Abstract
Sexually mature male beetles of the genus Nicrophorus (Coleoptera: Silphidae) exhibit a conspicuous behavior, recognized as pheromone-releasing activity. Laboratory and field studies demonstrated that females are attracted to males that exhibit this behavior, both on or off reproductive resources. Here, we report the results of a study in which volatile chemicals released by calling Nicrophorus vespilloides were collected by solid-phase microextraction and analyzed by using coupled gas chromatography–mass spectrometry. These analyses revealed that ethyl 4-methyl heptanoate and (E)-geranylacetone are emitted by males that engage in the behavior. In the field, traps baited with racemic ethyl 4-methyl heptanoate caught roughly equal numbers of male and female N. vespilloides. Some male and female Nicrophorus vespillo and male Nicrophorus humator were also caught in traps baited with this compound. Traps baited with (E)-geranylacetone did not catch significant numbers of beetles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the past few decades, the chemical signals and defenses produced by Coleoptera have been the subject of numerous studies that focus on occurrence, biosynthesis, and biological significance (Francke and Dettner 2005). However, some coleopteran families have received relatively little investigation. In particular, there have been few studies on the chemical ecology of the Silphidae. In Necrodes surinamensis, several terpenes (necrodols) with a repellent function have been identified (Eisner and Meinwald 1982; Eisner et al. 1986; Roach et al. 1990). Silpha americana (Meinwald et al. 1985) and Silpha novaboracensis (Meinwald et al. 1987) utilize steroids that act as defensive chemicals. A recent study analyzed the composition of anal secretions in Nicrophorus marginatus (Woodard 2006).
Beetles of the genus Nicrophorus (Coleoptera, Silphidae) search for small vertebrate carcasses that are suitable as a reproductive resource. Once a suitable carcass has been found, the beetles drive away intra- and interspecific competitors and bury the carrion, thus hiding it from other potential competitors. Although fights over the carcass often result between competing Nicrophorus beetles, various breeding associations may also form on the carcass, from monogamous pairs to polygynandrous groups (reviewed in Eggert and Müller 1997).
Males of most, if not all, Nicrophorus species engage in pheromone emission during a species-specific time of day (Pukowski 1933; Eggert and Müller 1989a, 1997; Scott 1998). Males select elevated sites and assume a headstand-like posture, pointing the head down and raising the tip of the abdomen. Males differ from females morphologically in that they have an additional distal abdominal segment, presumably the site of the pheromone gland (Mosebach 1936). Since Pukowski’s (1933) detailed study of burying beetles provided the first accurate description of this behavior, numerous studies have attempted to elucidate its biological implications. Pukowski (1933) called the behavior “sterzeln,” stating that Nicrophorus males exhibit sterzeln only when they have discovered a reproductive resource on which no female is present. Later observations revealed that male pheromone emission is not contingent on the presence of reproductive resources, at least in some species (Müller and Eggert 1987; Eggert and Müller 1989a; Beeler et al. 1999), with pheromone emission in the absence of carrion being interpreted as an alternative mate-finding behavior (Eggert 1992).
The time of day and the extent to which pheromone emission occurs is known for several Nicrophorus species (Müller and Eggert 1987). Furthermore, Müller and Eggert (1987) have demonstrated that individuals of congeneric species may also be attracted to pheromone-emitting males, although the ecological significance of such interspecific attraction is unclear. Beeler et al. (2002) proposed that females discriminate among potential mates by assessing pheromone signals, which would require that the signals contain information about the calling individual beyond sex and species. In this study, we report the identification of the male-produced pheromone of Nicrophorus vespilloides, the most abundant Nicrophorus species in Central Europe.
Methods and Materials
Beetles
Nicrophorus vespilloides males used for headspace analysis were first- or second-generation offspring of beetles caught in pitfall traps baited with pieces of pigs’ lung in a field near Freiburg, Germany (48°02′14″N, 7°50′52″E). The traps used were identical to those used in the experiment that tested the attractiveness of synthetic chemicals in the field (Fig. 1). Individual beetles were kept in transparent plastic boxes (100 × 100 × 65 mm) filled with moist peat under a 16:8 light/dark photoperiod. Some male beetles were provided with a small mouse carcass, whereas others were given decapitated mealworms.
