Abstract
The cuticular hydrocarbon profiles of emerald ash borers, Agrilus planipennis, were examined to determine if there are differences in these compounds between the sexes. We also assessed feral male EAB in the field for behavioral changes based on the application of a female-specific compound to dead, solvent-washed beetles. Males in the field spent significantly more time attempting copulation with dead, pinned female beetles coated with a three-beetle-equivalent dose of 3-methyltricosane than with solvent-washed beetles or those coated in 3-methyltricosane at lower concentrations. Males in the field spent the most time investigating pinned dead, unwashed female beetles. In the laboratory, sexually mature males were presented with one of several mixtures applied in hexane to filter paper disks or to the elytra of dead female beetles first washed in solvent. Male EAB also spent more time investigating dead beetles treated with solution applications that contained 3-methyltricosane than dead beetles and filter paper disks treated with male body wash or a straight-chain hydrocarbon not found on the cuticle of EAB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emerald ash borer (EAB), Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) is a growing threat to the ash (Fraxinus sp.) resource of North America (reviewed in Poland and McCullough 2006). The beetle is spreading rapidly, and efforts to detect new infestations are of paramount importance to slowing further spread. Thus far, no species-specific trap is available for wide deployment. The use of girdled “trap trees” has proven to be an effective but cost- and labor-intensive method of detecting new infestations (Cappaert et al. 2005). Progress toward development of a widely deployable trap has been made on the grounds of trap color (Francese et al. 2005), with purple traps being the most effective at capturing adult EAB. Induced volatiles from ash trees are antennally active in adult EAB (Rodriguez-Saona et al. 2007) and incorporated into purple prism traps for a significant gain in trapping effectiveness (Crook et al. 2008). Green leafy volatiles derived from ash trees also have been identified and shown to improve the capture of adult EAB on traps (De Groot et al. 2008).
Field and the laboratory studies on the mating system of this beetle found that vision plays a key role in male mate-location (Lelito et al. 2007), similar to the mating systems of other buprestids examined to date (Carlson and Knight 1969; Matthews and Matthews 1978; Gwynne and Rentz 1983). Further, males appear to discriminate between the sexes once contact is made; feral males spend significantly more time attempting copulation with females than males or solvent-washed beetles of both sexes, suggesting the use of a contact cue (Lelito et al. 2007).
In an effort to understand the role of any sex-specific compounds the beetle may employ in mate recognition, we conducted solvent dipping and SPME sampling of the cuticles of both immature and mature male, as well as female, beetles. These samples revealed characteristic differences between the sexes once they are mature. Here, we examine the behavioral role of 3-methyltricosane, a long-chain hydrocarbon present on the cuticles of mature females, but only in traces on the cuticles of male or immature female EAB. The compound was tested for behavioral activity in the field, using dead adult EAB as lures in a manner similar to that used in the past to identify precopulatory behavior (Lelito et al. 2007), as well as in a laboratory bioassay to assess the arrestant and/or attractant properties of the compound.
Methods and Materials
Insects
Newly emerged adult beetles were provided by the staff of the Brighton, MI (USA 48116) USDA APHIS PPQ laboratory. These were segregated by sex upon emergence from rearing barrels, maintained in separate rearing tubs, and fed on ash foliage obtained from trees grown indoors. Beetles used for solvent and SPME sampling were either 3 (“young”) or at least 10–12 days post-eclosion (“mature”). Live beetles used in laboratory behavior assessments were between 8 and 18 days of age and were considered “mature” (Bauer et al. 2004; Lyons et al. 2004). The beetles we used as field lures were also mature, killed by freezing, pinned through the thorax, and allowed to dry.
Solvent Dipping and SPME Sampling
Young and mature beetles of both sexes were extracted in groups of three in 300 μl of dichloromethane (B&J, High Purity Solvent) or hexane (B&J, Ultra Resi-Analyzed). We did not detect significant differences between the dichloromethane and hexane washes of the beetles; therefore, we used the hexane solutions for quantification and further analysis. Hexane was evaporated in a gentle stream of nitrogen and samples redissolved in 60 μl of 50 ng/μl of 16-methyl hexatriacontane as an internal standard in hexane. For each of the four treatments, we performed four replicates.
