Abstract
Plant chemistry can have deleterious effects on insect parasitoids, which include the reduction in body size, increased development time, and increased mortality. We examined the effects of xanthotoxin, a linear furanocoumarin, on the polyembryonic encyrtid wasp Copidosoma sosares, a specialist parasitoid that attacks the parsnip webworm, Depressaria pastinacella, itself a specialist on furanocoumarin-producing plants. Furanocoumarins, allelochemicals abundant in the Apiaceae and Rutaceae, are toxic to a wide range of herbivores. In this study, we reared parasitized webworms on artificial diets containing no xanthotoxin (control) or low or high concentrations of xanthotoxin. Clutch sizes of both male and female C. sosares broods were more than 20% smaller when they developed in hosts fed the diet containing high concentrations of xanthotoxin. Xanthotoxin concentration in the artificial diet had no effect on the development time of C. sosares, nor did it have an effect on the body size (length of hind tibia) of individual adult male and female C. sosares in single-sex broods. Webworms fed artificial diets containing low or high concentrations of xanthotoxin were not significantly smaller, and their development time was similar to that of webworms fed a xanthotoxin-free diet. Mortality of webworms was not affected by xanthotoxin in their artificial diet. Therefore, dietary xanthotoxin did not appear to affect C. sosares via impairment of host health. However, unmetabolized xanthotoxin was found in D. pastinacella hemolymph where C. sosares embryos develop. Hemolymph concentrations were fourfold greater in webworms fed the high-xanthotoxin-containing diet than in webworms fed the low-xanthotoxin-containing diet. We failed to detect any xanthotoxin metabolism by either C. sosares embryos or precocious larvae. Therefore, the observed tritrophic effects of xanthotoxin are likely to be due to the effects of xanthotoxin after direct contact in the hemolymph rather than to the effects of compromised host quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The development of insect parasitoids is often influenced by the diet of their hosts. When hosts are herbivorous insects, plant defensive chemistry can adversely affect the fitness of third and sometimes higher (e.g., Harvey et al. 2003) trophic levels (Ode 2006). The diet of the host insect may affect developing parasitoids indirectly by altering host suitability or directly by exposing the parasitoid to unmetabolized defensive chemicals.
Numerous studies suggest negative associations between plant chemistry and/or plant species identity and parasitoid fitness measures in the field (reviewed in Ode 2006). Whereas correlative studies are relatively numerous, few studies have explicitly demonstrated that plant chemistry is responsible for the oft-observed negative correlations between levels of plant defensive chemistry and parasitoid fitness proxies (Ode 2006). A handful of investigators have used artificial diets, to which known quantities of specific plant allelochemicals were added, to establish a cause-and-effect relationship between plant chemistry and parasitoid fitness. For instance, the ichneumonid Hyposoter exigua Viereck suffered morphological deformities, increased mortality and development time, and decreased body size and longevity when reared from the noctuid Helicoverpa zea (Boddie) larvae feeding on diets containing high concentrations of the alkaloid α-tomatine (Campbell and Duffey 1979, 1981). Another series of studies documented the negative effects of dietary nicotine supplements on the generalist parasitoid Hyposoter annulipes (Cresson) and the specialist braconid parasitoid Cotesia congregata (Say) (Thurston and Fox 1972; Barbosa et al. 1986, 1991; El-Heneidy et al. 1988).
In this study, we used artificial diets to explore the tritrophic effects of the furanocoumarin xanthotoxin on several fitness correlates of the polyembryonic wasp, Copidosoma sosares (Walker) (Hymenoptera: Encyrtidae). Like all copidosomatine encyrtids (Strand et al. 1991), C. sosares is a polyembryonic egg-larval parasitoid (Hardy 1996; Ode et al. 2004; Guerrieri and Noyes 2005). C. sosares produces three types of broods: all male, all female, and mixed sex. All-male and all-female broods result from a single unfertilized or fertilized egg, respectively; mixed-sex broods develop from one egg of each sex (Hardy 1996; Ode et al. 2004). As the host continues its larval development, C. sosares eggs proliferate clonally, resulting in 100 to 400 genetically identical offspring (Ode et al. 2004). The vast majority of embryos undergo morphogenesis during the host’s last instar, completely consuming the host except for the exoskeleton, thus forming a “mummy” (Hardy 1996). As the mummy is formed, the C. sosares larvae begin to pupate. Typically, adult wasps emerge 10–12 d later. One or two embryos develop early (during the earlier host instars) into “precocious” or “soldier” larvae (Hardy 1996). In the well-studied congener C. floridanum (Ashmead), soldier larvae are implicated in defending reproductive individuals against multiparasitoids (Harvey et al. 2000) and superparasitoids (Giron et al. 2004) as well as in mediating conflicts over sex ratio in mixed-sex broods (Grbić et al. 1992).
