Abstract
Neonate fall armyworms [FAW; Spodoptera frugiperda (Smith)] often encounter conspecific herbivore damage as they disperse from an egg mass to an initial feeding site. We investigated the orientation responses of dispersing neonates to herbivore damage in cowpea seedlings, specifically examining whether neonate behaviors were affected by inceptin, the primary elicitor of FAW-induced defenses in cowpea leaves. We focused on responses to damage caused by conspecific first instars, as might occur during the dispersal of siblings from an egg mass. Inceptin contents of damaging first instar FAW were controlled through their diets, with leaf-fed FAW producing inceptins in their oral secretions, and root-fed or starved FAW lacking these elicitors. In a bioassay designed to evaluate neonate dispersal off a host plant, a higher percentage of neonates remained on herbivore-induced or inceptin-treated plants than on undamaged plants, mechanically damaged plants, freshly damaged plants, or on plants damaged by FAW lacking inceptins. Further investigations of neonate responses to plant odors with a four-arm olfactometer demonstrated that neonate attraction to odors from 4-h old FAW damage was strongly dependent on previous diet of the damaging larvae. Neonates were attracted to odors from 4-h old FAW damage over odors from undamaged plants or fresh FAW damage, provided that the damaging larvae had previously ingested leaf material. In a direct comparison of odors from induced plants, plants damaged by leaf-fed FAW were as attractive as plants treated with synthetic inceptin. GC-MS analysis confirmed that (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) was the major volatile induced by FAW herbivory. While both DMNT and undamaged plant odors were more attractive than air, neonates preferred DMNT-supplemented plant odors. These results suggest that neonate FAW exploit herbivore-induced plant volatiles as host plant location and recognition cues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants respond to herbivore feeding through increased biosynthesis and emission of volatile compounds from the damaged tissues (Paré and Tumlinson 1997, 1999). While these volatiles often benefit the plant through enhanced attraction of natural enemies (i.e., parasitoids and predators; Dicke and Sabelis 1988; Kessler and Baldwin 2001; Turlings et al. 1990, 1995), the same volatiles can potentially serve as olfactory cues to the herbivores themselves by making the damaged tissues more apparent (Bolter et al. 1997; Dicke and Vet 1999; Carroll et al. 2006). Such enhanced detectability of host plant tissue may be particularly useful to herbivores with limited search capacities (Ward 1987; Stamps and Krishnan 2005), such as neonate caterpillars (Zalucki et al. 2002). Despite their greatly restricted mobility, neonates must locate and establish feeding sites on appropriate host tissues within a limited amount of time, or they will starve to death due to minimal energy reserves (Zalucki et al. 2002). In many species, location of an initial neonate feeding site is accomplished largely by female oviposition choice (Thompson 1988). However, the oviposition site may be inappropriate or insufficient for neonate development for a variety of reasons. Females may oviposit off the plant or on tissues that are not consumed by the neonate (Harris et al. 1999; Zalucki et al. 2002). Ovipositing females may be phenologically displaced from the tissues that their offspring will consume, particularly in rapidly developing tissues, and therefore, they are often unable to assess host quality or to lay eggs in an appropriate location (Zangerl and Berenbaum 1992). Females may also lay more offspring on the natal plant than can be supported by the available resources (Chapman et al. 1999; Prokopy and Roitberg 2001). In such cases, neonates may compensate for suboptimal conditions at the oviposition site by dispersing to other host tissues via ballooning or crawling (Roitberg and Mangel 1993; Harris et al. 1999; Doak 2000; Zalucki et al. 2002). Since location and recognition of appropriate host plant tissue by neonates involves chemosensory and tactile inputs (del Campo et al. 2001; Schoonhoven and van Loon 2002), herbivore damage may affect dispersal behavior and eventual recruitment of neonates to damaged tissues.
The fall armyworm (FAW) Spodoptera frugiperda (Smith) is a highly mobile lepidopteran that disperses as a neonate from its oviposition site (Yang et al. 1993), thus escaping from the frequent and negative effects of overcrowding (Chapman et al. 1999). Larval overcrowding can be limited through the use of larval frass as an oviposition deterrent, as in the related noctuid S. littoralis (Hilker and Klein 1989); however, an ovipositing FAW female lays up to 200 eggs in a single egg mass on a host plant, often exceeding the resources available (Sparks 1979; Pitre et al. 1983; Chapman et al. 1999). Larvae that remain in proximity to conspecifics face not only depletion of host plant material, but the threat of cannibalism (Chapman et al. 1999). To compensate for unfavorable conditions at the oviposition site, neonates disperse en masse several hours after hatching from the egg mass before initiating feeding on the natal host plant and on neighboring ones (Sparks 1979).
During dispersal from the egg mass, neonates often encounter various phases of herbivore damage caused by feeding conspecifics, from fresh damage inflicted at new feeding sites to older damage at abandoned feeding sites, to a combination of fresh and older damage at feeding sites established by actively feeding (often older) conspecifics. Eventually, the neonate will experience the whole phenological sequence of damage volatiles as it establishes its own feeding site. The volatile compositions emitted during these various damage phases change considerably over time as individual volatile components are induced at different rates (Turlings et al. 1998). In many plants, fresh damage volatiles that consist of lipoxygenase products (i.e., green leaf volatiles) rapidly released by mechanical damage differ from older damage volatiles consisting of terpenoids and other compounds slowly synthesized by the attacked plant (Hoballah and Turlings 2005). As such, the damage-induced volatiles encountered by dispersing neonates may convey information not only about host tissue location, but also the presence of other herbivores (Landolt 1993; Prokopy and Roitberg 2001; Carroll et al. 2006), host tissue quality vis-à-vis damage phenology (Bernasconi et al. 1998), and eventually the location of the larva’s own feeding site.
We examined the responses of dispersing neonate FAW to herbivore damage in cowpea [Vigna unguiculata (L.) Walp.], an occasional, yet marginal, host of the fall armyworm (Meagher et al. 2004). We compared neonate responses to both fresh damage and older damage separately. We also evaluated neonate responses to damage-induced volatiles alone. While host location, recognition, and eventual acceptance of host tissues involves several sensory inputs, the initial opportunity for interactions with damaged host tissues are likely to occur through long distance modalities such as olfaction and vision (Harris et al. 1999; Schoonhoven and van Loon 2002).
