Abstract
Neotropical attine ants live in obligatory symbiosis with a fungus that they grow for food on a substrate of primarily plant material harvested by workers. Nestmate recognition is likely based on chemical cues as in most other social insects, but recent studies have indicated that both the ants and their mutualistic fungi may contribute to the recognition templates. To investigate the within-colony variation in chemical profiles, we extracted and identified compounds from the cuticle of workers, the postpharyngeal gland of workers, ant pupae and larvae, and the fungal symbiont of three species of higher attine ants: Atta colombica, Acromyrmex echinatior, and Sericomyrmex amabilis. The relative proportions of identified compounds were compared and represented 11 classes: n-alkanes, alkenes, branched methylalkanes, branched dimethylalkanes, trimethylalkanes, branched alkenes, aldehydes, alcohols, acetates, acids, and esters. The chemical profiles in all three species are likely to be sufficiently different to allow discrimination at the species and colony level and sufficiently similar within colonies to generate a relatively constant colony-specific chemical gestalt. The relative likelihood of individual compounds being derived from the ants, the ant brood, or the fungal symbiont are discussed. We hypothesize that hydrocarbons are particularly important as recognition cues because they appear to simultaneously allow the assessment of developmental stages and the identification of symbiont, colony, and species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cuticular hydrocarbons profiles are thought to provide the main nestmate recognition templates in social insects (Singer 1998; Lahav et al. 1999), and many studies have shown that these compounds are involved in recognition processes between individuals, castes, and colonies (Vander Meer and Morel 1998). A colony’s “odor profile” is typically determined by specific proportions of a series of hydrocarbons that function as a blurred barcode and prompts colony members to expel non-nestmate individuals with a different odor profile (Wilson 1971; Breed 1983). The origin of the chemical compounds involved in recognition and discrimination processes may differ across species, both for idiosyncratic reasons and because of variation in the genetic (Hölldobler and Wilson 1990; Beye et al. 1997, 1998; Boomsma et al. 2003) and environmental components (Obin 1986; Crosland 1989) of odor profiles. Environmental factors that contribute to colony odor include ambient temperature, humidity, soil characteristics, food, and nest material (Jutsum et al. 1979; Obin 1986; Crosland 1989; Heinze et al. 1996; Singer and Espelie 1996; Nielsen et al. 1999; Liang and Silverman 2000; Silverman and Liang 2001; Richard et al. 2004; Howard and Blomquist 2005).
The fungus-growing ants are exclusively a New World tribe of myrmicine ants (Attini) and are restricted largely to the tropics and subtropics of Central and South America. The attine tribe contains 13 genera and more than 210 species (Brandão and Mayhé-Nunes 2001; Mueller et al. 2001). The tribe is subdivided into a derived monophyletic group called the “higher attines,” which includes the genera Atta, Acromyrmex (including Pseudoatta), Trachymyrmex, and Sericomyrmex, and a largely paraphyletic group called the “lower attines” that contain the remaining genera (Apterostigma, Cyphomyrmex, Mycetarotes, Mycetophylax, Mycetosoritis, Mycetoagroicus, Mycocepurus, and Myrmicocrypta; Chapela et al. 1994; Schultz and Meier 1995). While leaf-cutting ants collect fresh leaves for fungiculture, lower attines use feces or dead plant or arthropod material as substrate for their fungus gardens. Colony size is relatively small (around 10–100 workers) in the lower attines compare to the thousands of workers in a Sericomyrmex colony, the tens of thousands in an Acromyrmex colony, and the millions of workers in an Atta colony (Weber 1972; Hölldobler and Wilson 1990). All attine ants cultivate fungi that belong to the tribe Leucocoprineae; the association is believed to have originated in the Amazon Basin more than 50 million years ago (Chapela et al. 1994; Mueller et al. 1998). The symbiosis is obligate and has at least diffusely coevolved with respect to several nutritional, physiological, and antibiotic characteristics (Mueller 2002; Currie et al. 2003, 2006; Richard et al. 2005; Mikheyev et al. 2007).
