Abstract
Aminocyclopyrachlor is a persistent herbicide in soil; in the presence of organic matter, this persistence can be increased and can contribute to environmental contamination. The objective of this study was to verify the effects of organic materials derived from the sugarcane cultivation system on the mineralization and degradation of aminocyclopyrachlor in the soil. 14C-aminocyclopyrachlor was applied to soil with organic residues (sugarcane straw, filter cake and vinasse) and evaluated for 112 days of incubation. Mineralization was quantified by the weekly evolution of 14CO2, and the extracted, bound residues and the formation of metabolites of aminocyclopyrachlor were quantified. The mineralization was lower than 45% in all treatments. The degradation half-life (DT50) is being 187 days for unamended soil and > 240 days for soils amended with organic materials. For 90% of the herbicide to be degraded (DT90), between 622 and 921 days was required in all soils. Bound residues were formed in the soil (30% of the total applied in all treatments). No metabolites were formed during the whole incubation period, regardless of the treatment. The presence of organic materials in the soil increased the persistence of aminocyclopyrachlor in the soil, so that persistence of the herbicide may promote a residual effect on weeds and carryover in subsequent crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aminocyclopyrachlor is a preemergence herbicide, registered since 2010 in the USA (Lewis et al. 2013) and still in the process of being registered in Brazil. This herbicide binds weakly to the soil and therefore highly mobile on the soil profile (Oliveira Jr. et al. 2011; Francisco et al. 2017), promoting a high risk of groundwater contamination (Francisco et al. 2017). Aminocyclopyrachlor is a weak acid herbicide, leading to a low soil colloid sorption (Cabrera et al. 2012; Hall et al. 2015), making it more available to processes that can reduce the persistence of the molecule in the environment. However, even if the herbicide is not sorbed in the soil, the half-life dissipation time can be longer than 100 days (Finkelstein et al. 2008; Conklin and Lym 2013; Guerra et al. 2016; Francisco et al. 2018), being able to control weeds with residual effect for more than 6 months (Flessner et al. 2011).
Despite having low toxicity for mammals, aquatic invertebrates, fish, birds and being applied at low doses (35–315 g ai ha−1) (USEPA 2010) aminocyclopyrachlor has a high persistence on the environment. The persistence of the herbicide in the environment may contribute to the risk of environmental contamination. Its use requires caution, as it may affect subsequent crops, such as winter wheat (Kniss and Lyon 2011). The reported half-life (DT50) for aminocyclopyrachlor in soil was 72–128 days in bare ground field soil (Finkelstein et al. 2008), 22–126 days in studies provided by the manufacturer (USEPA 2010) and in uncovered soil reached up to 164 days (DuPont 2009). However, Westra et al. (2008) found that aminocyclopyrachlor was active for up to 2 years in the soil. This variation of the DT50, according to Conklin and Lym (2013), occurred due to a difference in temperature, humidity and components of each soil in particular. No report of DT50 of this herbicide has been found on tropical soils.
The microbiological pathway has been cited as one of the main routes of degradation of aminocyclopyrachlor in soil (Finkelstein et al. 2008; DuPont 2009). In this sense, the organic carbon content of the soil (OC), whether inherent to the soil or the addition of materials added to it, serves as an energy source for degrading microorganisms (Cox et al. 1997; Gómez et al. 2014). The addition of organic materials to the soil has been reported as an important factor in reducing herbicide persistence in soil (Fenoll et al. 2014a, b). However, the presence of organic materials may lead to a competition for energy source in the soil microbiota (Albarrán et al. 2003) or be responsible for the formation of bound residues of the herbicide (Prata and Lavorenti 2000), increasing the persistence of the herbicide in the environment. The addition of organic residues in the soil deserves attention regarding reported low toxicity for mammals, aquatic invertebrates and fish a the degradation of herbicides in the soil.