We also asked whether neonate responses to herbivore damage could be mediated by elicitor-induced defensive responses in cowpea. Inceptin, a peptide formed by the partial proteolysis of chloroplastic ATPase, is the primary elicitor of induced volatile responses to sixth instar FAW in cowpea leaves (Schmelz et al. 2006). We examined whether neonate responses to older damage were dependent on full induction of herbivore-induced responses (sensu Dicke et al. 1999 and Turlings et al. 2000), both through the application of synthetic inceptin to wounded plant tissues and the use of damaging FAW with different predicted inceptin contents. Since only FAW larvae that have previously ingested chloroplast-containing tissues produce inceptin in their oral secretions, the inceptin contents of damaging larvae, and therefore the extent of herbivore-induced responses in cowpea leaves, can be readily manipulated through the larval diet (Schmelz et al. 2006). We also examined whether neonate responses to whole plant odors could be altered by a single induced volatile. The terpene (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) is a major constituent of induced volatiles in many plants, not only in cowpea but also in preferred graminaceous hosts such as maize (Turlings et al. 1998; D’Alessandro and Turlings 2005; Carroll et al. 2006). Given the prominence of DMNT in the induced volatile profile of such unrelated host plants, we investigated the orientation responses of dispersing neonates to DMNT volatiles.
Methods and Materials
Plants
Seeds of cowpea [Vigna unguiculata (L.) Walp. var. Cal 5] were planted in professional growers mix potting soil (Metro Mix 300, SunGro Horticulture, Vancouver, British Columbia) in 4 in. square pots and initially maintained for the first 7 days under 350 μmol m-2 s-1 photosynthetic active radiation under a 12:12 h (L/D) photoperiod. On the eighth day, plants were transferred and maintained at 25 °C under ambient greenhouse light supplemented with high-pressure sodium lamps under a 12:12 h (L/D) photoperiod. Supplemental lighting was used from 6:00 a.m. to 6:00 p.m. Seedlings (14–17 days old) with two fully expanded V2 leaves were used in all experiments.
Insects
Fall armyworm eggs and neonates used in the behavioral bioassays were obtained from a colony maintained on an agar-based artificial diet at CMAVE, USDA-ARS (Gainesville, FL, USA). All insects were reared at 25°C under a 14:10 h (L/D) photoperiod.
Damage Treatments of Cowpea Seedlings
Herbivore damage was inflicted by first instar FAW that either contained or lacked inceptins in their oral secretions. Because inceptins are formed by the partial proteolysis of chloroplastic ATP synthase, only larvae that have previously ingested and partially digested chloroplast-containing plant material produce inceptins (Schmelz et al. 2006). To obtain damaging first instars with inceptins, neonates were prefed on cowpea leaves (LF-FAW) for 10 h before their use in the damage treatments. Larvae without inceptins in their oral secretions were acquired by prefeeding neonates on cowpea roots [which lack chloroplasts (RF-FAW)] for 10 h or starving the larvae (unfed; UF-FAW) before their use in the damage treatments (Schmelz et al. 2006). We also specifically included a treatment that stimulates fresh damage by unfed [UF-FAW (fresh)] first instars, as this type of herbivore damage is commonly encountered by dispersing neonates near the initial feeding sites of their neonate siblings. Herbivore damage to leaves was initiated by transferring 120 first instar FAW to the V2 leaves of each plant. To minimize the production of inceptin-related peptides from recently ingested foliage during the damage treatments, we restricted the time period that damaging larvae were allowed to feed on the plants to 60 min or less. In cowpea leaves, emissions of damage volatiles induced by FAW herbivory or inceptin treatment increase substantially 4 h after the initial attack (Schmelz et al. 2006). To obtain cowpea seedlings that emitted only fresh damage or older damage odor phenologies, bioassays or volatile collections were conducted on plants either 1 h (fresh) or 4 h (old) after feeding damage had been inflicted.
Artificial damage treatments were initiated by superficially scratching three small wound patches (about 2 cm2 each) with repeated shallow swipes of a razor blade on the adaxial side of a newly expanded V2 trifoliate. Each trifoliate was treated by applying either inceptin [2.5 ng synthetic inceptin in 10 μl 50 mM sodium phosphate pH 8.0 buffer, inceptin-treated (INC)] or a buffer solution [10 μl buffer, mechanically-damaged (MD)] across its wounds. To obtain cowpea seedlings that emitted older damage odors, all artificial damage treatments were conducted 4 h before use in bioassays or volatile collections. All damage treatments were inflicted under normal light conditions (previously described) to obtain maximal induction of photosynthesis-dependent volatiles (Turlings et al. 1995).
Plant Volatile Collections
Collections of headspace volatiles emitted by cowpea plants were performed in glass chambers with a push–pull system (Analytical Research Systems, Gainesville, FL, USA) modified after Heath and Manukian (1994). An excess of pre-humidified, carbon-filtered air was added to the top of the chamber at 3 l/min to ensure that outside air did not enter the chamber. Volatiles were sampled by pulling air over the plants and through a Super Q absorbent (80–100 mesh, Alltech Associates, Deerfield, IL, USA) filter at 600 ml/min for 1 h. Adsorbed volatiles were eluted from the filter with 200 μl dichloromethane that contained 400 ng nonyl acetate as an internal standard.
Identification of Plant Volatiles
Volatile analysis was performed by positive ion electron impact gas chromatography-mass spectrometry (EI GS-MS) on an HP 6890 gas chromatograph coupled to an HP 5973 MS detector. 1 μl of the sample was injected (240°C) onto an Agilent HP-5MS dimethylpolysiloxane column (30 m × 250 μm (i.d.) × 0.25 μm, Agilent Technologies, Palo Alto, CA, USA) and separated by temperatures programmed from 35°C (1.0 min hold) to 230°C at 10°C/min. Helium was used as a carrier gas at 1.2 ml/min. Volatiles were identified by comparison of mass spectra (a) with mass spectra libraries (NIST and Department of Chemical Ecology, Göteborg University, Sweden) and (b) with mass spectra and retention times of authentic standards.
