Abstract
In ca. 150 species of queenless ants, a specialized queen caste is rare or absent, and mated workers take over the role of the queen in some or all of the colonies. Previously, it has been shown that reproduction in queenless ants is regulated by a combination of dominance behavior and chemical fertility signaling. It is unknown, however, whether chemical signals alone can sufficiently regulate reproduction. To investigate this possibility, we studied reproductive regulation in the facultatively queenless ant Gnamptogenys striatula, a species where dominance behavior is rare or absent. Active egg layers and infertile workers showed qualitative and quantitative differences in their cuticular hydrocarbon profile. Five long-chain methyl alkanes, 3,13- and 3,15-dimethyl pentriacontane, 3,13- and 3,15-dimethyl heptentriacontane, and 3,11,15-trimethyl heptentriacontane occurred only on the cuticles of virgin and mated egg layers. Pronounced quantitative differences were found in a further 27 alkenes; alkanes; and mono-, di-, and trimethyl alkanes. Workers that had recently stopped laying eggs had profiles similar to infertile workers, and mating status did not affect this chemical pattern. We conclude that the cuticular hydrocarbon profiles of G. striatula workers provide reliable information about their current fertility. In the interest of colony productivity, this allows reproduction to be regulated without the use of aggression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The key characteristic of ant societies is a reproductive division of labor, with morphologically specialized queens producing most or all the offspring, and workers performing other tasks such as foraging or caring for the brood (Wilson, 1971). In ca. 150 ectatommine and ponerine species, however, the specialized queen caste is rare or absent, and mated workers or “gamergates” take over the role of the queen in some or all of the colonies (Peeters, 1991). Because all individuals in these species are totipotent, i.e., able to mate and reproduce, there is potential for reproductive conflict (Monnin and Ratnieks, 2001; Ratnieks et al., 2006). One expression of this conflict is dominance behavior, which in most species is critical in establishing and maintaining a reproductive division of labor (Ito, 1993; Liebig et al., 1999; Monnin and Peeters, 1999). Dominance behavior, however, is potentially costly, because individuals that engage in dominance interactions generally work at a lower rate than labor workers (Cole, 1986; Gobin et al., 2003; Ito and Higashi, 1991; Monnin and Peeters, 1999; Monnin and Ratnieks, 1999).
Dominance interactions are usually most prominent immediately after colonies are orphaned, when the reproductive dominance hierarchy is first established (Gobin et al., 2001; Monnin and Peeters, 1999). Aggression typically occurs only between individuals of similar dominance status and reduces to a low level as soon as one or a few dominant individuals start to reproduce (Monnin and Peeters, 1999; Gobin et al., 2001; Cuvillier-Hot et al., 2004). This reduction in aggression may coincide with the development of a specific cuticular hydrocarbon profile in egg-laying individuals. Indeed, consistent chemical differences in the cuticular profiles between fertile and infertile individuals have now been shown in Diacamma ceylonense (Cuvillier-Hot et al., 2001), Dinoponera quadriceps (Monnin et al., 1998), Harpegnathos saltator (Liebig et al., 2000), Myrmecia gulosa (Dietemann et al., 2003), Pachycondyla cf. inversa (Tentschert et al., 2001; Heinze et al., 2002), and Streblognathus peetersi (Cuvillier-Hot et al., 2004). By secreting specific pheromones, it is thought that active egg layers signal their high fertility (Cuvillier-Hot et al., 2001) so that low-ranking individuals concede reproduction to high-ranking fecund individuals. In this way, the reproductive division of labor would be reinforced.
It is currently unknown whether a reproductive division of labor in queenless colonies can be regulated purely through chemical signaling, without the use of aggression. In some species, dominance behavior appears to be lacking altogether, even after colonies have been orphaned (Gnamptogenys striatula, Blatrix and Jaisson, 2000; Pachycondyla berthoudi, Peeters and Crewe, 1985, 1984; Sledge et al., 2001; P. krugeri, Wildman and Crewe, 1988; Platythyrea lamellosa, Villet et al., 1990; P. schultzei, Villet, 1991; Rhytidoponera aurata, Komene et al., 1999; R. sp. 12, Tay and Crozier, 2000; Streblognathus aethiopicus, Ware et al., 1990). This suggests that in these species, reproductive regulation is entirely pheromone-based.