C. sosares is a specialist parasitoid of the parsnip webworm, Depressaria pastinacella (Duponchel) (Lepidoptera: Elachistidae). The parsnip webworm is a specialist herbivore that feeds on reproductive structures of plants in the genera Pastinaca and Heracleum (Apiaceae), both of which are rich in furanocoumarins (Berenbaum and Zangerl 2006). Field studies conducted in western Europe, where this system is native, showed that two furanocoumarins are negatively associated with fitness correlates of C. sosares (Ode et al. 2004). Webworms feeding on host plants containing higher levels of isopimpinellin were less likely to be successfully parasitized by C. sosares; survivorship as well as clutch size of C. sosares broods were reduced when developing in webworms that fed on plants containing higher xanthotoxin levels.
Furanocoumarins are toxic to a wide range of herbivores (Berenbaum 1990; Berenbaum and Zangerl 1996). In addition to tritrophic effects observed in the field (Ode et al. 2004), laboratory studies that use artificial diets demonstrate tritrophic effects on two other insect parasitoids. Survivorship of C. floridanum decreased when their generalist hosts Trichoplusia ni (Hübner) (Lepidoptera: Noctuidae) fed on an artificial diet with high concentrations of a mixture of three furanocoumarins: bergapten, psoralen, and xanthotoxin (Reitz and Trumble 1996). Similarly, Archytas marmoratus (Townsend) (Diptera: Tachinidae) experienced reduced survivorship when its host, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), was reared on an artificial diet with the same high-concentration mixture (Reitz and Trumble 1997).
The observed tritrophic effects of furanocoumarins on C. sosares may be the result of compromised webworm quality if the costs of detoxification or excretion are sufficient to reduce the nutritional quality or size of the webworm. Alternatively or in addition, some furanocoumarins may pass, unmetabolized, into the hemolymph of the webworm where developing C. sosares embryos would directly encounter these potentially toxic compounds. Webworms fed an artificial diet containing 0.3% fresh weight xanthotoxin had trace amounts of xanthotoxin in their hemolymph, and webworms feeding on P. sativa fruit had trace amounts of six furanocoumarins in their hemolymph. These findings suggest that immature C. sosares do encounter these compounds (McGovern et al. 2006). Moreover, metabolism assays failed to show any ability of C. sosares larva to metabolize xanthotoxin (McGovern et al. 2006).
We manipulated the amount of xanthotoxin present in artificial diets fed to parasitized webworms to establish the effects of xanthotoxin on C. sosares. Given the negative correlations between xanthotoxin and C. sosares fitness proxies observed in the field, we also measured the amount of unmetabolized xanthotoxin present in the webworm hemolymph. Although C. sosares larvae apparently are incapable of metabolizing xanthotoxin (McGovern et al. 2006), they spend a majority of their developmental time as polygerms (embryos) or precocious larvae. Host larvae continue to feed as the C. sosares polygerm proliferates, potentially exposing developing C. sosares directly to chemicals from the host’s diet. Precocious larvae could also potentially detoxify furanocoumarins that their reproductively destined clone mates would otherwise encounter. Therefore, we examined the ability of C. sosares embryos and precocious larvae to metabolize xanthotoxin.
Methods and Materials
Effect of Xanthotoxin on C. sosares
Approximately 200 adult female and male C. sosares were collected as they emerged from 100 field-collected mummies from 45 cowparsnip (Heracleum lanatum Michx.) plants in July 2005 from two sites (N 44°05′, W 103°38′ and N 44°25′, W 103°53′) 44.3 km apart in the in the Black Hills National Forest. Males and females were allowed to mate and placed at 5°C and a 8:16-hr light/dark photoperiod for 4 mo to break the reproductive diapause. After this period, C. sosares females were placed individually into 100-mm-diameter plastic Petri dishes for 24 hr and allowed to oviposit in freshly laid webworm eggs on parsnip leaves. Neonate parasitized webworms hatched 6–7 d later. Approximately 100 additional mated adult females were collected with a handheld aspirator from the same sites in the Black Hills National Forest on 18 June 2006. Females were transported back to the laboratory where they were allowed to oviposit in freshly laid webworm eggs as described above. Webworms collected from H. lanatum in the Black Hills National Forest were the source of the host eggs used in both years. Once webworm eggs hatched, a trimmed paintbrush was used to place individual neonate larvae randomly into 44-ml diet cups (Solo®). Diet cups contained ∼15 ml of semidefined artificial either with no xanthotoxin (control), a low (0.313 ng/μg fresh weight diet), or a high concentration of xanthotoxin (4.71 ng/μg fresh weight). These concentrations reflect the range of xanthotoxin concentrations observed in our field collections of H. lanatum seed material throughout the western USA (unpublished data). Furanocoumarins were dissolved in 50–150 ml acetone. α-Cellulose was placed in the furanocoumarin–acetone mixture and left in a fume hood 1–2 d to dry completely before incorporation into the artificial diet (Nitao and Berenbaum 1988). Parasitized webworms were allowed to develop in a growth chamber set to 27°C and a 16:8-hr light/dark photoperiod.