Studies reporting the relationship between organic carbon (OC) and behavior of aminocyclopyrachlor in the soil have focused only on aspects related to sorption and leaching. The addition of organic materials, such as bone char derived from cow bone, can increase the aminocyclopyrachlor sorption (Mendes et al. 2018), and also sugarcane straw can reduce the leaching potential in the soil profile (Silva et al. 2018). However, for the biodegradation and mineralization of aminocyclopyrachlor, there are no reports of the influence of organic materials. Since mineralization and degradation of aminocyclopyrachlor are low in soil (Francisco et al. 2018), organic waste may affect persistence of the herbicide in the environment. Further studies investigating this effect are needed for aminocyclopyrachlor.
Currently, aminocyclopyrachlor is in the process of being registered in Brazil for a tropical crop, such as sugarcane, but further research on the behavior of aminocyclopyrachlor under these conditions of cultivation system is required. Thus, the objective of this study was to evaluate the effect of the addition of organic materials, derived from the sugarcane cultivation system, into the soil on the mineralization and degradation processes of aminocyclopyrachlor.
Materials and Methods
Soil Sampling and Preparation
Soil samples were collected from the superficial layer (0–10 cm), with previous cleaning of the vegetal layer, in an experimental area of sugarcane, with no history of application of aminocyclopyrachlor, in the city of Piracicaba, SP, Brazil (22° 42′ 28″ S, 47° 37′ 50″ W). Subsequently, the soil samples were air-dried and sifted through a 2.0-mm mesh and stored. The physicochemical characteristics of the soil are presented in Table 2, in which it was classified as an Alfisol-Paleudult, “NITOSSOLO VERMELHO Eutroférrico típico” (Santos et al. 2018).
Organic Materials from the Sugarcane Cultivation System
The organic wastes were acquired from an agricultural experimental area and from a sugarcane mill in Piracicaba, SP, Brazil. The properties of these materials are presented in Table 2. Soil treatments consisted of an unamended soil (US), soil amended with sugarcane straw (12 t ha−1) (ASS), soil amended with filter cake (90 t ha−1) (ASFC) and soil amended with vinasse (200 m3 ha−1) (ASV). The amount of the materials was determined considering the soil density of 1.2 g dm3, according to the amount of material per ha. For the addition of the straw, the recommendations of the cultivation system reported by Leal et al. (2013) were followed, and the average recommendation of filter cake and vinasse by Raij et al. (1996) was followed. The physicochemical properties of the soil with and without addition of the organic materials are presented together in Table 1.
Aminocyclopyrachlor
The 14C-radiolabeled herbicide (pyrimidine-2-14C-aminocyclopyrachlor), provided by DuPont (Wilmington, DE, EUA), has a 99.5% radiochemical purity and a specific activity of 1.57 MBq mg−1. The working solution was prepared with a mixture of 14C-aminocyclopyrachlor in 0.01 M CaCl2 at 0.79 μg mL−1 (1222.37 Bq mL−1).
Biodegradation and Mineralization Studies
The biodegradation and mineralization studies were adapted from a guideline established by OECD-307 “Aerobic and Anaerobic Transformation in Soil” (OECD 2002). The experimental design was completely randomized, containing four treatments (soil without alteration, soil modified with straw, filter cake and sugarcane vinasse), in duplicate. Each experimental unit consisted of a biometric vial (250 mL) fitted with a side tube containing 10 mL of sodium hydroxide (0.2 N NaOH) as a carbon trap in the form of carbon dioxide for the collection of 14CO2 evolved with the process of mineralization of the herbicide in the soil (Fig. 1).

Source: Adapted of Dias (2012)
Schematic model of the biometric flask used in the biodegradation and mineralization studies of aminocyclopyrachlor in unamended soil (50 g dry base) and soil amended with organic residues from the sugarcane system.