Previous work on Acromyrmex species has shown that the workers and fungus gardens of the same colony have partly similar and partly different chemical profiles (Viana et al. 2001; Richard et al. 2004, 2007) and that nestmate recognition within and across closely related species is related to differences in surface chemistry (Lambardi et al. 2007; Richard et al. 2007). The purpose of the present study was to further investigate the magnitude and variability of these differences by: (1) comparing the qualitative and quantitative differences among three different sympatric species that belong to different genera of higher attine ants (Atta, Acromyrmex, and Sericomyrmex) and (2) comparing the chemical profiles of different colony members and developmental stages (workers, worker PPGs, brood, and the fungus garden) within and between these ant genera.
Methods and Materials
Biological Material
We used five colonies of Atta colombica (one without larvae), nine colonies of Acromyrmex echinatior (one without larvae; colony number Ae48, Ae109, Ae124, Ae132, Ae168, Ae210, Ae219, Ae221, Ae223), and four colonies of Sericomyrmex amabilis (two without larvae and pupae; colony numbers Sal220402-1, Sal210402, Sal110601-18, Sal030603-1). All colonies were collected in Gamboa, Panama, between 1994 and 2002, and had been maintained in the laboratory for a minimum of 2 years on a similar diet. At the time of sampling (July 2004), nest boxes were maintained at the University of Copenhagen under standardized conditions of approximately 70% relative humidity and 25°C. Colonies were provided with a diet of bramble leaves and rice grains, except for Sericomyrmex colonies that were provided with dried flower petals and small pieces of cut bramble leaves.
Chemical Analyses of Colonies
Extracts were obtained from 200 mg of larvae or pupae or ten workers immersed in 1 ml of pentane for 10 min. Another ten workers from each colony were frozen for later dissection of their postpharyngeal glands (PPGs). Fragments of fungus garden were taken from all colonies of Sericomyrmex with two replicates per colony and from all colonies of Atta and Acromyrmex with four replicates per colony. To minimize any effects of leaf material on the chemical profiles, the fungus garden samples were taken from the mature middle section of fungus gardens where all leaf material was degraded (cf. Richard et al. 2007). All brood and workers were removed from the fungus fragments before chemical extraction, which was performed on 200 mg of fungus material immersed in 3 ml pentane for 10 min.
Extracts were stored at −20°C until analyzed. Before analysis, any remaining pentane was allowed to evaporate, and dried extracts were redissolved in 50 μl pentane containing 10 ng/μl eicosane (n-C20) as an internal size standard. Two microliters of these mixtures were injected into a gas chromatograph coupled with a mass spectrometer, and compound quantification and identification were performed on an HP6890 gas chromatograph with an HP5973 mass-selective detector, equipped with an HP-5 column (30 m × 0.32 mm inner diameter, film thickness = 0.25 μm). The initial temperature was 80°C for 1 min with a subsequent gradual increase of 10°C/min until 200°C was reached and finally a gradual temperature increase of 3°C/min until the maximum temperature of 340°C was reached and maintained for 10 min. The mass spectrometer was operated at 70 eV, and scanning was done from 40 to 550 amu at 1.5 scans/sec.
Identification of Compound Classes
The objective of this study was to compare the chemical profiles of different species of ants, their larvae, pupae, and the fungus—not to provide absolute identifications of all of the different compounds that were found. Cuticular hydrocarbons (alkanes and mono-, di-, and trimethyl alkanes) were characterized to class level by using diagnostic ions, comparisons with standard mass spectrometry (MS) databases, and by determination of Kovats indices (see Carlson et al. 1998; Lommelen et al. 2006). The position of the double bond in the alkenes was not determined because our purpose was not to identify specific compounds. The identification of other compounds was based on comparison of retention times and diagnostic ions with NIST/EPA/NIH 172 mass spectral libraries. Our chemical profiles were also compared with previous studies where we did identify the chemicals associated with leaf-cutting ant fungus gardens (Richard et al. 2007).
