/ml of Artemisia oil or β-myrcene was in the range of 60–64% and 95–99%, respectively, of the maximum leakage (685.2 µS; observed upon boiling the root tissue) (Fig. 1).
Effect of Artemisia oil and β-myrcene on membrane integrity measured as electrolyte leakage from roots of Cyperus rotundus. The dotted line at the top represent maximum conductivity (685.2 µS) measured upon boiling of plant tissue. Vertical line marks the time period after which the samples were exposed to light
The increased conductivity of the bathing medium indicates cellular membrane disruption resulting in excessive solute leakage. These results are in agreement with earlier reports that essential oil and the monoterpenes (citronellal, α-pinene, and (+)-pulegone) cause ion leakage from plant tissue (Maffei et al. 2001; Singh et al. 2006a, b). Maffei et al. (2001) demonstrated that essential oil from Mentha × piperata and its pure component, (+)-pulegone, induced membrane depolarization in cucumber roots that resulted in an altered flux of ions across the membrane. Dayan et al. (2000) opined that a loss in membrane integrity relates to induction of oxidative stress. In fact, enhanced electrolyte leakage is an indicator of membrane damage due to generation of reactive oxygen species (Singh et al. 2006a). Montillet et al. (2005) reported that membrane disruption occurs due to peroxidation of polyunsaturated fatty acids or lipids in biomembranes and results in formation of byproducts such as malondialdehyde. Thus, we measured the MDA content, amount of conjugated dienes, and hydrogen peroxide content (as indicators of oxidative stress) in response to Artemisia oil or β-myrcene exposure.
Effect on Lipid Peroxidation
Exposure to Artemisia oil significantly (at P < 0.05) enhanced the MDA content in roots of C. rotundus (Table 6). It increased by 1.24- to 2.3-fold upon exposure to Artemisia oil or β-myrcene over that of control (Table 6). Increased content of MDA (a major thiobarbituric acid reactive substance) indicates enhanced lipid peroxidation and damage to membranes. These observations are in conformity with earlier reports that essential oil and monoterpenes induce membrane damage, and thus inhibit root growth (Scrivanti et al. 2003; Zunino and Zygadlo 2004; Singh et al. 2006a). The volatile oil from Tagetes minuta and Schinus areira and its monoterpenes (ocimene, ocimenone, α-pinene, 1,8-cineole, thymol, geraniol, menthol, and camphor) have been reported to inhibit maize growth by causing lipid peroxidation (Scrivanti et al. 2003; Zunino and Zygadlo 2004). Singh et al. (2006a) demonstrated that α-pinene induced severe lipid peroxidation in roots of C. occidentalis, damaged cellular membranes, and inhibited root growth. Enhanced MDA content due to lipid peroxidation indicates an induction of ROS-generated oxidative stress.
Membrane damage upon exposure to Artemisia oil or β-myrcene was confirmed further by a significant decline in conjugated dienes content (Table 6). Upon exposure to ≥0.14 mg/ml of Artemisia oil or β-myrcene, the conjugated dienes content in roots of C. occidentalis decreased by 21–59% and 9–13%, respectively (Table 6). Decreased conjugated dienes further indicate a ROS-induced damage to biological membranes (Singh et al. 2007).
Effect on H2O2 Content
Parallel to MDA accumulation, the amount of H2O2 increased significantly in roots of C. rotundus upon exposure to Artemisia oil or β-myrcene (Table 6). Artemisia oil and β-myrcene exposure increased H2O2 by 1.3- to 1.7-fold and 1.2- to1.5-fold, respectively, compared with control. At 0.35 mg/ml of Artemisia oil or β-myrcene, H2O2 content increased by 1.5- and 1.7-fold, respectively, over control (Table 6). Accumulation of H2O2 in C. rotundus roots further enhances lipid peroxidation, thus resulting in increased oxidative stress, and leading to disruption of metabolic activities in the cell. In general, H2O2 hinders the activity of ~SH group containing enzymes, impairs the photosynthetic activity in chloroplasts, and thereby reduces plant growth (Takeda et al. 1995). Among the different ROS produced in cells in response to environmental stresses, H2O2 acts as a signaling molecule. At low concentrations, it aids in cellular defense, and provides tolerance against stress, whereas at high concentrations, it induces cellular damage (Stone and Yang 2006). H2O2 is removed by antioxidant enzymes such as catalases, and ascorbate peroxidases, which were not studied in the present study.