The aim of the present study was to provide the first detailed test of the hypothesis that reproductive regulation is purely pheromone-based in a species that lacks dominance behavior. We used the facultatively queenless ant G. striatula (Blatrix and Jaisson, 2000) to examine the cuticular chemistry of fertile and infertile individuals with respect to different stages of ovarian development and mating status. As predicted, we showed that active egg layers have a unique cuticular hydrocarbon profile that, thus, can provide nestmates with reliable signals of fertility. In the interest of colony productivity, this allows reproduction to be regulated without the use of aggression.
Methods and Materials
Study Species
The ant G. striatula is a New World species, typically found in open habitats (Giraud et al., 2001) and humid forests (Lattke, 1995). Colonies are headed by either one or several queens, or more commonly, by several gamergates (up to 31; Blatrix and Jaisson, 2001). In our study population, 15 out of 23 colonies (65%) were queenless and headed by gamergates. Removing queens or gamergates from nests results in sexual calling and egg laying by some of the virgin workers inside the nest. The labor workers then transport nearby males to the nest, where the males will mate with any worker that assumes the sexual-calling position (Blatrix and Jaisson, 2000). The ability of workers to mate after loss of the queen or gamergates renders the colonies potentially everlasting.
Collection and Housing of Colonies
Colonies of G. striatula were collected in Santa Rosa National Park, Costa Rica, in October 1999 (four colonies) and October 2003 (six colonies). Colonies were transferred to the laboratory in Belgium, where they were housed in plaster of Paris nests with foraging arenas of 20 × 10 × 6 cm or 20 × 20 × 6 cm, depending on colony size, and glass covered nest cavities of 50 × 40 × 3 mm to 150 × 85 × 3 mm.
Collection of Individuals of Differing Reproductive Status
Five individuals were selected for each of the following categories from gamergate-right supply colonies and orphaned groups.
From gamergate-right colonies: G1—gamergates: mated workers, typically with well-developed ovaries, i.e., with many developing and mature oocytes, yellow bodies (an indicator of previous egg-laying; Billen, 1985; Fénéron and Billen, 1996), and the main or sole reproducers in the colony. The spermathecae of these individuals were opaque, indicating the presence of semen. G2—nonreproductive workers: workers with nonactive ovaries, i.e., without or with very few developing oocytes and without yellow bodies.
From orphaned colonies: O1—reproductive workers with fully developed ovaries: virgin workers with well-developed ovaries, i.e., with many developing and mature oocytes, and yellow bodies, an indication of high fertility and previous egg laying. A transparent spermatheca shows they were unmated. O2—reproductive workers with partly developed ovaries: virgin workers with numerous developing and mature oocytes but no yellow bodies. These intermediates were likely on the verge of oviposition or they might be ovipositing already (it is unclear how many ovipositions are required for the development of yellow bodies). O3—former reproductive workers: virgin workers with yellow bodies but only few developing oocytes. Video observation revealed that these individuals did not lay eggs and behaved like foragers. We therefore considered them former egg layers. O4—nonreproductive workers: virgin workers with nonactive ovaries, i.e., without or with very few developing oocytes and without yellow bodies.
To obtain workers of categories O1–O4, we created five orphaned groups of 20 virgin workers by isolating them from their natal gamergate colonies. When five eggs were present in the experimental chamber, each worker's cuticle was sampled for chemical analysis. Dissection of these workers showed they all had empty spermathecae. We also counted the number of ovarioles and the number of yolky and nonyolky oocytes, as oocytes progress from nonyolky to yolky during maturation (Peeters, 1993; Gobin et al., 1998) and noted whether yellow bodies were present. To obtain five individuals for each O1–O4 category, we chose for each category five workers out of the dissected ants that had ovary characteristics that best fit the description. For categories O1 and O2, we retained the individuals with the highest number of yolky oocytes, and for categories O3 and O4 the individuals with the least or preferably no oocytes (and never having yolky oocytes). Samples from these individuals were analyzed for their cuticular chemicals.