Statistical Analyses
The chemical profiles of the different colonies were obtained by gas chromatography (GC)–MS analysis and were compared within and among species. Quantification and chemical profile characterization were done by GC-MS peak integration using the relative abundance of the various peaks. To test for differences in chemical profiles among different groups, we used factorial analyses and nested two-way analyses of variance (ANOVAs) having ant species, ant colony nested within species, and development stage as main factors (using arcsine square root [X] transformed individual peak areas of all identified compounds; Statistica 6.0, StatSoft®). To evaluate the relative contribution of ant species origin, ant colony, and developmental stage or symbiont origin to the profiles, we performed type II ANOVAs on each of these factors individually, both for all compounds present in the profiles and for the class of hydrocarbons alone. Data are presented as median ± semi-interquartile ranges of the relative areas. To compare the relative quantities of the different compound categories between the different species, we used nonparametric Kruskal–Wallis tests on ranks.
Results
A total of 179 compounds, of which 139 hydrocarbons (16 n-alkanes, 22 alkenes, 49 Me-alkanes, 44 Dime-alkanes, and 8 Trime-alkanes), were identified (see Supplementary material: Appendix Table 1) and present in sufficient quantities to be analyzed for their contribution to the overall chemical profiles of the three focal species A. colombica, A. echinatior, and S. amabilis (Appendix Tables 2, 3 and 4). The cuticular profiles of worker ants contained a mixture of alkanes, alkenes, and mono- and dimethylalkanes with chain lengths ranging from C16 to C39 in A. colombica, C18 to C37 in A. echinatior, and C27 to C37 in S. amabilis (Appendix Tables 1, 2, 3, and 4). Considerable amounts of 5, 9 + 5, 11 Dime C35, and trimethylalkanes were present in A. colombica and four other compounds were specific for workers of A. echinatior: C20:2; C23:2, 11MeC21, and xMeC21. S. amabilis was characterized by the presence of 3MeC29 in all samples and 7MeC33 and 12,16 + 14,18 Dime C32 in all samples except larvae.
When evaluating compounds in the five major classes of hydrocarbons only, there were significant differences among the three species, with A. colombica having more alkenes and trimethylalkanes, A. echinatior having more alkanes, and S. amabilis having more methylalkanes and dimethylalkanes (Tables 1 and 3). No chemical compounds other than hydrocarbons were identified on S. amabilis worker cuticles, but A. echinatior cuticles contained an appreciable fraction of aldehydes, whereas A. colombica workers expressed small quantities of aldehydes, alcohols, and formates (Tables 2 and 3). Larval and pupal profiles consisted almost exclusively of hydrocarbons in all three species (Table 2). In general, brood profiles were less variable in chain length than worker profiles in A. colombica (C18 to C37) and A. echinatior (C18 to C33) and shorter than worker profiles in S. amabilis (C21 to C33). Finally, there was a striking difference between the very small total amounts of hydrocarbons on the bodies of larvae and pupae, relative to the much larger amounts on worker cuticles (Table 3). Thus, developmental stages and symbionts had a highly significant impact on all compound groups, while only some compound groups appeared affected by colony-of-origin (alkanes and alkenes) and ant species (hydrocarbons and branched alkanes). Because of the difficulty of standardizing tissue samples among groups, such absolute quantity estimates need to be interpreted with caution. However, the fact that the amount of compounds on larvae and pupae was an order of magnitude less than on worker cuticles and fungus garden material makes it reasonable to infer that these quantitative differences are real.
The fungus chemical profiles contained alkanes, alkenes, and methyl alkanes with chain lengths ranging from C18 to C36 for A. colombica, C18 to C33 for A. echinatior, and C25 to C35 for Sericomyrmex (Appendix Tables 1, 2, 3 and 4). The fungus garden methylalkanes were MeC23 and MeC26 for A. colombica and A. echinatior, and MeC27 to MeC35 for S. amabilis. Dimethylalkanes with chains ranging from DimeC33 to DimeC35 were found in A. colombica and DimeC31 to DimeC33 in S. amabilis, whereas trimethyl alkanes were only found in A. colombica fungus gardens. The diversity of chemical compounds in the fungus garden was higher than the diversity in brood, workers, and PPGs (see Supplemantary material: Appendix Tables 1, 2, 3, 4). However, hydrocarbons were more abundant than all other chemical classes in the fungi cultivated by A. colombica and A. echinatior, whereas the S. amabilis symbionts also had relative large amounts of acetates (Table 2).