Cups containing parasitized webworms were checked daily, and the dates at which mummies were formed were recorded. Mummies were placed individually into 18 × 180-mm glass tubes stopped with cloth plugs and returned to the growth chamber. They were checked daily until adult wasps emerged, whereupon the date was recorded, and the wasps were frozen at −20°C until clutch size and body size could be measured. The number of emerged adult wasps of each sex was counted, as were the numbers of unemerged adults, pupae, and larvae inside mummies. Clutch size was calculated as the total number of individual C. sosares per host (i.e., the primary clutch size (sensu Ode and Strand 1995)): emerged plus unemerged adults (secondary clutch size), pupae, and larvae. Within-brood mortality was calculated as the total number of dead pupae and larvae divided by the primary clutch size. Mummies that failed to produce any emerged adults by 30 d postmummy formation were dissected, and body size and sex of any unemerged adults were recorded along with primary clutch size and within-brood mortality. Both metatibiae were removed from 10 randomly selected wasps of each sex from each mummy and measured with an ocular micrometer at ×50 magnification. Lengths of both tibiae were averaged for each wasp, and the measurements from 10 wasps were averaged for each mummy. Tibial length was chosen as a measure of body size, as tibial length correlates strongly with egg load and male mating ability in the congener C. floridanum (Ode and Strand 1995).
The effects of diet composition and sexual composition (all male, all female, and mixed sex) on primary clutch size and development time were analyzed separately with two-way analyses of variance (ANOVAs; PROC GLM; SAS Institute 2003). The effect of diet composition on within-brood mortality was analyzed with a logistic model that treated the response, mortality, as a binomial count (PROC LOGISTIC; SAS Institute 2003). The effect of diet composition on body size was analyzed with an analysis of covariance, with clutch size as a covariate. Tukey’s honestly significant difference (HSD) multiple comparisons tests were performed when the overall test was significant (PROC GLM; SAS Institute 2003).
Xanthotoxin Effects on Host Quality
Effects of xanthotoxin on C. sosares may be the result of compromised host quality. To examine this possibility, we randomly assigned unparasitized neonate parsnip webworms individually to one of the three artificial diets as described. Cups with unparasitized webworms were examined each morning for the formation of pupae. The sex of pupae was determined by the location of the bursal scar (Nitao and Berenbaum 1988); the scar is on the ninth abdominal tergite in males and eighth abdominal tergite of females. Pupae whose sex could not be determined were removed from the analyses. Pupal mass was recorded to the nearest 0.0001 g with an analytical balance. After measurements, pupae were placed individually into empty diet cups in the growth chamber until moths emerged, and the total number of days from the time that neonates were placed on diet until emergence of adult moths was recorded. The effects of diet and gender on D. pastinacella pupal mass and development time were analyzed with separate two-way ANOVAs (PROC GLM; SAS Institute 2003). Pupae that failed to emerge after 30 d were dissected to determine whether death occurred before or after an adult developed inside. The mortality of D. pastinacella was calculated as the proportion of neonates that failed to develop into adult moths; mortality was compared across the three artificial diets with a likelihood ratio chi-square test (PROC FREQ; SAS Institute 2003).
Webworm Hemolymph Analysis
To quantify unmetabolized xanthotoxin encountered by developing C. sosares embryos and precocious larvae, we removed parasitized fourth-instar webworms (C. sosares individuals are either embryos or precocious larvae at this point) from each diet treatment (26 total = nine from low-xanthotoxin diets, 17 from high-xanthotoxin diets). Webworms were anesthetized with CO2, and prolegs were snipped with fine spring scissors. Hemolymph drained from these wounds was drawn with a pipette and transferred into 0.5-ml centrifuge tubes.