In each biometric vial, 50 g of air-dried soil was added with moisture adjusted to the field capacity of 75% with deionized water. The amount of water needed to reach half the soil field capacity was added, and the preincubation was performed in a dark room at 20 ± 2 °C for 7 days in order to reactivate the soil microbiota. After this period, the rest of the water was added for the beginning of the study: 200 μL was applied to the working solution per biometric bottle. The soils were homogenized with the aid of a spatula, and the vials were closed with blotting caps coupled to a soda lime filter, so there was no atmospheric CO2 exchange during the biodegradation and mineralization analysis. To quantify the mineralization of the herbicide, aliquots of 1 mL of NaOH were collected every 7 days during the period after application of the product, up to 112 days after herbicide application (DAA). The total volume of the solution was replaced with new solution for each collection. Each sample was added with 10 mL scintillation solution in scintillation flasks, and the radioactivity was quantified for 10 min in a liquid scintillation spectrometer (LSS) by a Tri-Carb 2910 TR meter (LSA PerkinElmer, Waltham, MA, EUA).
The biodegradation of 14C-aminocyclopyrachlor was determined by quantifying the extracted residue (residue obtained after solvent extraction) and the bound residue (any residue that remains in the soil even after solvent extraction) formed throughout the incubation period. Extractions were performed at 0, 7, 14, 28, 56 and 112 DAA. In each extraction, the soil samples were removed from the biometric vials and passed into Teflon tubes (250 mL) with 100 mL of acetonitrile/water extracting solution, acidified with formic acid (0.2%) (80:20 v/v) and then taken to ultrasound (Branson 2510 ultrasonic, Marshall Scientific, Hampton, NH, USA) at 60 °C for 30 min (Nanita et al. 2009). The samples were centrifuged (Hitachi CF16RXII centrifugal, Hitachi Koki Co., Ltd., Indaiatuba, SP, Brazil) for 10 min at 7000 rpm. The same procedure was performed a second time with the same extraction solution and a third time with 100 ml of the solution of acetonitrile/ammonium acetate (0.15 mol L−1) (70:30 v/v) (Francisco et al. 2018).
The supernatant removed after each extraction was packed into a Schott’s flask (500 mL), to check the amount of the total extracted liquid. Two aliquots of 1 mL of each sample were taken from the flasks after extraction, and 10 mL of scintillator solution was added for the measurement of the radioactivity in LSS for 5 min. The remaining extracted liquid was concentrated under vacuum conditions at 40 °C in a rotoevaporator (vacuum controller V-850, Rotavapor R-215, heating bath B-491, Buchi Brasil Ltda, Valinhos, SP, Brazil), for the application in plates of thin layer chromatography (TLC), that will be described later. The remaining soils in the Teflon tubes were oven-dried (40 °C) and then milled and homogenized in a mechanical mill (Marconi MA330, Piracicaba, SP, Brazil). The bound residues were quantified by burning the soil samples in a biological oxidizer for 3 min (OX500, R.J. Harvey Instrument Corporation, Tappan, NY, EUA), using aliquots of 0.2 g (in triplicate) of soil from each previously homogenized sample. The oxidation transformed all 14C contained in the soil sample into 14CO2, which was fixed in 10 mL of scintillation cocktail, already in the scintillation vials, and then was taken to the LSS, and the radioactivity was quantified for 5 min.
Metabolite Formation Analysis by Thin Layer Chromatography (TLC)
The TLC analysis followed the methodology proposed by the US Environmental Protection Agency method 507 (EPA 1998; Fried and Sharma 1999). On silica plates (20 × 20 cm, 60F254, EMD Millipore), previously activated at 250 °C for 2 h, with the aid of electron microspheres (Hamilton PB6000 Dispenser, Hamilton, CO, EUA), 0.1 mL aliquots of the concentrate extracted from each sample and the analytical standard of 14C-aminocyclopyrachlor were applied. The elution of the plates was adapted from Bell et al. (2011), which consisted of a 100-mL solution of methanol/isopropanol/ethyl acetate/water/acetic acid (70:10:10:9:1 v/v).