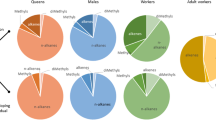
Factorial analyses showed that the chemical profiles of larvae and pupae lie between the profiles obtained from worker cuticles and the fungal symbionts (Fig. 1) and that PPG profiles are most different from those of the fungal profiles. Moreover, each of these developmental stage or symbiont components of the colony had its own specific chemical profile nested within the overall characteristic profile for the colony and species (Fig. 2). Type II ANOVAs were performed to evaluate how much of the variation in the complete chemical profiles and in the profiles including only hydrocarbons could be attributed to ant species, ant colony, and developmental stage or symbiont origin. While the species component only explained 0.34% (R 2) of the variation in the complete chemical profiles, colony-of-origin explained 21%, and developmental stage or symbiont origin explained 35% of the variance in profiles. When only hydrocarbon compounds were included in the analysis, 11.4, 23.7, and 23.0% of the variation could be attributed to species, colony, and developmental stage/symbiont origin, respectively. The low amount of variation explained by ant species in the former analyses indicates that the presence of abundant nonhydrocarbon compounds, particularly in the fungal samples of all three species (Table 2), tend to swamp the species differences that emerge when hydrocarbons are analyzed alone.
Factorial analyses showing the differences in chemical profiles between worker cuticles, workers PPGs, pupal cuticles, larval cuticles, and fungus garden mycelium in Atta colombica, Acromyrmex echinatior, and Sericomyrmex amabilis. All analyses were based on the relative proportions of all chemical compounds identified by GC/MS. Ellipses have been drawn to emphasize the categories but have no specific statistical meaning
Factorial analyses showing the differences in chemical profiles between the three species of fungus-growing ants Atta colombica, Acromyrmex echinatior, and Sericomyrmex amabilis for worker cuticles (top left), fungus gardens (top right), pupae (bottom left), and larvae (bottom right). All analyses were based on the relative proportions of all chemical compounds identified by GC/MS. Ellipses have been drawn to emphasize the categories but have no specific statistical meaning
Discussion
We have shown that: (1) a large variety of potential recognition compounds is present in the colonies of fungus-growing ants; (2) workers express mostly hydrocarbons, whereas fungus gardens have a number of additional compounds; (3) ants and fungal symbionts of the three species (genera) can be statistically distinguished based on their chemical profiles; and (4) larvae and pupae have much smaller absolute quantities of cuticular hydrocarbons than workers. In the following sections, we discuss some of the implications of these results.
The Spectrum of Surface Chemicals in Fungus-growing Ants
Both intra- and interspecific variation in the chemical profiles expressed by A. colombica, A. echinatior, and S. amabilis were found, but the intraspecific differences were much less than the interspecific differences both when complete and partial (hydrocarbons only) chemical profiles were considered. Moreover, species are characterized by some specific chemical compounds. Provisional data on Atta cephalotes (one colony: workers, larvae, and fungus) and Acromyrmex octospinosus (two colonies: workers, PPG, larvae, and fungus) from the same site in Gamboa, Panama, showed that differences between congeneric species are intermediate (Appendix Table 1). The species-specific chemical profiles are qualitatively similar among colonies (i.e., all colonies have the same compound composition), so that the differences obtained from multivariate statistics (Fig. 1) are mostly based on quantitative differences of the same compounds, except for the fungus gardens that harbor a number of unique nonhydrocarbon compounds. Differences in profiles were especially prominent among the hydrocarbons, which is consistent with these compounds having an important role in intra- and intercolony recognition (Lahav et al. 1999; Singer 1998; Wagner et al. 2000). Workers of all three species tended to have more (and more complex) hydrocarbons than the brood and the fungus gardens, which generally have simpler profiles consisting mostly of n-alkanes and n-alkenes. Similar differences in worker and brood chemical composition have been observed in other ant species (Myrmica [Elmes et al. 2002], Camponotus vagus [Bonavita-Cougourdan et al. 1989], and Lasius sakagamii [Akino and Yamaoka 1998]). However, it is remarkable that the larvae and pupae of fungus-growing ants have so few cuticular compounds and such low quantities of them. This finding is unlikely to be an artifact as it matches an earlier result on leaf-cutting ants by Viana et al. (2001). Whether this adult-brood difference is typical only for fungus-growing ants or for ants in general remains to be determined.