Chemical Analysis
Cuticular chemicals were extracted by placing ants in individual vials containing 10 μl of hexane for 30 min. The chemical extracts were analyzed by gas chromatography (GC) in splitless mode [Agilent 6850 Series, Agilent Technologies, USA, equipped with a capillary column (Agilent HP-1; coated with methyl siloxane; length: 30 m; I.D.: 0.32 mm; film thickness: 0.25 μm; Agilent Technologies) and flame ionization detector]. The injector and detector temperatures were 290°C and 300°C, respectively, with an inlet pressure of 6.74 psi and a constant flow of 1.1 ml/min. The oven was programmed from 90°C to 180°C at 20°C/min, then to 290°C at 5°C/min, and held for 30 min. We used helium as the carrier gas. Data were analyzed using Chemstation (Rev A.09.01, Agilent Technologies).
Gas chromatography/mass spectrometry analysis (GC-MS) was carried out on an HP 6890 GC (equipped with an HP-5MS column; length: 30 m; ID: 0.25 mm; film thickness: 0.25 μm) connected to an HP5973 MSD (quadrupole mass spectrometer with 70 eV electron impact ionization). Samples were injected in the splitless mode, and the oven was programmed from 60°C to 250°C at 10°C/min and then from 250°C to 320°C at 5°C/min, and held for 7 min at 320°C. Helium was used as carrier gas, at a constant flow rate of 1.0 ml/min. Cuticular hydrocarbons (alkanes and mono-, di-, and trimethyl alkanes) were characterized by the use of standard MS databases and diagnostic ions and by determining Kovats indices by the method of Carlson et al. (1998). The position of the double bond in nonacosene and hentriacontene was not determined.
Statistical Analysis
Before doing multivariate analysis, data were standardized by calculating relative peak areas, i.e., peak areas of compounds divided by the total sum of all peak areas for one individual. This was performed to ensure that each individual was given the same weight in the analysis. The total sum of all peak areas for each individual was added as a control variable. We only considered compounds with relative peak areas >1% in at least two individuals. This reduced the number of compounds to 51. In two cases, MS revealed two different compounds with the same retention time in two individuals (peaks 18 and 25; Table 1). Because these compounds could not be distinguished with GC only, they were excluded in all statistical analyses.
Data were subjected to principal component analysis (PCA) to allow compounds that differed between groups to be graphically identified. To focus on the compounds with the greatest difference between groups, we only considered compounds that had factor loadings with an absolute value >0.75 on the first axis.
To assess the significance of the differences in cuticular profiles between the different groups, we used MANOVA on the first four factors of the PCA, which together explained 73% of the observed variation. All statistical calculations were performed using Statistica 6.0 (Statsoft, Inc.).
Results
General Characteristics of Cuticular Chemical Profiles
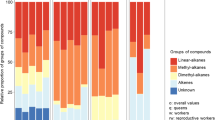
The cuticular profiles contained a mixture of alkanes; alkenes; and mono-, di-, and trimethyl alkanes with chain lengths ranging from C28 to C37 (Fig. 1; Table 1). Several compounds occurred in high proportions in all individuals (nonacosane, 12- and 18-methyl triacontane, and 3-methyl hentriacontane, peaks 3, 8, and 16). Other compounds were specific to gamergates or nonreproductive workers (see below).
Representative cuticular chemical profiles of a gamergate (a) and a worker with undeveloped ovaries (b). The peak numbers correspond to the identified compounds listed in Table 1.