Chemicals
Inceptin (ICDINGVCVDA) was synthesized and purified at the Protein Core Chemistry Facility (University of Florida, Gainesville, FL) as previously described by Schmelz et al. (2006). Synthetic stocks of 4,8-dimethyl-1,3,7-nonatriene were acquired as a gift from Robert J. Bartlett (Crop Bioprotection Research Unit, USDA-ARS, Peoria, IL, USA). To enrich the content of the naturally emitted (E) isomer over the (Z) isomer, the mixture was separated three times sequentially by normal phase HPLC on a AgNO3-coated (25%) silica gel column with benzene as an eluent (Heath and Sonnet 1980). Although we were unable to completely separate the isomers, the ratio of (E):(Z) DMNT was improved from 1:2 to approximately 20:1. The benzene was carefully removed under gentle N2 flow.
Neonate Dispersal Bioassay
To assess the effects of plant damage on neonate dispersal, we recorded the number of first instars remaining on a plant 24 h after transfer to leaves as dispersing neonates. We compared first instar retention on plants induced by inceptin [LF-FAW (old) and INC (old)] with retention on plants that were not induced by inceptin [CON (undamaged control), MD (old), UF-FAW (fresh), RF-FAW (old)]. The bioassay was conducted in a walk-in incubator (2.4 × 2.4 × 2.4 m, Aminco, Silver Springs, MD) maintained at 30°C and 80% RH under a 12:12 h (L/D) photoperiod. The ambient vibration and airflow within this incubator was minimal, thereby reducing the chance of disturbance or successful ballooning off the plant in a strong airflow. A group of 20 newly dispersing neonates were transferred by a camel hair brush to the adaxial side of V2 leaflets on each plant at 8:00 p.m. (2 h after the onset of scotophase). Retention of larvae was tested on three plants per treatment. One replicate set of plants containing one plant from each treatment (six plants total) was placed in the walk-in incubator at a time. Three replicate sets total were performed during separate overnight periods. To limit the exposure of neonates to volatiles from adjacent plants, the plants were placed in a single, staggered line that ran perpendicular to the reduced air flow. The order of plants in the linear array was randomly assigned by treatment in each replicate. Each plant was isolated from other plants by at least 50 cm and placed on a water-filled plastic saucer (25 cm diameter) to ensure that neonates that left the plant could not return. Larvae observed attempting to silk off the plant were unable to become aloft and simply lowered to the ground or returned to the plant above. The number of first instars left alive on each plant was recorded 24 h after introduction. No dead larvae were observed on any of the plants at the end of the bioassay.
Four-Arm Olfactometer Bioassays
Orientation responses of neonate FAW to plant and synthetic volatile odors were evaluated with a four-arm olfactometer (Suazo et al. 2003). The olfactometer consisted of a quadrilateral-shaped choice arena with an arm at each corner and a 3 cm diameter base for air outflow at the center. Air flow from distal odors sources through the olfactometer was maintained with a push–pull system. Volatiles were obtained in distal volatile collection systems (previously described, Heath and Manukian 1994) by passing 3 l/min of pre-humidified, carbon-filtered air over the odor source. A portion of these volatiles were relayed from the distal odor source to an arm by 1/4” OD corrugated Teflon tubing. Air was then pulled through the olfactometer and out of the bottom at 2.4 l/min (600 ml/min per arm) by a vacuum line attached to the central base. Neonates were prevented from entering the vacuum line by a fine nylon mesh screen and the odor source lines by a glass trap placed at the distal end of each arm.
We evaluated neonate responses to odors in the olfactometer by using groups of neonates rather than individuals, since neonate FAW disperse from egg masses containing many siblings. A group of 120–200 neonates was transferred by a camel hairbrush to a 10 ml glass scintillation vial. Each group of neonates was placed in the center of the olfactometer by rapidly inverting the vial directly over the base and gently tapping the larvae off the glass vial. To minimize phenological changes in odors from damaged plants, the neonates were given a limited amount of time (up to 1 h) to move toward an odor source in the arena. A choice preference for an odor source was scored only if a neonate entered an arm. Larvae that did not enter an arm (up to 65%) within the allotted time were scored as neutrals and excluded from the statistical analysis.
Whole plant odors were sampled by the methods previously described for volatile collection. Synthetic DMNT was introduced into the odor source airstream by capillary release, a method that allows for the predictable and stable release of volatile compounds from a capillary tube based on their physiochemical characteristics (Weatherston et al. 1985). We based our protocol on the modifications of D’Alessandro et al. (2006), who greatly improved handling ease and conservation of volatile compounds by creating a volatile-saturated atmosphere in an auxillary container rather than the capillary tube itself. A DMNT-saturated atmosphere was created within a 4 ml glass vial by placing 5 μl DMNT inside a 250 μl glass GC vial insert and sealing the vial with a GC septum. A defined capillary release of DMNT (80 ng/h) was achieved by inserting one end of a 50 μl capillary tube (Drummond Scientific Company, Broomall, PA, USA) through the vial septum and projecting the other end into the odor source airstream. The released DMNT was thoroughly mixed with the airstream through the use of multiple nylon mesh baffles.
The capillary-release rate of DMNT in odor source airstreams was estimated by analysis of SuperQ filter-trapped volatiles following the methods of Schmelz et al. (2001). Quantification of DMNT was performed on an Agilent 6890 gas chromatograph coupled to a flame ionization detector (250°C). One microliter of the sample was injected (splitless, 220°C) onto an Agilent DB-1 dimethylpolysiloxane column (15 m × 250 μm (i.d.) × 0.25 μm, Agilent Technologies, Palo Alto, CA, USA) and separated with temperatures programmed from 40°C (0.50 min hold) to 180°C at 12°C/min followed by a 220°C post run (2 min). Helium was used as a carrier gas at 1.2 ml/min.