Fungus Garden Chemistry
The fungus produces several classes of compounds that ants do not synthesize, which confirms previous findings that workers only have alkanes, alkenes, and branched alkanes (Viana et al. 2001; Lambardi et al. 2007), whereas fungus gardens of Acromyrmex contain both aldehydes, alcohols, acetates, acids, and esters (Richard et al. 2007). The latter study that compared the fungus garden chemistry of Panamanian A. octospinosus and A. echinatior leaf-cutting ants showed that these fungi express the same chemical compounds but in different relative quantities among ant species and colonies (Richard et al. 2007). The same profiles, with the same chemical classes, were found in two colonies of A. octospinosus (Appendix Table 1), and the single colony of A. cephalotes likewise had a fungal profile that was similar to that of A. colombica both characterized by the presence of trimethylalkanes (Appendix Table 1). Richard et al. (2007) also showed that the fungus-derived compounds in A. echinatior and A. octospinosus have a, albeit weak, genetic component and that they are shared between the two species (see also Bot et al. 2001). As Mikheyev et al. (2007) recently showed that the symbiont fungi of Panamanian Atta and Acromyrmex in Gamboa, Panama, are similar genetically, it appears that the Atta and Acromyrmex fungus gardens each express at least some compounds that are ant species specific but not shared by the ants. Given that all colonies were reared on the same laboratory diet, this suggests that the same fungi express different chemical (and thus possibly metabolic) traits depending on the ant genus that they are associated with. The degree to which symbionts of Sericomyrmex are related to those reared by Atta and Acromyrmex leaf-cutting ants is not clear (Chapela et al. 1994; Hinkle et al. 1994), but it seems unlikely that they belong to the same pool. Thus, the differences in chemistry between Sericomyrmex symbionts and symbionts of Atta and Acromyrmex leaf-cutting ants may, in part, be genetic.
Recognition in Fungus-growing Ants
Differences in the proportion of hydrocarbons in workers (cuticle and PPG), brood, and fungus garden material are consistent with these compounds playing a central role in caste recognition, nestmate recognition, and discrimination between resident and alien strains of the fungal symbiont (Bot et al. 2001; Viana et al. 2001; Poulsen and Boomsma 2005; Richard et al. 2007). The patterns observed (Figs. 1 and 2) and the partitioning of variance in chemical profiles suggest that chemistry differences may allow efficient within-colony distinction between brood and symbionts without jeopardizing efficient nestmate recognition as hypothesized by Vander Meer and Morel (1998). This agrees with findings in other social insects; for example, cuticular hydrocarbons in the ant Pachycondyla inversa vary within and among colonies but also with an individual’s social and reproductive status (Heinze et al. 2002). Similarly, cuticular hydrocarbons in the ant Formica truncorum vary among colonies, to some extent between patrilines within colonies, and among samples taken from the same colony in different seasons (Nielsen et al. 1999; Boomsma et al. 2003). In addition, in the termite Reticulitermes virginicus and the ant C. vagus, all castes carry the same hydrocarbons, but the caste-specific proportions differ (Howard et al. 1978, 1982; Blomquist et al. 1979; Bonavita-Cougourdan et al. 1989).