The spatial distribution of cuticular compounds on the surface of mature males and females was investigated by using SPME fibers coated with 7 μm of polydimethylsiloxane (Supelco, Bellefonte, PA, USA). Body tagmata (head, thorax, and abdomen) of three mature EAB of each sex were sampled for 2 min each by rubbing a SPME fiber gently against the cuticle of the beetle. We prepared three replicate SPME samples of each body part. Beetles were held in place with soft forceps during fiber application.
Chemical Analysis
All samples were analyzed with an Agilent 6890 GC-FID system equipped with an Equity-5 column (30 m × 0.2 mm × 0.2 μm; Supelco, Bellefonte, PA, USA) for quantification purposes. To identify components, selected samples were analyzed on an identical column in an Agilent 6890N GC coupled with a 5973N MSD system in EI mode (+70 eV). The oven temperature program was 50°C (1 min) − 20°C/min − 210°C − 3°C/min − 320°C (10 min) for all GC analyses. The temperature of the injector was held at 280°C, and the FID in the GC and the transfer line in the GC-MS were kept at 300°C. Samples were injected splitless (0.75 min) and run at an average linear flow velocity of 25 cm/s in the GC and 30 cm/s in the GC-MS. SPME samples were analyzed using the same settings.
Identification of the compounds was based on their MS spectra (NIST05, Masslib), their Kovats indices on the Equity-5 column described elsewhere (Böröczky et al. 2008), and by comparison with authentic standards. Quantification of hydrocarbons was based on their peak area values obtained from our data acquisition and analysis software (Chemstation, Agilent). Peak area values were corrected with the relative response factors described elsewhere (Böröczky et al. 2008). Percent composition was calculated as percentage of the sum of all identified compounds. Absolute amounts were calculated relative to the internal standard. The FID response was linear in the concentration range of the compounds we analyzed.
Synthesis of 3-Methyltricosane
A solution containing 0.78 g (2.8 mmol) of eicosanal in 30 ml of anhydrous ether was added to an excess of ethereal 2-butyl magnesium bromide under an argon atmosphere. The mixture was stirred overnight and after careful addition of 10% HCl; the ether layer was separated, washed with saturated NaHCO3, and dried over anhydrous MgSO4. After filtration, the residue was taken up in 7 ml of pyridine and treated with 0.31 ml of methane sulfonyl chloride at 0°C and stirred overnight. After the addition of 50 ml of ether, the mixture was washed with 10% HCl; the ether layer was separated, washed with saturated NaHCO3, and dried over anhydrous MgSO4. After filtration, the solvent was removed in vacuo, and the residue was taken up in 50 ml of ethyl acetate and hydrogenated over 100 mg of PtO2 under 3 Atm of hydrogen overnight. After the mixture was filtered and the solvent was removed, flash chromatography (silica gel/hexane) provided 521 mg of 3-methyltricosane, m/z 338 [M+](0.2), 309(10), 281(2), 25312), 239(2), 225(3), 211(3), 197(3), 183(3), 169(4), 155(5), 141(7), 127(9), 113(13), 99(19), 97(9), 85(41), 83(9), 71(65), 69(14), 57(100), 56(36), 55(22), 43(57), 41(24). GC-MS was carried out in the EI mode using a Shimadzu QP-2010 GC-MS equipped with an RTX-5, 30 m × 0.25 mm i.d. column. The instrument was programmed from 60°C to 250°C at 10°C/min and held at 250°C for 40 min.
The synthetic compound had a Kovats index (2371 on the Equity-5 column used above) and MS spectrum identical to that of the compound found in the body washes.