Webworms from which hemolymph was collected were dissected lengthwise in physiological saline, and any C. sosares precocious larvae and polygerms were removed and placed separately into 1.5-ml sample tubes. Precocious larvae (three samples from low-xanthotoxin diet and four from high-xanthotoxin diet) and polygerm (five samples from low-xanthotoxin diet and five from high-xanthotoxin diet) were frozen at −80°C until metabolic assays could be run (see below). Hemolymph samples plus the tubes in which they were contained were weighed to the nearest milligram and dried in a lyophilizer, and the residues were extracted with 30 μl methanol. The tubes from which the samples were drawn were washed, dried, and reweighed to quantify the fresh mass of hemolymph analyzed. Ten microliters of the extract was analyzed by high-pressure liquid chromatography (HPLC; Waters, Milford, MA, USA, gradient HPLC, with diode-array detector and autosampler). Solvent A was 1% formic acid in water, and solvent B was acetonitrile (linear gradient from 85% A to 100% B over 30 min, followed by 5 min at 100% B, flow rate 1 ml min−1). Furanocoumarins were separated with a C-18 column (Shiseido, Capcell, 4.5 × 250 mm, 5-μm particles). Differences in hemolymph titers of xanthotoxin (ng mg−1 fresh weight of hemolymph) among webworms reared on the different diets were analyzed with an ANOVA (PROC GLM; SAS Institute 2003).
Metabolism of Xanthotoxin by C. sosares Embryos and Precocious Larvae
Despite encountering furanocoumarins in host hemolymph, C. sosares larvae do not exhibit detectable metabolic detoxification of xanthotoxin (McGovern et al. 2006). To examine the possibility that embryos and/or precocious larvae are able to detoxify xanthotoxin, collected embryos and precocious larvae (see above) were analyzed according to McGovern et al. (2006). In brief, samples were homogenized and incubated for 30 min at 30°C in a reaction mixture that contained 500 ng xanthotoxin and an NADPH-regenerating system. The reactions were halted by adding 0.1 N HCl. Ten microliters of each reaction mixture was removed for protein quantification by using a dye assay (BioRad, Hercules, CA, USA) before HPLC analysis of unmetabolized furanocoumarins (extracted with 300 μl of ethyl acetate).
Results
Effect of Xanthotoxin on C. sosares
Experiments that examined the effects of xanthotoxin on C. sosares clutch size, survivorship, and body size were repeated in 2005 and 2006. In none of the analyses was the year in which the experiment was conducted significant (P > 0.2 in all tests). Therefore, we pooled data across 2005 and 2006 in the analyses presented below. A total of 269 C. sosares broods developed on the three diets: 28 all male, 27 all female, and three mixed sex on the control diet; 56 all male, 36 all female, and six mixed sex on low xanthotoxin; and 74 all male, 31 all female, and eight mixed sex on high-xanthotoxin diets.
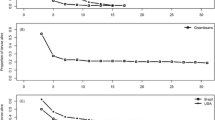
C. sosares that developed in webworms fed with the artificial diet with a high concentration of xanthotoxin produced smaller primary clutch sizes than those that developed in webworms fed with diets with low xanthotoxin concentrations or no xanthotoxin (Fig. 1; F 2, 260 = 24.60, P < 0.001). All-male and mixed-sex clutch sizes were larger than all-female clutch sizes irrespective of diet on which they were reared (F 2, 260 = 54.60, P < 0.001). Xanthotoxin concentration in the artificial diet had a similar effect on all-male, all-female, and mixed-sex broods (diet × sex interaction F 4, 260 = 0.70, P = 0.594).