Specificity of the chemical profiles of workers, brood, and fungi is intriguing because these different components of the same insect society interact constantly. For example, workers could transmit colony odor to the fungal substrate when they chew the harvested leaves before integrating them into the garden. Other workers may regurgitate the content of their PPG (which is known to be a reservoir of colony gestalt odor profiles) to nestmates by a process called trophallaxis (Soroker et al. 1994, 1995). Grooming and other social interactions may further promote the circulation of hydrocarbons through the colony. Given these close interactions, it is interesting that the hydrocarbon compounds present in fungus material were always present on workers and brood but that the opposite is not true (Viana et al. 2001; Lambardi et al. 2007; Richard et al. 2007). Previous studies combined with our present data, therefore, indicate that hydrocarbons are transferred by workers when moving around in the garden while grooming fungus fragments and other workers, but whether the ants or the fungus are the ultimate producers of these compounds is not yet clear.
References
Akino, T. and Yamaoka, R. 1998. Chemical mimicry in the root aphid parasitoid Paralipsis eikoae Yasumatsu (Hymenoptera: aphidiidae) of the aphid attending ant Lasius sakagamii Yamauchi and Hayashida (Hymenoptera: Formicidae). Chemoecology 8:153–161.
Beye, M., Neumann, P., and Moritz, R. F. A. 1997. Nestmate recognition and the genetic gestalt in the mound-building ant Formica polyctena. Insectes Soc. 44:49–58.
Beye, M., Neumann, P., Chapuisat, M., and Pamilo, P., and Moritz, R. F. A. 1998. Nestmate recognition and the genetic relatedness of nests in the ant Formica pratensis. Behav. Ecol. Sociobiol. 43:67–72.
Blomquist, G. J., Howard, R. W., and McDaniel, C. A. 1979. Structures of the cuticular hydrocarbons of the termites Zootermopsis angusticollis (Hagen). Insect Biochem. 9:365–370.
Bonavita-Cougourdan, A., Clément, J., and Lange, C. 1989. The role of cuticular hydrocarbons in the recognition of larvae by workers of the ant Camponotus vagus: changes in the chemical signature in response to social environment (Hymenoptera: Formicidae). Sociobiology 16:49–74.
Boomsma, J. J., Nielsen, J., Sundström, L., Oldham, N. J., Tentschert, J., Petersen, H. C., and Morgan, E. D. 2003. Informational contraints on optimal sex allocation in ant. Proc. Natl. Acad. Sci. USA 100:158799–8804.
Bot, A. N. M., Rehner, S. A., and Boomsma, J. J. 2001. Partial incompatibility between ants and symbiotic fungi in two sympatric species of Acromyrmex leaf-cutting ants. Evolution 55:1980–1991.
Brandão, C. R. F. and Mayhé-Nunes, A. J. 2001. A new fungus-growing genus, Mycetagroicus gen. n., with the description of three new species and comments on the monophyly of the Attini (Hymenoptera: Formicidae). Sociobiology 38:639–665.
Breed, M. D. 1983. Nestmate recognition in honey bees. Anim. Behav. 31:86–91.
Carlson, D. A., Bernier, U. R., and Sutton, B. C. 1998. Evolution patterns from capillary GC for methyl-branched alkanes. J. Chem. Ecol. 24:1845–1865.
Chapela, I. H., Rehner, S. A., Schultz, T. R., and Mueller, U. G. 1994. Evolutionary history of the symbiosis between fungus-growing ants and their fungi. Science 266:1691–1694.
Crosland, M. W. J. 1989. Kin recognition in the ant Rhytidoponera confusa. I. Environmental odour. Anim. Behav. 37:912–919.
Currie, C. R., Wong, B., Stuart, A. E., Schultz, T. R., Rehner, S. A., Mueller, U. G., Sung, G. H., Spatafora, J. W., and Straus, N. A. 2003. Ancient tripartite coevolution in the Attine ant–microbe symbiosis. Science 299:386–388.
Currie, C. R., Poulsen, M., Mendenhall, J., Boomsma, J. J., and Billen, J. 2006. Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311:81–83.