Field Behavior
The dead, pinned female “beetle-lures” used for these experiments were either: unwashed, “U”; washed in dichloromethane for 10 min and then dried for 24 h, “W”; or solvent-washed, dried, and then coated with one of our experimental treatments. Ten microliters of 4 ng/µl n-eicosane (“E”; an impurity resulting from our synthesis of 3-methyltricosane); 2 ng/μl 80%/20% 3-methyltricosane/n-eicosane mixture (“T1”); 6 ng/μl 80%/20% 3-methyltricosane/n-eicosane mixture (“T2”); or 20 ng/µl 80%/20% 3-methyltricosane/n-eicosane mixture (“T3”) were applied to the dorsal cuticle of a dead, dichloromethane-washed, and dried female EAB. T1, T2, and T3 represented 16, 48, and 160 ng of 3-methyltricosane, respectively. All applications were made with a 1-μl glass pipette, which was cleaned between applications by three separate washes with 10 μl of hexane. We prepared fresh beetle-lures for each replicate of the field experiment.
Field experiments took place between 7 June and 5 July 2007 between the hours of 1000 AM and 1600 PM EST, on days without heavy rain. Each 2-h replicate (one per suitable day, for a total of 17 replicates) was performed in an area of high-density EAB population located in Livingston County, MI, USA, south of the town of Howell. The site used was private agricultural and forest land, containing a large number of green ash trees (F. pennsylvanica). Experiments were conducted prior to and through the peak EAB flight period. Individual ash trees selected for these experiments were between 10 and 20 m tall and had healthy branches accessible at 2–3 m height from ground level for the pinning of beetle-lures. Each tree used was selected randomly on each day, with the precondition that at least five live EAB could be seen on that tree from ground level (i.e., a presumably infested tree).
For each replicate of field experiment, we pinned three beetle-lures of each treatment (U, W, E, T1, T2, and T3), on individual terminal leaflets of compound ash leaves on the sunny side of a selected ash tree, between 2 and 3 m from ground level. Beetle-lures were positioned randomly (not blocked by treatment), between 10 and 30 cm apart. We then observed these lures from the ground for a 2-h period and timed the duration of any feral EAB attempts at copulation and investigation of a lure. We defined “investigation” as a feral EAB remaining in contact with a pinned beetle-lure subsequent to an airborne approach and copulation attempt.
Laboratory Behavior
We prepared experimental arenas by using a plastic 100-mm diameter Petri dish (Cat. No. 08-757-12, Fisher Scientific) lined with a 100-mm diameter filter paper insert (Cat. No. 1001-100, Whatman). For each experiment, we applied 10 μl of hexane as a control to the filter paper at each of two opposite points along the diameter of the dish, both 40 mm from the center of the filter paper. We then applied 10 μl of one of the experimental solutions (in hexane; Table 1) to the paper at the other two opposite points along the perpendicular diameter. We prepared both male and female body washes by placing 12 mature individual EAB into 100 μl of hexane in a 2-ml glass vial, gently agitating for 2 min, and extracted the remaining liquid. We used the same approach for the lower dosage of one beetle-equivalent (1BE) but only extracted four mature beetles in 100 μl of hexane. We prepared fresh body wash solution at both dosages as needed and stored any remaining solution in a capped 2-ml glass vial in a standard freezer.
We performed 25 trials of each treatment at three and one BE. A new arena was prepared at the start of each trial, and the assay began when we placed individual mature (8–18 days post-eclosion) male EAB into the dish at the center-point of the filter paper. Each EAB male was then observed for a period of 10 min, while we recorded the number and duration of entries into the 1-cm diameter area centered on the application points.
We repeated the same assay but this time the solutions were applied to the dorsal cuticle of a dead, solvent-washed female EAB that had been hot-glued to the filter paper at the typical application points (Fig. 1) less than 24 h before and stored in a freezer prior to use. We recorded the number of direct male to female-beetle-lure contacts, the duration of each contact, and the number of times the male attempted to copulate.
The Petri dish used as an experimental arena for the Agrilus planipennis laboratory behavioral assay; the beetle in the center is a live male at the release point. The other four beetles are the dead, solvent-washed female beetles used as dummies for the application of either solvent controls or experimental lipid applications
We also conducted a series of trials to test mature female EAB for their response to beetle-lures using the same experimental treatments for mature males at the 3BE dosage described above. We recorded the number and duration of female-to beetle-lure contacts for each treatment.