Statistics
In both the neonate dispersal bioassay and the olfactometer bioassays, the proportion of neonates remaining on the plant or selecting the source were arcsin-transformed and compared either across treatments or odor sources by a one-way ANOVA (JMP 4.0.4, SAS Institute, Cary, NC, USA). In all bioassays, proportion means were compared by Tukey-Kramer HSD tests (JMP 4.0.4, SAS Institute, Cary, NC, USA).
Results
Retention of Dispersing Neonates on Herbivore-Induced Plants
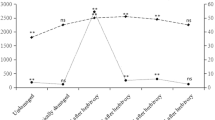
The percentage of dispersing neonates remaining on induced plants (plants damaged by leaf-fed first instar FAW or inceptin-treatment) was significantly higher than on undamaged plants or plants that experienced other forms of damage (Fig. 1). The retention of first instars on these plants could not be attributed to feeding damage alone, as plants that experienced fresh damage or damage by root-fed first instar FAW retained first instars in proportions similar to undamaged or mechanically damaged plants. These results are consistent with the hypothesis that neonate retention on damaged cowpea plants is largely mediated by inceptin-induced plant responses. Of all the damaging larvae, only leaf-fed first instar FAW would be expected to produce inceptins from previously ingested chloroplast-containing plant material. Curiously, we observed that the majority of first instars retained on the damaged plants established feeding sites in pre-existing wound sites, despite the fact that these tissues comprised less than 5% of the total leaf area.
Retention of Spodoptera frugiperda neonates on cowpea plants subjected to different leaf damage treatments: undamaged (CON), mechanically damaged (MD), inceptin-treated (INC), or FAW-damaged [unfed (UF-FAW), root-fed (RF-FAW), or leaf-fed (LF-FAW) first instars] plants. Mean percentage(±SE) of first instars represents the number of FAW remaining (out of 20 dispersing neonates introduced) on a plant 24 h after placement on the upper foliage (N = 3 plants per treatment). To obtain only fresh or older damage odor phenologies, FAW-damaged plants were attacked by damaging larvae either 1 h (fresh) or 4 h (old) before use in the bioassay. Treatments not sharing the same letter are significantly different by Tukey-Kramer HSD (P < 0.05; one-way ANOVA; P < 0.001, F = 32.55, df = 5)
Neonate Attraction to Herbivore-Induced Odors in Olfactometer Bioassays
In the olfactometry bioassays, dispersing neonates were strongly attracted to odors emitted by herbivore-induced plants (plants damaged by leaf-fed FAW), but not to odors from undamaged plants, plants with fresh damage, or plants damaged by larvae lacking inceptins (starved or root-fed FAW; Figs. 2 and 3). These results support the hypothesis that neonate attraction to cowpea volatiles induced by plant damage, like neonate retention, is strongly mediated by inceptin-induced plant responses. A direct comparison of the larval response to volatiles released from plants with old feeding damage and to odors from inceptin-treated plants revealed that neonates do not distinguish between these odor sources (Fig. 4).
Response of Spodoptera frugiperda neonates to different odor sources offered simultaneously in a four-arm olfactometer: undamaged cowpea plants (CON), or cowpea plants attacked by FAW [unfed (UF-FAW) or leaf-fed (LF-FAW) first instars]. Mean percentage (±SE) of neonates is the proportion of neonates entering a respective olfactometer arm out of the total number that responded positively (entered arms; N = 2 replicates, a total of 93 and 179 larvae responded in each replicate). To obtain only fresh or older damage odor phenologies, FAW-damaged plants were attacked by damaging larvae either 1 h (fresh) or 4 h (old) before use in the bioassay. Treatments not sharing the same letter are significantly different by Tukey-Kramer HSD (P < 0.05; one-way ANOVA; P < 0.001, F = 27.60, df = 3)
Response of Spodoptera frugiperda neonates to different odor sources offered simultaneously in a four-arm olfactometer: undamaged cowpea plants (CON), or cowpea plants attacked by FAW [unfed (UF-FAW), root-fed (RF-FAW), or leaf-fed (LF-FAW) first instars]. Mean percentage (±SE) of neonates is the proportion of neonates entering a respective olfactometer arm out of the total number that responded positively (entered arms; N = 3 replicates, a total of 95, 109, and 149 larvae responded in each replicate). To obtain only fresh or older damage odor phenologies, FAW-damaged plants were attacked by damaging larvae either 1 h (fresh) or 4 h (old) before use in the bioassay. Treatments not sharing the same letter are significantly different by Tukey-Kramer HSD (P < 0.05; one-way ANOVA; P = 0.003, F = 34.81, df = 3)
Response of Spodoptera frugiperda neonates to different odor sources offered simultaneously in a four-arm olfactometer: undamaged cowpea plants (CON), mechanically damaged cowpea plants (MD), inceptin-treated cowpea plants (INC), or cowpea plants damaged by leaf-fed first instar FAW (LF-FAW). Mean percentage (±SE) of neonates is the proportion of neonates entering a respective olfactometer arm out of the total number that responded positively (entered arms; N = 2 replicates, a total of 104 and 112 larvae responded in each replicate). To obtain only older damage odor phenologies, FAW-damaged plants were attacked by damaging larvae 4 h before use in the bioassay. Treatments not sharing the same letter are significantly different by Tukey-Kramer HSD (P < 0.05; one-way ANOVA; P = 0.014, F = 14.14, df = 3)
Herbivore-Induced Plants Emit DMNT
In a comparison of volatiles from FAW-damaged, inceptin-treated, and undamaged cowpea seedlings during the early afternoon, the homoterpene (E)-4,8-dimethyl-1,3,7-nonatriene (DMNT) was the major induced volatile produced by cowpea foliage (Fig. 5). DMNT emissions were strongly induced by inceptin-treatment or feeding damage by leaf-fed first instar FAW after 4 h, but only moderately by mechanical damage, feeding damage from unfed FAW, or fresh feeding damage. The greater induction of DMNT in inceptin-treated plants was likely due to the large amount of inceptin (2.2 pmol/trifoliate) applied, which equates to 4 μl of oral secretions by a 6th instar, a quantity that probably exceeds the amount contributed by leaf-fed first instars (Schmelz et al. 2006). Other volatiles commonly encountered in damaged leaf tissues, including lipoxygenase products (green leaf volatiles) from freshly damaged plants, were not detected by the methods used.