Elmes, G. W., Akino, T., Thomas, J. A., Clarke, R. T., and Knapp, J. J. 2002. Interspecific differences in cuticular hydrocarbon profiles of Myrmica ants are sufficiently consistent to explain host specificity by Maculinea (large blue) butterflies. Oecologia 130:525–535.
Heinze, J., Foitzik, S., Hippert, A., and Hölldobler, B. 1996. Apparent dear-enemy phenomenon and environment-based recognition cues in the ant Leptothorax nylanderi. Ethology 102:510–522.
Heinze, J., Stengl, B., and Sledge, M. F. 2002. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 52:59–65.
Hinkle, G., Wetterer, J. K., Schultz, T. R., and Sogin, M. L. 1994. Phylogeny of the attine ant fungi based on analysis of small subunit ribosomal RNA gene sequences. Science 266:1695–1697.
Hölldobler, B. and Wilson, E. O. 1990. The ants. 732 pp. Belknap, Cambridge, MA.
Howard, R. W. and Blomquist, G. J. 2005. Ecological, behavioral, and biochemical aspects of insect hydrocabons. Annu. Rev. Entomol. 50:371–393.
Howard, R. W., Mcdaniel, C. A., and Blomquist, G. J. 1978. Cuticular hydrocarbons of the eastern subterranean termite, Reticulitermes flavipes (Kollar) (Isoptera: Rhinotermitidae). J. Chem. Ecol. 4:233–245.
Howard, R. W., Mcdaniel, C. A., Nelson, D. R., Blomquist, G. J., Gelbaum, L. T., and Zalkow, L. H. 1982. Cuticular hydrocarbons of Reticulitermes virginicus (BANKS) and their role as potential species and caste-recognition cues. J. Chem. Ecol. 8:1227–1239.
Jutsum, A. R., Saunders, T. S., and Cherrett, J. M. 1979. Intraspecific aggression in the leaf-cutting ant Acromyrmex octospinosus. Anim. Behav. 27:839–844.
Lahav, S., Soroker, V., Hefetz, A., and Vander Meer, R. K. 1999. Direct behavioral evidence for hydrocarbons as ant recognition discriminators. Naturwissenschaften 86:246–249.
Lambardi, D., Dani, F. R., Turillazzi, S., and Boomsma, J. 2007. Incipient social parasites of Acromyrmex leaf-cutting ants avoid host aggression by being chemically inconspicuous. Behav. Ecol. Sociobiol. 61:843–851.
Liang, D. and Silverman, J. 2000. You are what you eat: diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87:412–416.
Lommelen, E., Johnson, C. A., Drijfhout, F. P., Billen, J., Wenseleers, T., and Gobin, B. 2006. Cuticular hydrocarbons provide reliable cues of fertility in the ant Gnamptogenys striatula. J. Chem. Ecol. 32:2023–2034.
Mikheyev, A. S., Mueller, U. G., and Boomsma, J. J. 2007. Population genetic signatures of diffuse co-evolution between leaf-cutting ants and their cultivar fungi. Mol. Ecol. 16:209–216.
Mueller, U. G. 2002. Ant versus fungus versus mutualism: ant cultivar conflict and the deconstruction of the Attine ant–fungus symbiosis. Am. Nat. 160:S67–S98.
Mueller, U. G., Rehner, S. A., and Schultz, T. R. 1998. The evolution of agriculture in ants. Science 281:2034–2038.
Mueller, U. G., Schultz, T. R., Currie, C. R., Adams, R. M. M., and Malloch, D. 2001. The origin of the Attine ant–fungus mutualism. Q. Rev. Biol. 76:169–197.
Nielsen, J., Boomsma, J. J., Oldham, N. J., Petersen, H. C., and Morgan, E. D. 1999. Colony-level and season-specific variation in cuticular hydrocarbon profiles of individual workers in the ant Formica truncorum. Insect. Soc. 46:58–65.
Obin, M. S. 1986. Nestmate recognition cues in laboratory and field colonies of Solenopsis invicta Buren (Hymenoptera: Formicidae): effect of environment and the role of cuticular hydrocarbons. J. Chem. Ecol. 12:1965–1975.