Statistical Analysis
Principal component analysis shows that differences in fertility explain most of the variation in cuticular hydrocarbon profiles, with the first axis of the PCA clearly differentiating active egg layers from nonreproductive workers (percentage of the variance explained: 44%; Fig. 2). Reproductive workers with fully and partly developed ovaries (O1 and O2) cluster together with gamergates (G1; Fig. 2). This shows that mating as such did not influence cuticular hydrocarbon profiles. Former reproductive workers (O3) cluster together with nonreproductive workers (O4 and G2; Fig. 2), and reproductive workers with fully developed ovaries (O1 and G1) cluster with reproductive workers with partly developed ovaries (O2). This demonstrates that chemical profiles accurately reflect current fertility independent of previous egg laying. A MANOVA on the first four factors of the PCA confirms that there were significant differences in the chemical profile of ants with high and low fertility (groups O1, O2, and G1 vs. groups O3, O4, and G2; F 4,23 = 57.3; P < 10−6) but that there were no significant differences detectable between mated (G1) and unmated egg layers (O1 and O2; F 4,23 = 1.42; P = 0.26) nor between workers with (O1, O3, and G1) and without yellow bodies (O2, O4, and G2; F 4,23 = 0.78; P = 0.55).
Biplot showing the first two factors of a PCA. The symbols represent the factor scores of individuals belonging to different groups. The lines radiating from the origin indicate the factor loadings of the different compounds. The overlap between both plots shows the correlation between the two. Factor scores were reduced 10-fold to facilitate comparison with the factor loadings. Compound numbers correspond to those listed in Fig. 1 and Table 1. “Sum” is the total quantity of chemicals on the cuticle, i.e., the sum of all compounds. Symbols refer to the following groups: from gamergate-right colonies: (○) gamergates (G1) and (▴) nonreproductive workers (G2); from orphaned colonies: (△ and •) reproductive workers with fully and partly developed ovaries (O1 and O2), (▪) former reproductive workers (O3), and (□) nonreproductive workers (O4).
Chemical Differences between Fertile and Infertile Individuals
To determine which compounds correlate most with differences in fertility, we can look at those compounds that show the strongest correlation with the first factor of the PCA (compounds with factor loadings with an absolute value >0.75; Fig. 2). In this way, it can be seen that there are 18 compounds that are strongly negatively correlated with factor 1, and occur in higher concentration on the cuticle of fertile individuals, i.e., gamergates and unmated egg-laying workers. These compounds comprise one monomethyl alkane: 3-methyl nonacosane (peak 5; Table 1); 15 dimethyl alkanes: 3,15-dimethyl nonacosane (peak 7), 3,11-, 3,13-, and 3,15-dimethyl hentriacontane (peak 17), 4,10-, 4,12-, and 4,14-dimethyl dotriacontane (peak 21), 3,11-, 3,13-, and 3,15-dimethyl tritriacontane (peak 24), 4,x-dimethyl tetratriacontane (peak 27), 3,13- and 3,15-dimethyl pentatriacontane (peak 28), and 3,13- and 3,15-dimethyl heptatriacontane (peak 30); and two trimethyl alkanes: 3,11,15-trimethyl pentatriacontane (peak 29) and 3,11,15-trimethylheptatriacontane (peak 31). In this group, peaks 17, 24, and 28 show the strongest negative correlation with factor 1 (factor loading <−0.95). The compounds 3,13- and 3,15-dimethyl pentatriacontane (peak 28), 3,13- and 3,15-dimethyl heptatriacontane (peak 30), and 3,11,15-trimethyl heptatriacontane (peak 31) are unique for active egg layers (Table 1).
Similarly, 14 compounds are strongly positively correlated with factor 1 (factor loadings >0.75), and occur in higher concentration on the cuticle of nonreproductive workers. These compounds comprise one alkene: hentriacontene (peak 11); eight monomethyl alkanes: 11- and 13-methyl nonacosane (peak 4), 9-, 11-, and 13-methyl hentriacontane (peak 13), 9-, 11-, and 13-methyl tritriacontane (peak 22); and five dimethyl alkanes: 10,14- and 8,12-dimethyl dotriacontane (peak 20) and 9,21-, 9,23-, and 11,23-dimethyl tritriacontane (peak 23). Peak 13 shows the strongest correlation with factor 1 (factor loading 0.89). None of the compounds included in the PCA were unique to nonreproductive workers. The total of all compounds (“sum”) was independent of the first principal component, and hence was independent of fertility (Fig. 2).