Headspace Sampling
Headspace chemicals were obtained from 15 individual males, in separate plastic boxes, by using solid-phase microextraction fibers (SPME, coating polydimethylsiloxane/divinylbenzene, Supelco, Bellefonte, PA, USA) and analyzed by gas chromatography–mass spectrometry (GC-MS). GC-MS analysis was performed on a Hewlett Packard (Palo Alto, CA, USA) 6890-5973 system equipped with a DB-1 column (30 m × 0.25 mm ID; df = 0.25 μm; J & W, Folsom, CA, USA). Conditions were as follows: injector temperature 250°C, splitless mode, oven temperature 50–250°C at 10°C min−1. The electron impact mass spectra (EI-MS) were recorded with an ionization voltage of 70 eV and a source temperature of 230°C.
For collection of volatile chemicals, small holes were bored into the lids of boxes such that fibers could be placed close to the male during pheromone emission. Sampling took place from just before to the end of a beetle assuming the headstand posture typical of “sterzeln.” Pheromone emission typically occurred between 1 hr before and 3 hr after lights off. After lights off, sampling continued under red light. We sampled the headspace of males with or without access to a carcass.
Chemicals
Synthesis of racemic ethyl 4-methyl heptanoate was carried out by the method of Joung et al. (1998). The product was purified by liquid chromatography. (E)-geranylacetone was purchased from Fluka (Deisenhofen, Germany).
Field Experiment
The field experiment was carried out in the oak-dominated mixed forest near Freiburg, southwestern Germany, from which our laboratory colonies originated. Five pitfall traps (Fig. 1) were arranged in a circle equidistant (ca. 50 m) from each other. A dispenser consisted of a 1.5 ml glass vial filled with silica gel (Merck, Darmstadt, Germany), 0.7 ml distilled water for wetting, and a 50-μl solution of the test chemicals. Vials were secured inside a tea ball with a small amount of liquid plaster. Each trap was baited with a tea ball placed above the center of the trap opening (Fig. 1). The vial remained closed until the bait was placed above a trap, at which point the cap was removed. The following treatments were tested: bait 1, ethyl 4-methyl heptanoate (34 nmol); bait 2 (control A), silica gel with distilled water; bait 3 (control B), same as control A but with 50 μl of n-pentane/dichloromethane (9:1); bait 4, (E)-geranylacetone (2.6 nmol); and bait 5, ethyl 4-methyl heptanoate and (E)-geranylacetone (34 and 2.6 nmol, respectively). The ratio of methyl heptanoate to geranylacetone in bait 5 was similar to that observed in the SPME analyses.
Fresh baits were placed above the traps an hour before sunset and left for 24 hr, after which the baits and the trapping containers were removed. Each trap was left without bait for 1 d before adding a new bait and trapping container. The position of each treatment was moved to the next trap in a clockwise direction. This procedure was repeated until the dispensers had rotated through all the traps twice; i.e., a total of 10 d of trapping, alternating with a total of 10 d without baits. The field trial was carried out between June 26 and July 14, 2006.
Results
Gas Chromatography–Mass Spectrometry
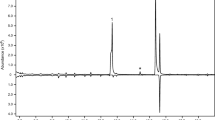
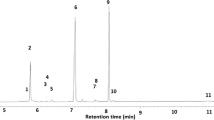
SPME GC-MS analysis of the SPME samples revealed two volatile compounds that were detectable only when Nicrophorus males assumed the typical “sterzeln” posture. Based on mass spectra and retention times of the two compounds, and comparison with those of the synthetic versions, we concluded that the compounds were ethyl 4-methyl heptanoate {m/z (relative abundance): 172 [M+] (<1), 143 (6), 129 (13), 127 (28), 115(15), 109 (26), 101 (100), 88 (88), 73 (30); RT 7.99 min} and (E)-geranylacetone {m/z (relative abundance): 194 [M+] (2), 176 (2), 161 (3), 151 (28), 136 (24), 125 (12), 107 (22), 93 (13), 69 (64), 43 (100); RT 11.91 min}, in a ratio of approximately 1:13. No differences were detected in the composition of pheromone blends of males with and without carcasses.
Attractiveness of Compounds
In the field, the number of N. vespilloides captured (Table 1) differed significantly among bait types (Friedman test, P < 0.0001), as did the number of successful vs. unsuccessful trapping days (χ 2 = 26.3, df = 4, P < 0.001). Traps baited with either ethyl 4-methyl heptanoate or a mixture of ethyl 4-methyl heptanoate and geranylacetone caught the greatest number of beetles (Wilcoxon–Wilcox-test, P < 0.05); both of these treatments caught N. vespilloides on all but one of the trapping days (Table 1). The catches in traps baited with either of these two treatments did not differ (Wilcoxon–Wilcox-test, P > 0.1). Traps with treatments that lacked ethyl 4-methyl heptanoate (i.e., the two controls and geranylacetone) caught few beetles. Comparison of the daily catches of N. vespilloides showed similar numbers of males and females trapped (Wilcoxon MPSR, P > 0.3, Table 2). Most of the beetles caught in the trial (80.5%) were N. vespilloides, but some Nicrophorus vespillo (17.1% of total) and Nicrophorus humator (2.4%) were also caught in traps that contained ethyl 4-methyl heptanoate (Table 2). There was no effect of trap position on the number of beetles trapped (Friedman test, P > 0.25).