In summary, the present study concludes that Artemisia oil inhibits germination and plant root growth through generation of ROS-induced oxidative stress associated with membrane disruption, enhanced lipid peroxidation, and accumulation of H2O2. Whether this induction of oxidative stress in response to Artemisia oil was accompanied by any alteration of antioxidant/scavenging enzymatic machinery in the plant roots remains to be studied.
References
Abrahim, D., Braguini, W. L., Kelmer Bracht, A. M., and Ishi-Iwamoto, E. L. 2000. Effects of four monoterpenes on germination primary root growth and mitochondrial respiration of maize. J. Chem. Ecol. 26:611–623.
Adams, R. P. 1995. Identification of Essential Oil Components by Gas Chromatography Mass Spectroscopy. Allured Publishing, Carol Stream, Illinois.
Anonymous 1993. Artemisia Linn, pp. 434–442, in G. P. Phondke (ed.). The Wealth of India—Raw Materials, vol. III (Ca–Ci), revised series. Council of Scientific and Industrial Research, New Delhi, India.
Bakkali, F., Averbeck, S., Averbeck, D., and Idaomar, M. 2008. Biological effects of essential oils—a review. Food Chem. Toxicol. 46:446–475.
Barney, J. N., Hay, A. G., and Weston, L. A. 2005. Isolation and characterisation of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 31:247–265.
Basher, K. H. C., Ozek, T., Demirehakmak, B., Nuriddinov, K. H. R., Abduganiev, B. Y. O., Aripov, K. H. N., Khodzimatov, K. K. H., Nigmatullaev, O. A., and Shamyanov, E. D. 1997. Essential oils of some Artemisia species from Central Asia. Chem. Nat. Comp. 33:383–385.
Batish, D. R., Setia, N., Singh, H. P., and Kohli, R. K. 2004. Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicide. Crop Prot. 23:1209–1214.
Batish, D. R., Singh, H. P., Setia, N., Kaur, S., and Kohli, R. K. 2006a. Chemical composition and inhibitory activity of essential oil from decaying leaves of Eucalyptus citriodora. Z. Naturforsch. 61c:52–56.
Batish, D. R., Singh, H. P., Setia, N., Kaur, S., and Kohli, R. K. 2006b. Chemical composition and phytotoxicity of volatile essential oil from intact and fallen leaves of Eucalyptus citriodora. Z. Naturforsch. 61:465–471.
Batish, D. R., Lavanya, K., Singh, H. P., and Kohli, R. K. 2007a. Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul. 51:119–128.
Batish, D. R., Singh, H. P., Setia, N., Kohli, R. K., Kaur, S., and Yadav, S. S. 2007b. Alternative control of littleseed canary grass using eucalypt oil. Agron. Sustain. Dev. 27:171–177.
Batish, D. R., Singh, H. P., Kohli, R. K., and Kaur, S. 2008. Eucalyptus essential oil as natural pesticide. For. Ecol. Manage. 256:2166–2174.
Dayan, F. E., Romagni, J., and Duke, S. O. 2000. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 26:2079–2094.
Duke, S. O., and Kenyon, W. H. 1993. Peroxidizing activity determined by cellular leakage, pp. 61–66, in P. Böger, and G. Sandmann (eds.). Target Assays for Modern Herbicides and Related Phytotoxic Compounds. CRC Press, Boca Raton, FL.
Ens, E. J., Bremner, J. B., French, K., and Korth, J. 2008. Identification of volatile compounds released by roots of an invasive plant, bitou bush (Chrysanthemoides monilifera spp. rotundata), and their inhibition of native seedling growth. Biol. Inv. 11:275–287. doi:10.1007/s10530-008-9232-3.
Heath, R. L., and Packer, L. 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125:189–198.
Kong, C. H., Hu, F., Xu, T., and Lu, Y. H. 1999. Allelopathic potential and chemical constituents of volatile oil from Ageratum conyzoides. J. Chem. Ecol. 25:2347–2356.
Langenheim, J. H. 1994. Higher plant terpenoids: a phytocentric overview of their ecological roles. J. Chem. Ecol. 20:1223–1280.
Maffei, M., Camusso, W., and Sacco, S. 2001. Effect of Mentha × piperita essential oil and monoterpenes on cucumber root membrane potential. 2001. Phytochemistry 58:703–707.
McLafferty, F. W. 1989. Registry of Mass Spectral Data. 5th edn.John Wiley and Sons, New York.
Mirjalili, M. H., Tabatabaei, S. M. F., Hadian, J., Ebrahimi, S. N., and Sonboli, A. 2007. Phenological variation of the essential oil of Artemisia scoparia Waldst. et Kit from Iran. J. Essential Oil. Res. 19:326–329.