In summary, there were at least 32 compounds strongly correlated with reproductive status, 18 of which were more abundant on the cuticle of gamergates and reproductive workers, and 14 of which were more abundant in nonreproductive workers. In addition to these compounds, MS indicated that 10-, 12-, 14-, and 20-methyl dotriacontane (peak 18), and 12-, 14-, and 20-methyltetratriacontane (peak 25) were unique to gamergates, and that 12,x-dimethyl dotriacontane (peak 18) and 3,11,15-trimethyl tritriacontane (peak 25) were unique for nonreproductive workers. Unfortunately, because the retention times of monomethyl dotriacontane and dimethyl dotriacontane (peak 18), and of monomethyl tetratriacontane and trimethyl tritriacontane (peak 25), were identical, these differences could not be statistically confirmed in a larger sample by using GC.
Discussion
Our results show that there are pronounced differences in the cuticular chemistry of fertile and infertile individuals in queenless colonies of the ant G. striatula. Both qualitative and quantitative differences in over 32 hydrocarbons contributed to this result. Previously, cuticular hydrocarbons linked to fertility have also been found in ant species that form aggressive dominance hierarchies, for example, D. ceylonense (Cuvillier-Hot et al., 2001, 2002), D. quadriceps (Monnin et al., 1998), and S. peetersi (Cuvillier-Hot et al., 2004). These fertility signals are supposed to help in resolving intracolony conflicts by stimulating low-rankers to concede reproduction to more fecund high-ranking ones (Cuvillier-Hot et al., 2001). Our species, however, is unique in that it lacks any observable dominance behavior. Hence, our study is the first to show that chemical fertility cues may alone regulate reproduction. It remains to be elucidated which of the identified compounds act as the main signal. As several compounds are either up- or down-regulated depending on status, it is likely that more than one compound is involved in fertility regulation in G. striatula. In P. inversa, however, D'Ettorre et al. (2004) showed that the ants are able to detect a single compound that is specifically associated with ovarian activity.
Overall, the chemicals characterizing fertile workers are hydrocarbons with longer chain lengths compared with the compounds found on the cuticle of infertile workers. A shift toward heavier hydrocarbons in ants with well-developed ovaries has also been found in other ponerine species, e.g., P. inversa (Heinze et al., 2002) and H. saltator (Liebig et al., 2000). The elongation reactions, following a switch to reproductive maturation, have been well studied in other insect taxa (Tillman-Wall et al., 1992; Blomquist et al., 1995, 1998; Tillman et al., 1999), and it is likely that a similar mechanism is acting in the species studied here, G. striatula. In two species, the dolichoderine ant Linepithema humile and the myrmeciine ant M. gulosa, the shift has been shown to proceed in the opposite direction (de Biseau et al., 2004; Dietemann et al., 2003), with queens being characterized by shorter-chain hydrocarbons than workers. Mechanistically, the chemical differences between fertile and infertile individuals might be caused by the differential activity of oenocytes, which are involved in yolk production (Jensen and Borgesen, 2000) and are also the producers of cuticular hydrocarbons (Diehl, 1975; Fan et al., 2003).
A major conclusion of our results is that the chemical profiles accurately reflect the current fertility of an individual. This was shown by the fact that workers with yellow bodies in their ovaries, which are thought to be workers that have recently stopped laying eggs, had the same cuticular profiles as nonlaying workers. By contrast, it is clear that mating did not produce any detectable changes in cuticular chemistry, as gamergates and unmated workers with active ovaries had essentially the same chemical profiles. This also appears to be true for other queenless ants (D. ceylonense, Cuvillier-Hot et al., 2002; H. saltator, Liebig et al., 2000) and it suggests that signaling fertility is generally more important than signaling mating status.