Discussion
The data from both the headspace analysis and the field experiment suggest that ethyl 4-methyl heptanoate is a male-produced pheromone of N. vespilloides. The role of geranylacetone, also released by male N. vespilloides, remains unclear; catches with this compound alone were not different from those of the controls. Furthermore, addition of this compound to ethyl 4-methyl heptanoate did not yield increased catches compared to those with ethyl 4-methyl heptanoate alone. Interestingly, ethyl 4-methyl heptanoate has also been found as a minor component of volatile emissions from the beetle Oryctes rhinoceros (Coleoptera: Scarabaeidae), a widespread coconut pest in Asia. The principal pheromone component of O. rhinoceros is, however, ethyl 4-methyl octanoate (Hallett et al. 1995). Behavioral data suggest that the beetles produce a mix of both enantiomers of ethyl 4-methyl octanoate (Hallett et al. 1995). We did not determine the stereochemistry of ethyl 4-methyl heptanoate in N. vespilloides, but will do so in future studies.
Following the definition of Wertheim et al. (2005), ethyl 4-methyl heptanoate should be classified as an aggregation pheromone, rather than as a sex pheromone, of N. vespilloides because beetles of both sexes are attracted to this compound. Interestingly, in the field, males appear to attract primarily females (Müller and Eggert 1987). Males of the rove beetle Aleochara curtula also release a pheromone that attracts both sexes (Peschke et al. 1999).
Pheromone emission without carrion has been interpreted as an alternative to the primary mate-finding tactic of searching for carcasses (Eggert 1992; Beeler et al. 1999; Müller et al. 2007). According to Eggert (1992), pheromone emission benefits males irrespective of the presence of a reproductive resource. Males that have access to a suitable carcass can attempt copulation once a female arrives, and males without such a resource can secure matings with attracted females. In a field study of N. vespilloides, males that had buried a carcass prior to pheromone emission attracted equal numbers of females as males that had not (Eggert and Müller 1989b). Responding to the pheromone also benefits females by allowing them access to a carcass and/or a mate. Benefits for males that are attracted to males are less clear, especially if the pheromone-emitting male does not have a carcass. One possibility is that the attracted male could mate with surplus females that the pheromone emitter attracts. If the calling male has a carcass, then presumably the attracted male would fight the caller for the resource.
In the field trial, two other Nicrophorus species were attracted to ethyl 4-methyl heptanoate, consistent with earlier findings that calling male Nicrophorus attract members of other congeneric species (Müller and Eggert 1987). There is some evidence (Haberer, Schmitt, and Müller, unpublished) that the attracted Nicrophorus species use similar (i.e., 4-methyl branched heptanoate and octanoate) esters for their own sex pheromones. Müller and Eggert (1987) suggested that the response of larger congeners to the pheromones of a smaller species might be adaptive. If the pheromone-emitting male has access to a carcass, members of larger species would be able to displace the original owner and utilize the resource for their own reproduction. The two additional species, N. vespillo and N. humator, that were attracted to ethyl 4-methyl heptanoate are both larger than and competitively superior to N. vespilloides (Pukowski 1933; Otronen 1988). Future investigations of other Nicrophorus pheromones will aid our understanding of cross-attraction within the genus.
References
Beeler, A. E., Rauter, C. M., and Moore, A. J. 1999. Pheromonally mediated mate attraction by males of the burying beetle Nicrophorus orbicollis: Alternative calling tactics conditional on both intrinsic and extrinsic factors. Behav. Ecol. 10:578–584.
Beeler, A. E., Rauter, C. M., and Moore, A. J. 2002. Mate discrimination by females in the burying beetle Nicrophorus orbicollis: The influence of male size on attractiveness to females. Ecol. Entomol. 27:1–6.
Eggert, A.-K. 1992. Alternative male mate-finding tactics in burying beetles. Behav. Ecol. 3:243–254.