Effects of dietary xanthotoxin on the mean (±sem) primary clutch sizes (see text for explanation) of all-male, all-female, and mixed-sex broods of Copidosoma sosares. Clutch sizes from webworms fed a high-xanthotoxin diet were significantly smaller than clutch sizes from either the low-xanthotoxin or xanthotoxin-free diets; clutch sizes from the low-xanthotoxin and xanthotoxin-free diets did not differ (Tukey’s HSD multiple comparisons test)
Within-brood mortality was affected by the concentration of xanthotoxin in the diet (diet term: Wald χ 2 = 45.58, P < 0.001), and this effect differed among the three types of C. sosares broods (diet × sex interaction term: Wald χ 2 = 71.85, P < 0.001; Fig. 2). Whereas the odds of an individual female surviving to adulthood in an all-female brood were not influenced by diet composition (Wald χ 2 = 4.02, P = 0.134), this was not the case for individuals in all-male or mixed-sex broods. The odds of an individual male failing to complete development to adulthood in a host on a high-xanthotoxin diet were 3.3 times greater than the odds for developing in a host fed a xanthotoxin-free diet (Wald χ 2 = 69.37, P < 0.001) but did not differ between low-xanthotoxin and xanthotoxin-free diets (Wald χ 2 = 3.10, P = 0.078). Likewise, the odds of an individual in a mixed-sex brood failing to complete development in a webworm fed a high-xanthotoxin diet was nearly 58 times greater than the odds of failing to complete development on a xanthotoxin-free diet (Wald χ 2 = 16.25, P < 0.001); the odds of larval mortality did not differ between the low-xanthotoxin and xanthotoxin-free diets (Wald χ 2 = 0.0123, P = 0.912). Larval survivorship to adulthood was 89% or above in all cases (Fig. 2).
Effects of dietary xanthotoxin on the proportion of the primary clutch that failed to develop into adults of all-male, all-female, and mixed-sex broods of Copidosoma sosares. Within each brood type (all-male, all-female, and mixed-sex), bars with different letters indicate significantly different odds of larval mortality as a function of diet type. Data were analyzed with logistic regression models (see text)
After accounting for the effects of clutch size and diet, individual females were larger than males (male tibial length = 0.54 ± 0.01 mm; female = 0.48 ± 0.01 mm; F 1, 241 = 233.72, P < 0.001). Neither artificial diet type nor the interaction with sex affected body size (diet: F 2, 241 = 1.37, P = 0.256; diet × sex: F 2, 241 = 0.72, P = 0.486).
Although individuals in all-female and mixed-sex broods developed more quickly than individuals in all-male broods (male = 40.6 ± 3.4 d; female = 37.7 ± 3.4 d; mixed = 34.0 ± 3.2 d; F 2, 257 = 13.87, P < 0.001), xanthotoxin concentration had no effect on the amount of time parasitized webworms fed on diet (diet: F 2, 261 = 2.32, P = 0.101; diet × sex: F 4, 261 = 2.05, P = 0.088).
Xanthotoxin Effects on Host Quality
A total of 241 D. pastinacella successfully pupated on the experimental diets: 23 male and 21 female moths on the xanthotoxin-free diet, 41 male and 58 female moths on the low-xanthotoxin diet, and 46 male and 52 female moths on the high-xanthotoxin diet.
The amount of xanthotoxin added to the artificial diet had no effect on the mass of D. pastinacella pupae (F 2, 234 = 2.15, P = 0.520). Likewise, after adjusting for the effects of xanthotoxin, males and females did not differ in pupal mass nor in how mass responded to dietary xanthotoxin (sex: F 1, 234 = 0.11, P = 0.743; sex × diet: F 2, 234 = 0.66, P = 0.520). The number of days from when neonates were placed on the diet until moth emergence was not influenced by xanthotoxin in the diet (F 2, 154 = 0.25, P = 0.779). Development time from neonates until adulthood did not differ between males and females (F 1, 154 = 1.05, P = 0.306). Development time of males and females did not differentially respond to diet composition (F 2, 154 = 0.70, P = 0.497). Finally, mortality rates of D. pastinacella were similar across the control, low-xanthotoxin, and high-xanthotoxin diets (likelihood ratio χ 2 = 3.47, 2 df, P = 0.177).
Webworm Hemolymph Analysis
Hemolymph from webworms fed with the artificial diet high in xanthotoxin (4.71 ng/μg fresh weight) had almost four times the unmetabolized xanthotoxin concentration as hemolymph from webworms fed with the artificial diet low in xanthotoxin (0.313 ng/μg fresh weight; Fig. 3; F 1, 15 = 7.11, P = 0.018).
Metabolism of Xanthotoxin by C. sosares Embryos and Precocious Larvae
Neither C. sosares embryos nor precocious larvae showed any detectable levels of metabolic detoxification of xanthotoxin. Of 34 reactions, only 17 showed detectable levels of protein ranging from 34 to 4,700 mg. The amount of unmetabolized xanthotoxin remaining after the metabolic assays of both C. sosares embryos and precocious larvae was no different from the mean of the four time-zero controls (t test, t = 0.013, P = 0.99). Comparison of the eight reactions with more than 1 mg of protein to time-zero controls gave a similar result (t = 0.252, P = 0.807).