Representative GC-FID chromatographic profiles of headspace volatiles collected in the early afternoon from cowpea plants subjected to different leaf damage treatments: inceptin-treated (INC), mechanically damaged (MD), FAW-damaged [unfed (UF-FAW) or leaf-fed FAW (LF-FAW)], or undamaged (control) plants. The homoterpene [E]-4,8-dimethyl-1,3,7-nonatriene (DMNT, RT 5.22 min) is the most abundant volatile emitted by both the induced and uninduced cowpea plants used in the bioassays. Samples represent 1/5× the total headspace emissions collected over 60 min. Octane (280 ng, RT 2.38 min) and nonyl acetate (400 ng, RT 7.53 min) were added separately as internal standards (I.S.). To obtain only fresh damage or older damage odor phenologies, FAW-damaged plants were attacked by damaging larvae either 1 h (fresh) or 4 h (old) before the volatile collections
Attraction of Neonates to DMNT
DMNT was an attractant to dispersing neonates in the four-arm olfactometer, both by itself and as a supplement to undamaged cowpea plant odors (Fig. 6). The combination of supplementary DMNT and undamaged plant odors was significantly more attractive than either odor source alone. This observation, combined with the equivalent response of neonates to DMNT or undamaged plant odors (which contain much lower amounts of DMNT than damaged plants), indicates that undamaged leaves may emit volatiles other than DMNT that are also attractive to dispersing neonates.
Response of Spodoptera frugiperda neonates to different odor sources offered simultaneously in a four-arm olfactometer: filtered air (AIR), undamaged cowpea plant (PLANT), DMNT-supplemented filtered air (DMNT), or a DMNT-supplemented air stream from an undamaged cowpea plant (PLANT & DMNT). Mean percentage (±SE) of neonates is the proportion of neonates entering a respective olfactometer arm out of the total number that responded positively (entered arms; N = 3 replicates, a total of 97, 107, and 123 larvae responded in each replicate). For the DMNT supplemented odor sources, 80 ng/h of synthetic DMNT was introduced into the air stream by capillary release. Treatments not sharing the same letter are significantly different by Tukey-Kramer HSD (P < 0.05; one-way ANOVA; P < 0.001, F = 27.60, df = 3)
Discussion
Neonate FAW are attracted to volatiles released from herbivore-damaged cowpea leaves. In cowpea leaves, inceptin induces both volatile and nonvolatile anti-herbivore defenses against FAW larvae, as implicated by phenylpropanoid markers and protease inhibitor transcripts (Schmelz et al. 2006). Sixth instar FAW fed on inceptin-induced cowpea leaves endure a significant reduction in growth rate (17%) compared to larvae given undamaged leaves (Schmelz et al. 2006). Furthermore, herbivore-induced volatiles are well-known to serve as signals to natural enemies that forage for plants infested with herbivores (Kessler and Baldwin 2001).
Despite the role of herbivore-induced plant volatiles as cues for natural enemies, some lepidopteran neonates orient to herbivore-damaged hosts during the search for an initial feeding site. Many neonates are not positioned on preferred host tissues during oviposition, but move within the plant to an appropriate feeding site after hatching (Zalucki et al. 2002). Movement of codling moth (Cydia pomonella; Lepidoptera: Tortricidae) neonates from oviposition sites on foliage to feeding sites on apple fruit is enhanced by conspecific damage and induction of the fruit volatile [E,E]-α-farnesene (Landolt et al. 2000), despite the fact that herbivore damage attracts the larval parasitoid Hyssopus pallidus (Hymenoptera: Eulophidae; Rott et al. 2005). In this case, the utility of induced volatiles as olfactory attractants to appropriate host tissue may outweigh secondary consequences of induced defenses or enhanced recruitment of natural enemies.
Noticeably, dispersing FAW neonates are more attracted to odors from plants with old (4 h) neonate feeding damage than to odors from plants with fresh (1 h) neonate feeding damage. These olfactory preferences occur despite an absence of prior feeding experience. Unlike older instars, neonates have not yet been exposed to plant odors during feeding. Experience-based olfactory preferences are thought to be an important component in the development of host preferences in related polyphagous noctuids, such as Spodoptera littoralis (Anderson et al. 1995; Carlsson et al. 1999). However, an innate lack of attraction to fresh damage volatiles would enhance dispersal of FAW neonates away from the egg mass and limit orientation to new feeding sites established by neonate siblings. Neonate attraction might also be targeted toward host plant volatiles induced by oviposition itself. Recent studies indicate that some plants respond to insect oviposition by emitting volatiles that attract egg parasitoids to the oviposition site (Meiners and Hilker 1997, 2000; Colazza et al. 2004). Although induction of volatiles by oviposition has not been examined in either cowpea or the grasses preferred by FAW, the large size of the egg mass and the female’s preference for readily inducible leaf tissue (leaf vein creases) as a preferred oviposition site (Sparks 1979) are factors that merit further investigation.
The use of herbivore-induced plant volatiles as neonate orientation cues may facilitate and reinforce a broad host range in polyphagous herbivores such as FAW. Although FAW has been reported from over 80 plant species, the preferred oviposition hosts are overwhelmingly grasses (Luginbill 1928; Sparks 1979; Meagher et al. 2004). On grasses, FAW neonates feed preferentially and performed better on younger tissues, especially leaf whorls (Yang et al. 1993; Davis et al. 1999), developing tissues that usually emit higher levels of constitutive and induced volatiles than comparable mature tissues (Hoballah et al. 2004). While unrelated hosts such as cowpea and maize differ considerably in plant architecture and overall secondary chemistry, these plants share some similarities in damage odors derived from a relatively restricted pool of volatiles (Gouinguené et al. 2003; D’Alessandro and Turlings 2005). DMNT is one of the few major induced volatiles emitted by both cowpea and maize (Carroll et al. 2006). FAW attraction to the major induced volatile emitted by a marginal host (cowpea) may reflect its co-occurrence in the preferred tissues of a major host (maize). A polyphagous caterpillar therefore may exploit induced volatile odors, not only because of their high emission rates, but also because qualitatively these compounds are fairly ubiquitous components of diverse host volatile profiles. The use of induced volatiles as cues may be further promoted by their familiar presence around actively feeding FAW at their own feeding sites, as larvae routinely encounter induced volatiles emitted by host tissues damaged by themselves.