Eggert, A.-K., and Müller, J. K. 1989a. Pheromone-mediated attraction in burying beetles. Ecol. Entomol. 14:235–238.
Eggert, A.-K., and Müller, J. K. 1989b. Mating success of pheromone-emitting Necrophorus males: Do attracted females discriminate against resource owners? Behaviour 110:248–257.
Eggert, A.-K., and Müller, J. K. 1997. Biparental care and social evolution in burying beetles: Lessons from the larder, pp. 216–236, in: J. C. Choe and B. J. Crespi (eds.). Social Behavior in Insects and Arachnids. Cambridge University Press, Cambridge.
Eisner, T., and Meinwald, J. 1982. Defensive spray mechanism of a silphid beetle (Necrodes surinamensis). Psyche 89:357–368.
Eisner, T., Deyrup, M., Jacobs, R., and Meinwald, J. 1986. Necrodols: Anti-insectan terpenes from defensive secretion of carrion beetle (Necrodes surinamensis). J. Chem. Ecol. 12:1407–1415.
Francke, W., and Dettner, K. 2005. Chemical signalling in beetles. Topics in current chemistry, pp. 85–166. Springer-Verlag, Berlin.
Hallett, R. H., Perez, A. L., Gries, G., Gries, R., Pierce, H. D., Jr., Yue, J., Oehlschlager, A. C., Gonzalez, L. M., and Borden, J. H. 1995. Aggregation pheromone of coconut rhinoceros beetle, Oryctes rhinoceros (L.) (Coleoptera: Scarabaeidae). J. Chem. Ecol. 21:1549–1570.
Joung, M. J., Ahn, J. H., Lee, D. W., and Yoon, N. M. 1998. Coupling reaction of alkenes with α-bromo carboxylic acid derivatives using nickel boride and borohydride exchange resin in methanol. J. Org. Chem. 63:2755–2757.
Meinwald, J., Roach, B., Hicks, K., Alsop, D., and Eisner, T. 1985. Defensive steroids from a carrion beetle (Silpha americana). Experientia 41:516–519.
Meinwald, J., Roach, B., and Eisner, T. 1987. Defensive steroids from a carrion beetle (Silpha novaboracensis). J. Chem. Ecol. 13:35–38.
Mosebach, E. 1936. Aus dem Leben des Totengräbers (Necrophorus). Nat. Volk 66:222–231.
Müller, J. K., and Eggert, A.-K. 1987. Effects of carrion-independent pheromone emission by male burying beetles (Silphidae: Necrophorus). Ethology 76:297–304.
Müller, J. K., Braunisch, V., Hwang, W., and Eggert, A.-K. 2007. Alternative tactics and individual reproductive success in natural associations of the burying beetle, Nicrophorus vespilloides. Behav. Ecol. 18:196–203.
Otronen, M. 1988. The effect of body size on the outcome of fights in burying beetles (Nicrophorus). Ann. Zool. Fenn. 25:191–201.
Peschke, K., Friedrich, P., Kaiser, U., Franke, S., and Francke, W. 1999. Isopropyl (Z9)-hexadecenoate as a male attractant pheromone from the sternal gland of the rove beetle Aleochara curtula (Coleoptera: Staphylinidae). Chemoecology 9:47–54.
Pukowski, E. 1933. Ökologische Untersuchungen an Necrophorus F. Z. Morphol. Okol. Tiere 27:518–586.
Roach, B., Eisner, T., and Meinwald, J. 1990. Defense mechanisms of arthropods. 83. Alpha- and beta-necrodol, novel terpenes from a carrion beetle (Necrodes surinamensis, Silphidae, Coleoptera). J. Org. Chem. 55:4047–4051.
Scott, M. P. 1998. The ecology and behavior of burying beetles. Annu. Rev. Entomol. 43:595–618.
Wertheim, B., Van Baalen, E. J. A., Dicke, M., and Vet, L. E. M. 2005. Pheromone-mediated aggregation in nonsocial arthropods: An evolutionary ecological perspective. Annu. Rev. Entomol. 50:321–346.
Woodard, C. B. S. 2006. Odor masking of a vertebrate carcass by a burying beetle (Nicrophorus marginatus). Master’s thesis, Texas Tech University.
Acknowledgments
We thank Anne-Katrin Eggert and Stephen Ellis for valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haberer, W., Schmitt, T., Peschke, K. et al. Ethyl 4-Methyl Heptanoate: A Male-Produced Pheromone of Nicrophorus vespilloides . J Chem Ecol 34, 94–98 (2008). https://doi.org/10.1007/s10886-007-9406-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9406-y