Discussion
A previous field study that examined the correlation between the concentration of seven furanocoumarins in the host plants, Pastinaca sativa and Heracleum sphondylium (a common host plant in Europe), and the fitness measures of C. sosares showed that one furanocoumarin, xanthotoxin, was negatively correlated with clutch size as well as within-brood survivorship (Ode et al. 2004). In this study, we demonstrate that xanthotoxin added to an artificial diet consumed by webworms is responsible for both of these tritrophic correlations observed in the field. Clutch sizes from webworms reared on an artificial diet with a high level of xanthotoxin were 20–30% smaller than those reared on diets containing 15-fold less xanthotoxin or on a xanthotoxin-free diet. Further corroborating observed correlations in the field (Ode et al. 2004), our results demonstrate that within-brood survivorship of all-male and mixed-sex broods but not of all-female broods was reduced by high levels of xanthotoxin in diets compared to low levels or xanthotoxin-free diets. However, while the effects of xanthotoxin are statistically significant, within-brood larval mortality was low across all brood type and diet combinations relative to observed survivorship of field-collected broods (cf. Ode et al. 2004), suggesting that factors other than xanthotoxin have an impact on larval mortality of C. sosares.
Specialist parasitoids are less strongly affected by plant defensive chemistry than generalist parasitoids (Gunasena et al. 1990; Barbosa et al. 1991; Harvey et al. 2003, 2005; Sznajder and Harvey 2003). However, despite being a highly specialized parasitoid of a specialist herbivore, C. sosares exhibited significant variation in clutch size and survivorship in response to variation in the concentration of xanthotoxin in the host diet. Similarly, Barbosa et al. (1991) found that nicotine resulted in increased mortality in the specialist parasitoid C. congregata, whereas its sphingid host, Manduca sexta L., was not as strongly affected. Such studies suggest that specialist parasitoids are not necessarily immune to the negative effects of plant chemistry.
Whereas xanthotoxin has demonstrable effects on C. sosares fitness proxies, we were unable to detect any negative effects on host quality in terms of reduced webworm mass, increased development time, or increased mortality. It does not appear that the observed effects of xanthotoxin on C. sosares are the result of compromised host quality in response to dietary xanthotoxin. However, it is possible that other furanocoumarins or synergistic combinations, not considered in this study, could reduce host quality. For instance, the angular furanocoumarin sphondin is negatively correlated with the proportion of the linear furanocoumarin bergapten in seeds, and both are associated with resistance to webworms (Berenbaum et al. 1986). The concurrent presence of bergapten and xanthotoxin may compromise the ability of D. pastinacella to metabolize xanthotoxin. Diets high in such furanocoumarins conceivably could reduce host quality to the point that C. sosares fitness is also reduced. On the other hand, previous field correlative studies (Ode et al. 2004) indicate that xanthotoxin alone, out of seven analyzed furanocoumarins, was negatively correlated with C. sosares fitness.
Xanthotoxin likely affects C. sosares fitness by directly acting as a toxin. Although webworms are remarkable for their efficiency in metabolizing xanthotoxin (Zangerl and Berenbaum 1993), our results, in addition to those of McGovern et al. (2006), show that developing C. sosares do encounter unmetabolized xanthotoxin in the hemolymph of their webworm hosts. Furthermore, hemolymph titers of xanthotoxin increased fourfold in webworms fed with a diet that contained high levels of xanthotoxin compared to webworms consuming a diet low in xanthotoxin, indicating that changes in webworm diet, be they artificial or natural, correspond directly to the amount of xanthotoxin encountered by developing C. sosares.
Despite the presence of potentially toxic levels of xanthotoxin during development, we found no evidence that either C. sosares embryos or precocious larvae are able to metabolize it. Successful development of C. sosares embryos and larvae (see McGovern et al. 2006) likely depends in part on the ability of the webworm to detoxify furanocoumarins as well as the furanocoumarin content of the host plant tissues consumed by the webworms. Webworms from different geographical regions vary in their metabolic capacities (Berenbaum and Zangerl 1998; Zangerl and Berenbaum 2003; Berenbaum and Zangerl 2006). Similarly, plant populations vary considerably in terms of the furanocoumarin profiles produced, both within a species (Berenbaum and Zangerl 1998; Zangerl and Berenbaum 2003) and among species (Zangerl and Berenbaum 2003; Ode et al. 2004). In western Europe, where this tritrophic interaction is native, C. sosares clutch size and survivorship are greater on H. sphondylium (a host plant that contains relatively low levels of xanthotoxin) than it is on P. sativa (which typically contains 10 times the levels of xanthotoxin as H. sphondylium). Together, variation among webworm populations in terms of detoxification abilities and among host plant populations/species in terms of furanocoumarin profiles may have a strong influence on the establishment success of C. sosares.