A total of 25 individual 10-min trials were conducted for each treatment/lure combination (e.g., 3BE dosage, filter paper application) from 10 February to 15 March 2008, between 900 AM and 1400 PM EST. Three trials were run concurrently, with arenas approximately 10 cm apart under full-spectrum fluorescent lighting at 25°C. The lighting was situated on wooden supports such that all arenas were lit from directly overhead the center of the arena from a height of approximately 40 cm. Within each experiment, treatments were completely randomized within a day so that all treatments had an equal chance of occurring at a given time of day. The live EAB of both sexes were used only once, and following the assay were frozen and discarded.
For the laboratory experiments and the field experiments outlined above we purchased n-eicosane and n-tetracosane from Aldrich Chemical Co. (99% purity, St. Louis, MO, USA) and synthesized the 3-methyltricosane mixtures as outlined above.
Statistical Analyses
We compared the mean investigation time of each treatment of beetle-lure in the field experiment time using a two-way ANOVA (analysis of variance), with replicate and treatment of the lure as factors. Treatment means were separated by in pair-wise comparisons by Tukey’s Honestly Significant Differences test. We employed a binomial test of proportions to detect any treatments that were contacted more often than their paired hexane control (a significant deviation from a 50–50 ratio could indicate either attraction to, or avoidance of, a given treatment). Laboratory bioassay data were log-transformed prior to analysis to achieve a normal distribution. We utilized PROC GLM in SAS for comparisons of mean times of investigation between treatments by male EAB, using both the lure type and the chemical treatment of the lure as factors with investigation time as the dependent variable. We performed all statistical analyses in SAS Version 9.1.3 (SAS Institute 2006).
Results
Solvent Dipping and SPME Sampling
Major components of the cuticle of both male and female EAB were found to be saturated and monomethyl branched odd-chain (C23–C29) hydrocarbons. The methyl branch typically was in the middle of the chain. Terminally branched monomethyl alkanes, dimethyl alkanes, and unsaturated hydrocarbons were minor components. More polar lipids, such as fatty acids, ethers, and acetate and butyrate esters of long-chain alcohols, were also detected in the body wash samples of both sexes.
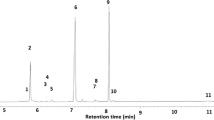
There were characteristic differences between the cuticular chemistry of mature male and female beetles (Fig. 2). 3-Methyltricosane is a minor component of the female body wash but is present in only trace amounts in that of males. Furthermore, it was found in limited quantity on the cuticle of the young female EAB, but it increased to approximately 50 ng of material coincident with sexual maturity. Other terminally branched mono-methylalkanes showed a similar trend but not as strongly as 3-methyltricosane (Table 2). We did not detect any variation in expression of 3-methyltricosane between the tagmata of the females with SPME.
The section of the gas chromatographic profile of the hexane body wash of mature Agrilus planipennis females, a young females, b young males, c and mature males, d containing 3-methyltricosane (indicated by the asterisk). 3-Methyltricosane is a minor component of the cuticular lipid profile of mature females; it occurs as a trace, if at all, in male and immature female beetles
Field Behavior
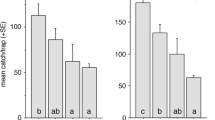
Replicates were not a significant source of variation (ANOVA, df = 16, F = 1.65, P = 0.104), and male EAB flew to and landed on all types of lures equally (ANOVA, df = 5, F = 0.536, P = 0.739). However, there was a significant treatment effect (ANOVA, df = 5, F = 20.66, P < 0.001) once contact was made, with unwashed females being investigated significantly longer than all other lures (Fig. 3). Treatments T3 and E were investigated for significantly longer than the washed female control, while T1 and T2 were not (Fig. 3).