Compared with other hosts of FAW, herbivore-damaged cowpea releases relatively few major volatile components, yet is readily detected by both feeding herbivores and parasitoids (Hoballah et al. 2002). It is unclear if herbivores and parasitoids are attracted to exactly the same induced volatiles. FAW neonates orient toward herbivore-induced volatiles that may be poorly perceived by some parasitoids (Gouinguené et al. 2005) but used as cues by others (Pickett et al. 2006). For example, DMNT, the major volatile induced in cowpea leaves, is readily detected (as measured by GC-EAG) in Spodoptera littoralis-damaged cowpea leaves by the parasitoid Campoletis sonerensis (Hymenoptera: Ichneumonidae) but not Cotesia marginiventris (Hymenoptera: Braconidae) or Microplitis rufiventris (Hymenoptera: Braconidae; Gouinguené et al. 2005). Other induced volatile components from cowpea leaves may be used as olfactory cues by both herbivores and parasitoids. For example, the monoterpene linalool, which has been detected as a minor component of herbivore-damaged cowpea leaves by several authors (Hoballah et al. 2002; D’Alessandro and Turlings 2005) but not in one of our previous studies (Schmelz et al. 2006), is highly attractive to both sixth instar FAW (Carroll et al. 2006) and several noctuid parasitoids (Hoballah et al. 2002; Gouinguené et al. 2005). Neonates and parasitoids have likely converged on a limited subset of induced plant volatiles as olfactory cues based on their functionality (Stamps and Krishnan 2005)—as ubiquitous and reliable markers of herbivore damage, induced volatiles are among the most apparent indicators of host plant location available to a forager. The extent of overlap and exclusion between herbivores and parasitoids in their use of particular volatiles as cues remains to be determined.
While much research has focused on how plants respond to herbivory and how natural enemies react to induced plant volatiles (Kessler and Baldwin 2002; Dicke et al. 2003; Paré et al. 2005), little is known about responses of feeding herbivores themselves. The few studies that have been performed on generalist responses to induced volatiles have focused primarily on ovipositing females for the simple reason that oviposition is often a critical determinant of host plant selection (De Moraes et al. 2001; Kessler and Baldwin 2001). However, we should not ignore the fact that it is neonate establishment of an initial feeding site on a host, rather than oviposition alone, that is the sine qua non of host acceptance by herbivores. Paradoxically, it is often the limited and unspectacular search capabilities and acceptance behaviors of neonates that determine whether host acceptance or rejection occurs after oviposition (del Campo et al. 2001; Renwick 2001). Despite their underlying simplicity (Bernays et al. 2004), caterpillar feeding behaviors can be readily influenced by host plant volatiles (Shiojiri et al. 2006a).
Given that the induced volatile profiles of herbivore-damaged plants often differ considerably from undamaged plants and change rapidly over time, it is necessary to consider both the role of volatile induction in herbivore host plant acceptance as well as the reciprocal impact of herbivore behavioral responses on the plant. Recent studies with parasitoids demonstrate a surprising complexity in the functions attributed to individual components of an attractive herbivore-induced plant odor, with some compounds being attractive, others repellent, some potentially masking the effects of others, and some inaffectably neutral (D’Alessandro et al. 2006). Comparable studies need to be conducted to determine to what extent these volatiles are directed against herbivores. In particular, the potential of induced volatile components to mask or alter the attractive qualities of a host plant to an herbivore should be further explored. Future attempts to improve plant resistance through alteration of induced volatiles must take into account the different roles of individual components, not only for natural enemies but also for direct mediation of herbivore behavior (Zangerl 2003; Shiojiri et al. 2006a, b).
References
Anderson, P., Hilker, M., and Löfqvist, J. 1995. Larval diet influence on oviposition behavior in Spodoptera littoralis. Entomol. Exp. Appl 74:71–82.
Bernasconi, M. L., Turlings, T. C. J., Ambrosetti, L., Bassetti, P., and Dorn, S. 1998. Herbivore-induced emissions of maize volatiles repel the corn leaf aphid, Rhopalosiphum maidis. Entomol. Exp. Appl 87:133–142.
Bernays, E. A., Singer, M. S., and odrigues, D. 2004. Foraging in nature: foraging efficiency and attentiveness in caterpillars with different diet breadths. Ecol. Entomol 29:389–397.
Bolter, C. J., Dicke, M., Vanloon, J. J. A., Visser, J. H., and Posthumus, M. A. 1997. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J. Chem. Ecol 23:1003–1023.
Carroll, M. J., Schmelz, E. A., Meagher, R. L., and Teal, P. E. A. 2006. Attraction of Spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. J. Chem. Ecol 32:1911–1924.
Carlsson, M. A., Anderson, P., Hartlieb, E., and Hansson, B. S. 1999. Experience-dependent modification of orientational response to olfactory cues in larvae of Spodoptera littoralis. J. Chem. Ecol 25:2445–2454.
Chapman, J. W., Williams, T., Escribano, A., Caballero, P., Cave, R. D., and Goulson, D. 1999. Fitness consequences of cannibalism in the fall armyworm, Spodoptera frugiperda. Behav. Ecol 10:298–303.
Colazza, S., Fucarino, A., Peri, E., Salerno, G., Conti, E., and Bin, F. 2004. Insect oviposition induces volatile emission in herbaceous plants that attracts egg parasitoids. J. Exp. Biol 207:47–53.