The establishment success of C. sosares may be influenced ultimately by the trophic complexity in which the selective interactions between wild parsnip and parsnip webworm are embedded. The interaction intensity between parsnip and webworm determines the variation in wild parsnip furanocoumarin profiles and parsnip webworm detoxification abilities (Berenbaum and Zangerl 2006). The level of trophic complexity involving parsnips and webworms in The Netherlands is, by and large, greater than in midwestern North America. Higher trophic complexity in The Netherlands populations is indicated by the presence of alternative host plants and attacks by parasitic wasps, both of which are largely absent in midwestern North American parsnip populations. As a possible consequence of decreased trophic complexity, xanthotoxin concentrations in midwestern North American populations of parsnip are generally higher than in populations in The Netherlands (Ode et al. 2004; Berenbaum and Zangerl 2006). If C. sosares was introduced to midwestern North America (whether intentionally, accidentally, or naturally), it may experience, to its detriment, significantly elevated levels of xanthotoxin in its webworm hosts. Alternatively, such effects of xanthotoxin may be negated by the elevated detoxification abilities of midwestern North American webworms. An understanding of the effects of reduced trophic complexity on the selective intensity between plant and herbivore is important not only for interpreting the evolution of trophic relationships but also for predicting and understanding the outcome of biological control introductions.
References
Barbosa, P., Gross, P., and Kemper, J. 1991. Influence of plant allelochemicals on the tobacco hornworm and its parasitoid, Cotesia congregata. Ecology 72:1567–1575.
Barbosa, P., Saunders, J. A., Kemper, J., Trumbule, R., Olechno, J., and Martinat, P. 1986. Plant allelochemicals and insect parasitoids: effects of nicotine on Cotesia congregata (Say) (Hymenoptera: Braconidae) and Hyposoter annulipes (Cresson) (Hymenoptera: Ichneumonidae). J. Chem. Ecol. 12:1319–1328.
Berenbaum, M. R. 1990. Evolution of specialization in insect-umbellifer associations. Annu. Rev. Entomol. 35:319–343.
Berenbaum, M. R., and Zangerl, A. R. 1996. Phytochemical diversity: adaptation or random variation?, pp. 1–24, in J.T. Romeo, J.A. Saunders, and P. Barbosa (eds.). Recent Advances in Phytochemistry: Phytochemical Diversity and Redundancy in Ecological Interactions, vol. 30. Plenum, New York, NY.
Berenbaum, M. R., and Zangerl, A. R. 1998. Chemical phenotype matching between a plant and its insect herbivore. Proc. Natl. Acad. Sci. USA 95:13743–13748.
Berenbaum, M. R., and Zangerl, A. R. 2006. Parsnip webworms and host plants at home and abroad: trophic complexity in a geographic mosaic. Ecology 87:3070–3081.
Berenbaum, M. R., Zangerl, A. R., and Nitao, J. K. 1986. Constraints on chemical coevolution: wild parsnips and the parsnip webworm. Evolution 40:1215–1228.
Campbell, B. C., and Duffey, S. S. 1979. Tomatine and parasitic wasps: potential incompatibility of plant antibiosis with biological control. Science 205:700–702.
Campbell, B. C., and Duffey, S. S. 1981. Alleviation of a-tomatine-induced toxicity to the parasitoid, Hyposoter exiguae, by phytosterols in the diet of the host, Heliothis zea. J. Chem. Ecol. 7:927–946.
El-Heneidy, A. H., Barbosa, P., and Gross, P. 1988. Influence of dietary nicotine on fall armyworm, Spodoptera frugiperda and its parasitoids, the ichneumonid wasp Hyposoter annulipes. Entomol. Exp. Appl. 46:227–232.
Giron, D., Dunn, D. W., Hardy, I. C. W., and Strand, M. R. 2004. Aggression by polyembryonic wasp soldiers correlates with kinship but not resource competition. Nature 430:676–679.
Grbić, M., Ode, P. J., and Strand, M. R. 1992. Sibling rivalry and brood sex ratios in polyembryonic wasps. Nature 360:254–256.
Guerrieri, E., and Noyes, J. 2005. Revision of the European species of Copidosoma Ratzeburg (Hymenoptera: Encyrtidae), parasitoids of caterpillars (Lepidoptera). Syst. Entomol. 30:97–174.