Poulsen, M. and Boomsma, J. J. 2005. Mutualistic fungi control crop diversity in fungus-growing ants. Science 307:741–744.
Richard, F. J., Hefetz, A., Christides, J. P., and Errard, C. 2004. Food influence on colonial recognition and chemical signature between nestmates in the fungus-growing ant Acromyrmex subterraneus. Chemoecology 14:9–16.
Richard, F. J., Mora, P., Errard, C., and Rouland, C. 2005. Digestive capacities of leaf-cutting ants and the contribution of their fungal cultivar to the degradation of plant material. J. Comp. Physiol. B 175:297–303.
Richard, F. J., Poulsen, M., Hefetz, A., Errard, C., Nash, D. R., and Boomsma, J. J. 2007. The origin of chemical profiles of fungal symbionts and their significance for nestmate recognition in Acromyrmex leaf-cutting ants. Behav. Ecol. Sociobiol. 61:1637–1649.
Schultz, T. R. and Meier, R. 1995. A phylogenetic analysis of the fungus-growing ants (Hymenoptera: Formicidae: attini) based on morphological characters of the larvae. Syst. Entomol. 20:337–370.
Silverman, J. and Liang, D. 2001. Colony disassociation following diet partitioning in a unicolonial ant. Naturwissenschaften 88:73–77.
Singer, T. L. 1998. Roles of hydrocarbons in the recognition systems of insects. Am. Zool. 38:394–405.
Singer, T. L. and Espelie, K. E. 1996. Nest surface hydrocarbons facilitate nestmate recognition for the social wasp, Polistes metricus Say (Hymenoptera, Vespidae). J. Insect Behav. 9:857–870.
Soroker, V., Vienne, C., Hefetz, A., and Nowbahari, E. 1994. The postpharyngeal gland as a “Gestalt” organ for nestmate recognition in the ant Cataglyphis niger. Naturwissenschaften 81:510–513.
Soroker, V., Vienne, C., and Hefetz, A. 1995. Hydrocarbon dynamics within and between nestmates in Cataglyphis niger (Hymenoptera: Formicidae). J. Chem. Ecol. 21(3):365–378.
Vander Meer, R. K. and Morel, L. 1998. Nestmate recognition in ants, pp. 79–103, in R. K. Vander Meer, M. Breed, K. E. Espelie, M. Winston, (eds.). Pheromone Communication in Social Insects. Westview, Boulder, CO.
Viana, A. M. M., Frézard, A., Malosse, C., Della Lucia, T. M. C., Errard, C., and Lenoir, A. 2001. Colonial recognition of fungus in the fungus-growing ant Acromyrmex subterraneus subterraneus (Hymenoptera: Formicidae). Chemoecology 11:29–36.
Wagner, D., Tissot, M., Cuevas, W., and Gordon, D. M. 2000. Harvester ants utilize cuticular hydrocarbons in nestmate recognition. J. Chem. Ecol 26:2245–2257.
Weber, N. A. 1972. Gardening ants, the attines. Memoirs of the American Philosophical Society, Philadelphiap xvii + 146.
Wilson, E. O. 1971. The insect societies. 548 pp. Belknap, Cambridge, MA.
Acknowledgements
We thank the Smithsonian Tropical Research Institute (STRI) for providing logistic help and facilities to work in Gamboa and the Autoridad Nacional del Ambiente y el Mar (ANAM) for permission to sample ant colonies in Panama and export them to Denmark. Fieldwork was supported by grants from the Carlsberg Foundation and the Danish Natural Science Research Council to JJB. All experiments performed in this manuscript comply with current Danish and USA law.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Richard, FJ., Poulsen, M., Drijfhout, F. et al. Specificity in Chemical Profiles of Workers, Brood and Mutualistic Fungi in Atta, Acromyrmex, and Sericomyrmex Fungus-growing Ants. J Chem Ecol 33, 2281–2292 (2007). https://doi.org/10.1007/s10886-007-9385-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-007-9385-z