In summary, our results demonstrate that in the ant G. striatula, cuticular hydrocabon profiles can provide nestmates with reliable information about each individual's fertility. We suggest that in this way, reproduction can be regulated without the use of aggression. The ability to regulate reproduction without the use of aggression has a large colony-level benefit, as costs associated with fighting, such as reduced colony efficiency and mortality, can be avoided. Nevertheless, most species go through an aggressive phase before chemical signals take over regulation of the reproductive conflict (among others Monnin and Peeters, 1999; Cuvillier-Hot et al., 2001, 2002, 2004; Gobin et al., 2001). G. striatula apparently lacks this aggressive phase, for a reason that remains unclear. Further studies are required to answer this.
References
Billen, J. 1985. Ultrastructure of the worker ovarioles in Formica ants (Hymenoptera: Formicidae). Int. J. Insect Morphol. Embryol. 14:21–32.
Blatrix, R. and Jaisson, P. 2000. Optional gamergates in the queenright ponerine ant Gnamptogenys striatula Mayr. Insect. Soc. 47:1–5.
Blatrix, R. and Jaisson, P. 2001. Reproductive strategy of the ponerine ant Gnamptogenys striatula Mayr (Hymenoptera: Formicidae). Sociobiology 37:147–161.
Blomquist, G. J., Tillman, J. A., Reed, J. R., Gu, P., Vanderwel, D., Choi, S., and Reitz, R. C. 1995. Regulation of enzymatic activity involved in sex pheromone production in the housefly, Musca domestica. Insect Biochem. Mol. Biol. 25:751–757.
Blomquist, G. J., Tillman, J. A., Mpuru, S., and Seybold, S. J. 1998. The cuticle and cuticular hydrocarbons of insects: structure, function, and biochemistry, pp. 34–54, in R. K. Vandeer Meer, M. D. Breed, M. L. Winston, and K. E. Espelic (eds.). Pheromone communication in social insects: ants, wasps, bees, and termites. Oxford: Westview Press, Boulder.
Carlson, D. A., Bernier, U. R., and Sutton, B. D. 1998. Elution patterns form capillary GC for methyl-branched alkanes. J. Chem. Ecol. 24:1845–1865.
Cole, B. J. 1986. The social behavior of Leptothorax allardycei (Hymenoptera, Formicidae): Time budgets and the evolution of worker reproduction. Behav. Ecol. Sociobiol. 18:165–173.
Cuvillier-Hot, V., Cobb, M., Malosse, C., and Peeters, C. 2001. Sex, age and ovarian activity affect cuticular hydrocarbons in Diacamma ceylonense, a queenless ant. J. Insect Physiol. 47:485–493.
Cuvillier-Hot, V., Gadagkar, R., Peeters, C., and Cobb, M. 2002. Regulation of reproduction in a queenless ant: Aggression, pheromones and reduction in conflict. Proc. R. Soc. Lond. B. 269:1295–1300.
Cuvillier-Hot, V., Lenoir, A., Crewe, R., Malosse, C., and Peeters, C. 2004. Fertility signalling and reproductive skew in queenless ants. Anim. Behav. 68:1209–1219.
de Biseau, J.-C., Passera, L., Daloze, D., and Aron, S. 2004. Ovarian activity correlates with extreme changes in cuticular hydrocarbon profile in the highly polygynous ant, Linepithema humile. J. Insect Physiol. 50:585–593.
D'Ettorre, P., Heinze, J., Schulz, C., Francke, W., and Ayasse, M. 2004. Does she smell like a queen? Chemoreception of a cuticular hydrocarbon signal in the ant Pachycondyla inversa. J. Exp. Biol. 207:1085–1091.
Diehl, P. A. 1975. Synthesis and release of hydrocarbons by the oenocytes of the desert locust, Schistocerca gregaria. J. Insect Physiol. 21:1237–1246.