Gunasena, G. H., Vinson, S. B., and Williams, H. J. 1990. Effects of nicotine on growth, development, and survival of the tobacco budworm (Lepidoptera, Noctuidae) and the parasitoid Campoletis sonorensis (Hymenoptera, Ichneumonidae). J. Econ. Entomol. 83:1777–1783.
Hardy, I. C. W. 1996. Precocious larvae in the polyembryonic parasitoid Copidosoma sosares (Hymenoptera: Encyrtidae). Entomol. Ber. 56:88–92.
Harvey, J. A., Corley, L. S., and Strand, M. R. 2000. Competition induces adaptive shifts in caste ratios of a polyembryonic wasp. Nature 406:183–186.
Harvey, J. A., Dam, N. M., and Gols, R. 2003. Interactions over four trophic levels: foodplant quality affects development of a hyperparasitoid as mediated through a herbivore and its primary parasitoid. J. Anim. Ecol. 72:520–531.
Harvey, J. A., Nouhuys, S., and Biere, A. 2005. Effects of quantitative variation in allelochemicals in Plantago lanceolata on development of a generalist and a specialist herbivore and their endoparasitoids. J. Chem. Ecol. 31:287–302.
McGovern, J. L., Zangerl, A. R., Ode, P. J., and Berenbaum, M. R. 2006. Furanocoumarins and their detoxification in a tri-trophic interaction. Chemoecology 16:45–50.
Nitao, J. K., and Berenbaum, M. R. 1988. Laboratory rearing of the parsnip webworm, Depressaria pastinacella (Lepidoptera: Oecophoridae). Ann. Entomol. Soc. Am. 81:485–487.
Ode, P. J. 2006. Plant chemistry and natural enemy fitness: effects on herbivore and natural enemy interactions. Annu. Rev. Entomol. 51:163–185.
Ode, P. J., and Strand, M. R. 1995. Progeny and sex allocation decisions of the polyembryonic wasp Copidosoma floridanum. J. Anim. Ecol. 64:213–224.
Ode, P. J., Berenbaum, M. R., Zangerl, A. R., and Hardy, I. C. W. 2004. Host plant, host plant chemistry, and the polyembryonic parasitoid Copidosoma sosares: indirect effects in a tritrophic interaction. Oikos 104:388–400.
Reitz, S. R., and Trumble, J. T. 1996. Tritrophic interactions among linear furanocoumarins, the herbivore Trichoplusia ni (Lepidoptera: Noctuidae), and the polyembryonic parasitoid Copidosoma floridanum (Hymenoptera: Encyrtidae). Environ. Entomol. 25:1391–1397.
Reitz, S. R., and Trumble, J. T. 1997. Effects of linear furanocoumarins on the herbivore Spodoptera exigua and the parasitoid Archytas marmoratus: host quality and parasitoid success. Entomol. Exp. Appl. 84:9–16.
Sas Institute 2003. SAS/STAT User’s Guide, Version 9.1.3. Cary, NC, SAS Institute.
Strand, M. R., Baehrecke, E. H., and Wong, E. A. 1991. The role of host endocrine factors in the development of polyembryonic parasitoids. Biol. Control 1:144–152.
Sznajder, B., and Harvey, J. A. 2003. Second and third trophic level effects of differences in plant species reflect dietary specialisation of herbivores and their endoparasitoids. Entomol. Exp. Appl. 109:73–82.
Thurston, R., and Fox, P. M. 1972. Inhibition by nicotine of emergence of Apanteles congregatus from its host, the tobacco hornworm. Ann. Entomol. Soc. Am. 65:547–550.
Zangerl, A. R., and Berenbaum, M. R. 1993. Plant chemistry, insect adaptations to plant chemistry, and host plant utilization patterns. Ecology 74:47–54.
Zangerl, A. R., and Berenbaum, M. R. 2003. Phenotype matching in wild parsnip and parsnip webworms: causes and consequences. Evolution 57:806–815.
Acknowledgments
This research was supported by NSF grant 0321028 to PJO and MRB. We thank Chris Asmundsen for assistance with colony maintenance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lampert, E.C., Zangerl, A.R., Berenbaum, M.R. et al. Tritrophic Effects of Xanthotoxin on the Polyembryonic Parasitoid Copidosoma sosares (Hymenoptera: Encyrtidae). J Chem Ecol 34, 783–790 (2008). https://doi.org/10.1007/s10886-008-9481-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-008-9481-8