Columns represent the mean time (in seconds) spent by feral male Agrilus planipennis in investigation of beetle-lures in the field in response to different treatments during each 2-h period of observation, with a total of 17 replicates. Treatments are listed in the following order on the x-axis: W a solvent-washed dead female EAB; T1 2 ng/μl 3-methyltricosane mixture application onto a washed female EAB; T2 6 ng/μl 3-methyltricosane mixture application; E 4 ng/μl eicosane application; T3 20 ng/μl 3-methyltricosane mixture application; U a nonsolvent-washed dead female EAB. Bars represent one standard error of the mean. Columns having no letters in common are significantly different (df = 5, F = 19.73, P < 0.001). This experiment was performed between 7 June and 5 July 2007 South of Howell, Michigan
Laboratory Behavior
Both lure type (ANOVA, df = 1, F = 12.30, P < 0.001) and treatment (ANOVA, df = 25, F = 4.34, P < 0.001) had a significant effect on male behavior so data were separated by lure type and analyzed separately for treatment effects by using a one-way ANOVA. The chemical treatment applied to the lure had an effect on male behavior for both beetle-lures (Table 3; ANOVA, df = 12, F = 4.79, P < 0.001) and filter paper applications (Table 3; ANOVA, df = 12, F = 2.72, P = 0.002). A greater number of cuticular lipid applications onto dead female EAB (treatments FBW3, TRM3, TRP3; Table 3) evoked significant differences in male behavior over their respective controls than did the corresponding lipid applications directly onto filter paper (treatment FBW3 only; Table 3).
The number of contacts made by male EAB with the different beetle-lures or the number of male EAB entering the treated areas on the filter paper, did not differ significantly between treatments (binomial test of proportions, P > 0.05 for all treatment-control pairs). However, treatment affected the incidence of copulation attempts to beetle-lures (ANOVA, df = 12, F = 6.032, P < 0.001) with FBW3 and TRP3 eliciting more than other treatments and hexane-only controls (Fig. 4, black columns). At 1BE, no significant differences were observed (Fig. 4, grey columns).
Columns represent the total number of copulation attempts performed by male Agrilus planipennis to each experimental treatment of beetle-lure. Black columns indicate three beetle-equivalent dosage, grey columns indicate one beetle-equivalent dosage. Treatment abbreviations on the x-axis are the same as in Fig. 4. Bars represent two standard errors of the mean. Columns having no letters in common are significantly different from one another (df = 12, F = 6.03, P < 0.001)
Female EAB did not exhibit a significant preference for any treatment either with respect to initial contact (Binomial test of proportions, P > 0. 05 for all treatment-control pairs) or time remaining at the lure (ANOVA, df = 6, F = 0.472, P = 0.829).
Discussion
Although the approach phase of the EAB mating system relies on visual cues, our results confirm the earlier suggestion that a female contact sex pheromone influences male behavior (Lelito et al. 2007). Males in the laboratory spent more time investigating beetle-lures to which we had reapplied female body wash or synthetic mixtures containing 3-methyltricosane than solvent-only controls, a preference we observed among feral male EAB tested in the field as well. However, males still spent significantly more time investigating unwashed female EAB than any dosage of 3-methyltricosane. Therefore, it is likely that the behavioral effect of 3-methyltricosane may be synergized by the perception of other compounds naturally secreted onto the cuticular surface of the unwashed beetle-lures.
The results of our assays support the pheromonal role of the cuticular hydrocarbon 3-methyltricosane, and the identification of this compound represents the first contact sex pheromone identified in the family Buprestidae. However, this compound has a chiral center at position 3, and it will be necessary to synthesize and test both enantiomers to determine if chirality is important.
Males do not differentiate over a distance between beetles treated with 3-methyltricosane and those treated with female body wash or solvent only in either the laboratory or the field, suggesting that 3-methyltricosane is likely perceived only as a contact cue. Although it is possible that males might be able to detect the presence of females from outside their visual range either directly or indirectly, through olfactory cues such as volatiles given off by the damaged host (Crook et al. 2008), 3-methyltricosane is unlikely to serve as a long-range attractant.
The continuous antennation of the treated substrate, especially obvious when the male encountered an affixed beetle to which female extract or 3-methyltricosane had been applied, suggests that the specific sensillae are on the antennae. Male chemoreception may not be limited to the antennae: males were also observed to “scratch” their tarsi against both the filter paper and the affixed beetles in many cases. This behavior was often followed by renewed bouts of vigorous antennation. This needs to be examined in greater detail.