Dietemann, V., Peeters, C., Liebig, J., Thivet, V., and Hölldobler, B. 2003. Cuticular hydrocarbons mediate discrimination of reproductives and non-reproductives in the ant Myrmecia gulosa. Proc. Natl. Acad. Sci. U. S. A. 100:10341–10346.
Fan, Y., Zurec, L., Dykstra, M. J., and Schal, C. 2003. Hydrocarbon synthesis by enzymatically dissociated oenocytes of the abdominal integument of the German cockroach, Blattella germanica. Naturwissenschaften 90:121–126.
Fénéron, R. and Billen, J. 1996. Ovarian cycle in Ectatomma tuberculatum workers (Formicidae, Ponerinae). Invertebr. Reprod. Dev. 29:79–85.
Giraud, T., Blatrix, R., Poteaux, C., Solignac, M., and Jaisson, P. 2001. High genetic relatedness among nestmate queens in the polygynous ponerine ant Gnamptogenys striatula in Brazil. Behav. Ecol. Sociobiol. 49:128–134.
Gobin, B., Peeters, C., and Billen, J. 1998. Production of trophic eggs by virgin workers in the ponerine ant Gnamptogenys menadensis. Physiol. Entomol. 23:329–336.
Gobin, B., Billen, J., and Peeters, C. 2001. Dominance interactions regulate worker mating in the polygynous ponerine ant Gnamptogenys menadensis. Ethology 107:495–508.
Gobin, B., Heinze, J., Strätz, M., and Roces, F. 2003. The energetic cost of reproductive conflicts in the ant Pachycondyla obscuricornis. J. Insect Physiol. 49:747–752.
Heinze, J., Stengl, B., and Sledge, M. F. 2002. Worker rank, reproductive status and cuticular hydrocarbon signature in the ant, Pachycondyla cf. inversa. Behav. Ecol. Sociobiol. 52:59–65.
Ito, F. 1993. Functional monogyny and dominance hierarchy in the queenless ponerine ant Pachycondyla (= Bothroponera) sp. in West Java, Indonesia (Hymenoptera, Formicidae, Ponerinae). Ethology. 95:126–140.
Ito, F. and Higashi, S. 1991. A linear dominance hierarchy regulating reproduction and polyethism of the queenless ant Pachycondyla sublaevis. Naturwissenschaften 78:80–82.
Jensen, P. V. and Borgesen, L. W. 2000. Regional and functional differentiation in the fat body of pharaoh's ant queens, Monomorium pharaonis (L.). Arthropod Struct. Develop. 29:171–184.
Komene, Y., Higashi, S., Ito, F., and Miyata, H. 1999. Effect of colony size on the number of gamergates in the queenless ponerine ant Rhytidoponera aurata. Insect. Soc. 46:29–33.
Lattke, J. E. 1995. Revision of the ant genus Gnamptogenys in the New World (Hymenoptera: Formicidae). J. Hymenopt. Res. 4:137–193.
Liebig, J., Peeters, C., and Hölldobler, B. 1999. Worker policing limits the number of reproductives in a ponerine ant. Proc. R. Soc. London B. 266:1865–1870.
Liebig, J., Peeters, C., Oldham, N. J., Markstadter, C., and Hölldobler, B. 2000. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc. Natl. Acad. Sci. U.S.A. 97:4124–4131.
Monnin, T. and Peeters, C. 1999. Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav. Ecol. 10:323–332.
Monnin, T. and Ratnieks, F. L. W. 1999. Reproduction versus work in queenless ants: When to join a hierarchy of hopeful reproductives? Behav. Ecol. Sociobiol. 46:413–422.
Monnin, T. and Ratnieks, F. L. W. 2001. Policing in queenless ponerine ants. Behav. Ecol. Sociobiol. 50:97–108.
Monnin, T., Malosse, C., and Peeters, C. 1998. Solid-phase microextraction and cuticular hydrocarbon differences related to reproductive activity in queenless ant Dinoponera quadriceps. J. Chem. Ecol. 24:473–490.