D’alessandro, M., Held, M., Triponec, Y., and Turlings, T. C. J. 2006. The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J. Chem. Ecol 32:2733–2748.
D’alessandro, M., and Turlings, T. C. J. 2005. In situ modification of herbivore-induced plant odors: A novel approach to study the attractiveness of volatile organic compounds to parasitic wasps. Chem. Senses 30:739–753.
Davis, F. M., Williams, W. P., Chang, Y. M., Baker, G. T., and Hedin, P. A. 1999. Differential growth of fall armyworm larvae (Lepidoptera: Noctuidae) reared on three phenotypic regions of whorl leaves from a resistant and a susceptible maize hybrid. Fla. Entomol 82:248–254.
De Moraes, C. M., Mescher, M. C., and Tumlinson, J. H. 2001. Caterpillar-induced nocturnal plant volatiles repel nonspecific females. Nature 410:577–580.
Del Campo, M. L., Miles, C. I., Schroeder, F. C., Mueller, C., Booker, R., and enwick, J. A. 2001. Host recognition by the tobacco hornworm is mediated by a host plant compound. Nature 411:186–189.
Dicke, M., Gols, R., Ludeking, D., and Posthumus, M. A. 1999. Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J. Chem. Ecol 25:1907–1922.
Dicke, M., and Sabelis, M. W. 1988. How plants obtain predatory mites as bodyguards. Neth. J. Zool 38:148–165.
Dicke, M., Van Poecke, R. M. P., and De Boer, J. G. 2003. Inducible indirect defence of plants: from mechanisms to ecological functions. Bas. Appl. Ecol 4:27–42.
Dicke, M., and Vet, L. E. M. 1999. Plant-carnivore interactions: evolutionary and ecological consequences for plant, herbivore and carnivore, pp. 483–520, in H. Olff, V. K. Brown, and R. H. Drent (eds.). Herbivores: Between Plants and PredatorsOxford, Blackwell Science Oxford.
Doak, P. 2000. Population consequences of restricted dispersal for an insect herbivore in a subdivided habitat. Ecology 81:1828–1841.
Gouinguené, S., Alborn, H., and Turlings, T. C. J. 2003. Induction of volatile emissions in maize by different larval instars of Spodoptera littoralis. J. Chem. Ecol 29:145–162.
Gouinguené, S., Pickett, J. A., Wadhams, L. J., Birkett, M. A., and Turlings, T. C. J. 2005. Antennal electrophysiological responses of three parasitic wasps to caterpillar-induced volatiles from maize (Zea mays mays), cotton (Gossypium herbaceum), and cowpea (Vigna unguiculata). J. Chem. Ecol 31:1023–1038.
Harris, M. O., Sandanayake, M., and Foster, S. P. 1999. Chemical stimuli from apple influence the behavior of neonate caterpillars of the generalist herbivore, Epiphyas postvittana. J. Chem. Ecol 25:1717–1738.
Heath, R. R., and Manukian, A. 1994. An automated-system for use in collecting volatile chemicals released from plants. J. Chem. Ecol 20:593–608.
Heath, R. R., and Sonnet, P. E. 1980. Technique for in situ coating of Ag+ onto silica gel in HPLC columns for the separation of geometrical isomers. J. Liquid Chromatogr 3:1129–1135.
Hilker, M., and Klein, B. 1989. Investigation of oviposition deterrent in larval frass of Spodoptera littoralis (Boisd.). J. Chem. Ecol 15:929–938.
Hoballah, M. E., Kollner, T. G., Degenhardt, J., and Turlings, T. C. J. 2004. Costs of induced volatile production in maize. Oikos 105:168–180.
Hoballah, M. E., and Turlings, T. C. J. 2005. The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbivore-induced maize volatiles. J. Chem. Ecol 31:2003–2018.
Hoballah, M. E. F., Tamo, C., and Turlings, T. C. J. 2002. Differential attractiveness of induced odors emitted by eight maize varieties for the parasitoid Cotesia marginiventris: Is quality or quantity important? J. Chem. Ecol 28:951–968.
Kessler, A., and Baldwin, I. T. 2001. Defensive function of herbivore-induced plant volatile emissions in nature. Science 291:2141–2144.
Kessler, A., and Baldwin, I. T. 2002. Plant responses to insect herbivory: The emerging molecular analysis. Annu. Rev. Plant Biol 53:299–328.
Landolt, P. J. 1993. Effects of host plant leaf damage on cabbage moth attraction and oviposition. Entomol. Exp. Appl 67:79–85.
Landolt, P. J., Brumley, J. A., Smithhisler, C. L., Biddick, L. L., and Hofstetter, R. W. 2000. Apple fruit infested with codling moth are more attractive to neonate codling moth larvae and possess increased amounts of (E,E)-alpha-farnesene. J. Chem. Ecol 26:1685–1699.
Luginbill, P. 1928. The fall armyworm. U.S. Department of Agriculture Technical Bulletin 34:1–91.
Meagher, R. L., Nagoshi, R. N., Stuhl, C., M, and E. R. 2004. Larval development of fall armyworm (Lepidoptera: Noctuidae) on different cover crop plants. Fla. Entomol 87:454–460.
Meiners, T., and Hilker, M. 1997. Host location in Oomyzus gallerucae (Hymenoptera: Eulophidae), an egg parasitoid of the elm leaf beetle Xanthogaleruca luteola (Coleoptera, Chrysomelidae). Oecologia 112:87–93.
Meiners, T., and Hilker, M. 2000. Induction of plant synomones by oviposition of a phytophagous insect. J. Chem. Ecol 26:221–232.
Paré, P. W., Farag, M. A., Krishnamachari, V., Zhang, H. M., yu, C. M., and Kloepper, J. W. 2005. Elicitors and priming agents initiate plant defense responses. Photosynth. Res 85:149–159.
Paré, P. W., and Tumlinson, J. H. 1997. Induced synthesis of plant volatiles. Nature 385:30–31.
Paré, P. W., and Tumlinson, J. H. 1999. Plant volatiles as a defense against insect herbivores. Plant Physiol 121:325–331.