References
Bauer, L. S., Haack, R. A., Miller, D. L., Petrice, T. R., and Liu, H. 2004. Emerald ash borer life cycle, p. pp. 8, in V. C. Mastro, and R. Reardon (eds.). Emerald Ash Borer Research and Technology Development Meeting. FHTET-2004-02USDA Forest Service, Morgantown.
Böröczky, K., Minard, R. D., Park, K.-C., Jones, T. J., Baker, T. C., and Tumlinson, J. H. 2008. Differences in cuticular lipid composition of the antennae of Helicoverpa zea, Heliothis virescens, and Manduca sexta. J. Ins. Physiol. 54:1385–1391.
Cappaert, D., Mccullough, D. G., Poland, T. M., and Siegert, N. W. 2005. Emerald ash borer in North America: A research and regulatory challenge. Am. Entomol 51:152–165.
Carlson, R. W., and Knight, F. B. 1969. Biology, taxonomy, and evolution of four sympatric Agrilus beetles (Coleoptera: Buprestidae). Contrib. Am. Entomol. Inst 4:1–105.
Crook, D. J., Khrimian, A., Francese, J. A., Fraser, I., Poland, T. M., Sawyer, A. J., and Mastro, V. C. 2008. Development of a host-based semiochemical lure for trapping emerald ash borer Agrilus planipennis (Coleoptera: Buprestidae). Envir. Ent. 37:356–365.
De Groot, P., Grant, G. G., Poland, T. M., Scharbach, R., Buchan, L., Nott, R. W., Macdonald, L., and Pitt, D. 2008. Electrophysiological response and attraction of emerald ash borer to green leaf volatiles (GLVs) emitted by host foliage. J. Chem. Ecol. 34:1170–1179.
Francese, J. A., Mastro, V. C., Oliver, J. B., Lance, D. R., Youssef, N., and Lavallee, S. G. 2005. Evaluation of colors for trapping Agrilus planipennis (Coleoptera: Buprestidae). J. Entomol. Sci 40:93–95.
Gwynne, D. T., and Rentz, D. C. F. 1983. Beetles on the bottle: male buprestids mistake stubbies for females (Coleoptera). J. Aust. Entomol. Soc 23:79, 80.
Lelito, J. P., Fraser, I., Mastro, V. C., Tumlinson, J. H., Böröczky, K., and Baker, T. C. 2007. Visually mediated ‘paratrooper copulations’ in the mating behavior of Agrilus planipennis (Coleoptera: Buprestidae), a highly destructive invasive pest of North American ash trees. J. Insect Behav 20:537–552.
Lyons, D. B., Jones, G. C., and Wainio-Keizer, K. 2004. The biology and phenology of the emerald ash borer, p. pp. 5, in V. C. Mastro, and R. Reardon (eds.). Emerald Ash Borer Research and Technology Development Meeting. FHTET-2004-02USDA Forest Service, Morgantown.
Matthews, R. W., and Matthews, J. R. 1978. Insect BehaviorWiley, New York.
Poland, T. M., and Mccullough, D. G. 2006. Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J. Forest. 104:118–124.
Rodriguez-Saona, C. R., Poland, T. M., Miller, J. R., Stelinksi, L. L., Grant, G. G., De Groot, P., Buchan, L., and Macdonald, L. 2007. Behavioral and electrophysiological responses of the emerald ash borer, Agrilus planipennis, to induced volatiles of Manchurian ash, Fraxinus mandshurica. Chemoecology 16:75–86.
SAS Institute 2006. SAS Software Version 9.1.3. SAS Institute, Cary.
Acknowledgements
The authors extend their thanks to M. Rietz, N. Smith, and B. Banks for assistance during this project. We also thank two anonymous reviewers, whose comments significantly improved an earlier draft of this manuscript. This work was supported by Cooperative Agreement Number 06-8100-1091-CA between J.H. Tumlinson and T.C. Baker at The Pennsylvania State University and USDA-APHIS-PPQ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lelito, J.P., Böröczky, K., Jones, T.H. et al. Behavioral Evidence for a Contact Sex Pheromone Component of the Emerald Ash Borer, Agrilus Planipennis Fairmaire. J Chem Ecol 35, 104–110 (2009). https://doi.org/10.1007/s10886-008-9583-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9583-3