Peeters, C. 1991. The occurrence of sexual reproduction among ant workers. Biol. J. Linn. Soc. 44:141–152.
Peeters, C. 1993. Monogyny and polygyny in ponerine ants with or without queens, pp. 234–261, in L. Keller (ed.). Queen Number and Sociality in Insects. Oxford University Press, Oxford.
Peeters, C. and Crewe, R. 1984. Insemination controls the reproductive division of labour in a ponerine ant. Naturwissenschaften 71:50–51.
Peeters, C. and Crewe, R. 1985. Worker reproduction in the ponerine ant Ophthalmopone berthoudi: An alternative form of eusocial organization. Behav. Ecol. Sociobiol. 18:29–37.
Ratnieks, F. L. W., Foster, K. R., and Wenseleers, T. 2006. Conflict resolution in insect societies. Annu. Rev. Entomol. 51:581–608.
Sledge, M. F., Peeters, C. and Crewe, R. M. 2001. Reproductive division of labour without dominance interactions in the queenless ponerine ant Pachycondyla (=Ophthalmopone) berthoudi. Insect. Soc. 48:67–73.
Tay, W. T. and Crozier, R. H. 2000. Nestmate interactions and egg-laying behaviour in the queenless ponerine ant Rhytidoponera sp. 12. Insect. Soc. 47:133–140.
Tentschert, J., Kolmer, K., Hölldobler, B., Bestmann, H.-J., Delabie, J. H. C., and Heinze, J. 2001. Chemical profiles, division of labor and social status in Pachycondyla queens (Hymenoptera: Formicidae). Naturwissenschaften 88:175–178.
Tillman, J. A., Seybold, S. J., Jurenka, R. A., and Blomquist, G. J. 1999. Insect pheromones—an overview of biosynthesis and endocrine regulation. Insect Biochem. Mol. Biol. 29:481–514.
Tillman-Wall, J. A., Vanderwel, D., Kuenzli, M. E., Reitz, R. C., and Blomquist, G. J. 1992. Regulation of sex pheromone biosynthesis in the housefly, Musca domestica: Relative contribution of the elongation and reductive steps. Arch. Biochem. Biophys. 299:92–99.
Villet, M. 1991. Social differentiation and division of labour in the queenless ant Platythyrea schultzei Forel 1910 (Hymenoptera: Formicidae). Trop. Zool. 4:13–29.
Villet, M., Hart, A., and Crewe, R. 1990. Social organisation of Platythyrea lamellosa (Roger) (Hymenoptera: Formicidae) I. Reproduction. S. Afr. J. Zool. 25:250–253.
Ware, A. B., Compton, S. G., and Robertson, H. G. 1990. Gamergate reproduction in the ant Streblognathus aethiopicus Smith (Hymenoptera: Formicidae: Ponerinae). Insect. Soc. 37:189–199.
Wildman, M. H. and Crewe, R. M. 1988. Gamergate number and control over reproduction in Pachycondyla krugeri (Hymenoptera: Formicidae). Insect. Soc. 35:217–225.
Wilson, E. O. 1971. The Insect Societies. Harvard University Press, Cambridge, MA.
Acknowledgments
We are grateful to T. Lauwers for ant care and K. Collart for technical assistance. The Ministry of Environment and Energy granted permission to collect the ants in Costa Rica (resolution no. 131-2002-OFAU and 282-2003-OFAU). We also thank the Research Council of the University of Leuven (research project 01.24), the Fund for Scientific Research-Flanders (Belgium, research project G.0247.02), and the European Community's Improving Human Potential Programme (contract HPRN-CT-2000-00052 “INSECTS”) for financial support. This study complies with Belgian and Costa Rican law.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lommelen, E., Johnson, C.A., Drijfhout, F.P. et al. Cuticular Hydrocarbons Provide Reliable Cues of Fertility in the Ant Gnamptogenys striatula . J Chem Ecol 32, 2023–2034 (2006). https://doi.org/10.1007/s10886-006-9126-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9126-8