Pickett, J. A., Bruce, T. J. A., Chamberlain, K., Hassannali, A., Khan, Z. R., Matthes, M. C., Napier, J. A., Smart, L. E., Wadhams, L. J., and Woodcock, C. M. 2006. Plant volatiles yielding new ways to exploit plant defence, pp. 161–173, in M. Dicke, and W. Takken (eds.). Chemical Ecology: from Gene to EcosystemSpringer, Wageningen, The Netherlands.
Pitre, H. N., Mulrooney, J. E., and Hogg, D. B. 1983. Fall armyworm (Lepidoptera: Noctuidae) oviposition: crop preferences and egg distribution on plants. J. Econ. Entomol 76:463–466.
Prokopy, R. J., and Roitberg, B. D. 2001. Joining and avoidance behavior in nonsocial insects. Annu. Rev. Entomol 46:631–665.
Renwick, J. A. A. 2001. Variable diets and changing taste in plant-insect relationships. J. Chem. Ecol 27:1063–1076.
Roitberg, B. D., and Mangel, M. 1993. Parent-offspring conflict and life history consequences in herbivorous insects. Am. Nat 1442:443–456.
Rott, A. S., Hackermann, J., Brand, N., Vallat, A., and Dorn, S. 2005. Parasitoid exploitation of the seasonal variation in host plant volatile emission for herbivore location. Entomol. Exp. Appl 115:199–205.
Schmelz, E. A., Alborn, H. T., and Tumlinson, J. H. 2001. The influence of intact-plant and excised-leaf bioassay designs on volicitin- and jasmonic acid-induced sesquiterpene volatile release in Zea mays. Planta 214:171–179.
Schmelz, E. A., Carroll, M. J., Leclere, S., Phipps, S. M., Meredith, J., Chourey, P. S., Alborn, H. T., and Teal, P. E. A. 2006. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. U.S.A 103:8894–8899.
Schoonhoven, L. M., and Van Loon, J. J. A. 2002. An inventory of taste in caterpillars: Each species its own key. Acta Zool. Acad. Sci. H 48:215–263.
Shiojiri, K., Ozawa, R., and Takabayashi, J. 2006a. Plant volatiles, rather than light, determine the nocturnal behavior of a caterpillar. Plos Biology 4:1044–1047.
Shiojiri, K., Kishimoto, K., Ozawa, R., Kugimiya, S., Urashimo, S., Arimura, G., Horiuchi, J., Nishioka, T., Matsui, K., and Takabayashi, J. 2006b. Changing green leaf volatile biosynthesis in plants: An approach for improving plant resistance against both herbivores and pathogens. Proc. Natl. Acad. Sci. U.S.A 103:16672–16676.
Sparks, A. N. 1979. A review of the biology of the fall armyworm. Fla. Entomol 62:82–86.
Stamps, J., and Krishnan, V. V. 2005. Nonintuitive cue use in habitat selection. Ecology 86:2860–2867.
Suazo, A., Torto, B., Teal, P. E. A., and Tumlinson, J. H. 2003. Response of the small hive beetle (Aethina tumida) to honey bee (Apis mellifera) and beehive-produced volatiles. Apidologie 34:525–533.
Thompson, J. N. 1988. Evolutionary ecology of the relationship between oviposition preference and performance of offspring in phytophagous insects. Entomol. Exp. Appl 47:3–14.
Turlings, T. C. J., Alborn, H. T., Loughrin, J. H., and Tumlinson, J. H. 2000. Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: Isolation and bioactivity. J. Chem. Ecol 26:189–202.
Turlings, T. C. J., Lengwiler, U. B., Bernasconi, M. L., and Wechsler, D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207:146–152.
Turlings, T. C. J., Loughrin, J. H., Mccall, P. J., ose, U. S., Lewis, W. J., and Tumlinson, J. H. 1995. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. U.S.A 92:4169–4174.
Turlings, T. C. J., Tumlinson, J. H., and Lewis, W. J. 1990. Exploitation of herbivore-induced plant odors by host-seeking wasps. Science 250:1251–1253.
Ward, S. A. 1987. Optimal habitat selection in time-limited dispersers. Am. Nat 129:568–579.
Weatherston, I., Miller, D., and Dohse, L. 1985. Capillaries as controlled-release devices for insect pheromones and other volatile substances - a reevaluation. Part I. Kinetics and development of predictive model for glass capillaries. J. Chem. Ecol 11:953–965.
Yang, G., Wiseman, B. R., and Espelie, K. E. 1993. Movement of neonate fall armyworm (Lepidoptera: Noctuidae) larvae on resistant and susceptible genotypes of corn. Environ. Entomol 22:547–553.
Zalucki, M. P., Clarke, A. R., and Malcolm, S. B. 2002. Ecology and behavior of first instar larval Lepidoptera. Annu. Rev. Entomol 47:361–393.
Zangerl, A. R. 2003. Evolution of induced plant responses to herbivores. Bas. Appl. Ecol 4:91–103.
Zangerl, A. R., and Berenbaum, M. R. 1992. Oviposition patterns and hostplant suitability - parsnip webworms and wild parsnip. Am. Midl. Nat 128:292–298.
Acknowledgment
We thank Sean Collins and Art Zangerl for comments that improved the manuscript. We especially thank Rob Meagher, Charlie Dillard, and Nancy Lowman for providing the insects used in these experiments. We also thank Julia Meredith and Mary Searle for assistance in the maintenance of plants used in this experiment.
Author information
Authors and Affiliations
Corresponding author
Additional information
The use of trade, firm, or corporation names in this publication (or page) is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Rights and permissions
About this article
Cite this article
Carroll, M.J., Schmelz, E.A. & Teal, P.E.A. The Attraction of Spodoptera frugiperda Neonates to Cowpea Seedlings is Mediated by Volatiles Induced by Conspecific Herbivory and the Elicitor Inceptin. J Chem Ecol 34, 291–300 (2008). https://doi.org/10.1007/s10886-007-9414-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9414-y