Montillet, J.-L., Chamnongpol, S., Rustérucci, C., Dat, J., Van de Cotte, B., Agnel, J.-P., Battesti, C., Inzé, D., Van Breusegem, F., and Triantaphylidès, C. 2005. Fatty acid hydroperoxides and H2O2 in the execution of hypersensitive cell death in tobacco leaves. Plant Physiol. 138:1516–1526.
Muller, C. H., Muller, W. H., and Haines, B. L. 1964. Volatile growth inhibitors produced by aromatic shrubs. Science 143:471–473.
Nishida, N., Tamotsu, S., Nagata, N., Saito, C., and Sakai, A. 2005. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 31:1187–1203.
Romagni, J. G., Allen, S. N., and Dayan, F. E. 2000. Allelopathic effects of volatile cineoles on two weedy plant species. J. Chem. Ecol. 26:303–313.
Safaei-Ghomi, J., Bamoniri, A., Sarafraz, M. B., and Batooli, H. 2005. Volatile components from Artemisia scoparia Waldst. et Kit. growing in central Iran. Flavour Fragr. J. 20:650–652.
Scrivanti, L. R., Zunino, M., and Zygadlo, J. A. 2003. Tagetes minuta and Schinus areira essential oils as allelopathic agents. Biochem. Syst. Ecol. 31:563–572.
Singh, H. P., Batish, D. R., Kaur, S., Ramezani, H., and Kohli, R. K. 2002. Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann. Appl. Biol. 141:111–116.
Singh, H. P., Batish, D. R., and Kohli, R. K. 2003. Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. Crit. Rev. Plant Sci. 22:239–311.
Singh, H. P., Batish, D. R., Setia, N., and Kohli, R. K. 2005. Herbicidal activity of volatile essential oils from Eucalyptus citriodora against Parthenium hysterophorus. Ann. Appl. Biol. 146:89–94.
Singh, H. P., Batish, D. R., Kaur, S., Arora, K., and Kohli, R. K. 2006a. α-pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 98:1261–1269.
Singh, H. P., Batish, D. R., Kaur, S., Kohli, R. K., and Arora, K. 2006b. Phytotoxicity of volatile monoterpene citronellal against some weeds. Z. Naturforsch. 61c:334–340.
Singh, H. P., Batish, D. R., Kohli, R. K., and Arora, K. 2007. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul. 53:65–73.
Singh, H. P., Kaur, S., Mittal, S., Batish, D. R., and Kohli, R. K. 2008. Phytotoxicity of major constituents of volatile oil from leaves of Artemisia scoparia Waldst. & Kit. Z. Naturforsch. 63c:663–666.
Singh, H. P., Mittal, S., Kaur, S., Batish, D. R., and Kohli, R. K. 2009. Chemical composition and antioxidant activity of essential oil from residues of Artemisia scoparia. Food Chem. 57:21–30. doi:10.1007/s10725-008-9314-3.
Stein, S. E. 1990. National Institute of Standards and Technology (NIST). Mass Spectral Data Base and Software, Ver. 3.02. Gaithersburg, Maryland, USA.
Stone, J. R., and Yang, S. 2006. Hydrogen peroxide: a signaling messenger. Antioxidant Redox Signal. 8:243–270.
Takeda, T., Yokota, A., and Shigeoka, S. 1995. Resistance of photosynthesis to hydrogen peroxide in algae. Plant Cell. Physiol. 36:1089–1095.
Vaughn, S. F. 1991. Natural compounds from spices could replace potato sprouting inhibitors. Ind. Bioprocess. 13:5.
Weaver, T., and Klarich, D. 1977. Allelopathic effects of volatile substances from Artemisia tridentata Nutt. Am. Midl. Nat. 97:508–512.
Yun, K. W., Kil, B. S., and Han, D. M. 1993. Phytotoxic and antimicrobial activity of volatile constituents of Artemisia princeps var. orientalis. J. Chem. Ecol. 19:2757–2766.
Zunino, M. P., and Zygadlo, J. A. 2004. Effect of monoterpenes on lipid peroxidation in maize. Planta 219:303–309.
Acknowledgement
Shalinder Kaur and Sunil Mittal are thankful to Department of Science and Technology, Government of India, New Delhi, and University Grants Commission, New Delhi, India, respectively, for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, H.P., Kaur, S., Mittal, S. et al. Essential Oil of Artemisia scoparia Inhibits Plant Growth by Generating Reactive Oxygen Species and Causing Oxidative Damage. J Chem Ecol 35, 154–162 (2009). https://doi.org/10.1007/s10886-009-9595-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-009-9595-7