The plates, after elution and drying at room temperature, were placed under phosphorescent film, for 24 h, for the sensitization and elaboration of autoradiography images. The images were generated on a radio scanner (Packard Cyclone—PerkinElmer, Shelton, CT, EUA). For each sample, the solvent front (elution limit) and the distance covered by aminocyclopyrachlor on the plate were used to calculate the retention factor (Rf).
Model of Degradation of Aminocyclopyrachlor
The 14CO2 values derived from the degradation of 14C-aminocyclopyrachlor were adjusted in the first-order kinetic model: C = C0 e−kt, where C is the aminocyclopyrachlor concentration at time t (%); C0 is the initial concentration of aminocyclopyrachlor (days−1); and t is the incubation time (days). DT50 and DT90 were determined using the following equations: DT50 = ln 2/k and DT90 = ln 10/k, where DT50 and DT90 determine the time at which 50 and 90% of the herbicide, respectively, were degraded from the herbicide applied initially.
Statistical Analysis of Data
The 14CO2, DT50 and DT90 values of aminocyclopyrachlor, as well as the values of accumulated mineralization, extracted residue and bound residue, using the mean and standard deviation of the mean (n = 2), were plotted using SigmaPlot (version 10.0 for Windows, Systat Software Inc., Point Richmond, CA, EUA).
Results and Discussion
Mass Balance
The mass balance of 14C-aminocyclopyrachlor (sum of mineralized total, extracted residue and bound residue), considering the entire period after application of the herbicide (0–112 days), was 95.35–108.41% for the soil without addition of organic material, 98.4–108.89% for the addition of straw, 93.44–108,68% for filter cake and 100.42–108% for sugarcane vinasse. The values are in accordance with the values recommended by the OECD (2002), of 90–110%, suggesting an adequate recovery of the herbicide.
Aminocyclopyrachlor Mineralization, Extract and Bound Residues in Soil Amended with Organic Materials Derived from the Sugarcane System
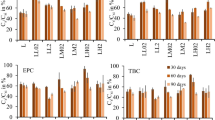
Total accumulation values of 14CO2 were found in the range of 36.84–44.27% for treatments with and without addition of organic materials to the soil, in descending order of ASS > US > ASFC > ASV (Fig. 2). In soils without addition of organic material, Francisco et al. (2018) found less than 10% of mineralization for aminocyclopyrachlor at the end of 126 DAA. Durkin (2012) also found lower values than those obtained in the present study, with 23.1% of 14CO2 accumulated at aminocyclopyrachlor 360 DAA, indicating a low mineralization rate of herbicide in soils. However, the presence of straw in the soil can promote improvements in microbiological activity, through the availability of water, temperature and favorable substrates (Souza et al. 2012; Araújo et al. 2013), possibly resulting in greater microbiological activity and mineralization of aminocyclopyrachlor when this organic material was present in this study, in relation to the other treatments.
Distribution of 14C-aminocyclopyrachlor in unamended soil (a), and soil amended with sugarcane straw (b), filter cake (c), and vinasse (d) between extractable residue, bound residues, and mineralized 14CO2 (%) during the 112 days of incubation of aminocyclopyrachlor. Vertical bars represent the standard deviations (± SD) of means (n = 2)
The mineralization of aminocyclopyrachlor was more pronounced at 28 DAA, with accumulation values of 14CO2 of 3.05% for unchanged soils, 2.27% for the addition of straw and 1.90% for the addition of filter cake (Fig. 2). However, there was no lag phase (phase of adaptation of the microbiota without any microbiological activity, mineralization and degradation of the herbicide), since there was mineralization from the beginning of incubation, even if low. On the other hand, Francisco et al. (2018) found a lag phase of 28 DAA for aminocyclopyrachlor in soils with lower OC content (0.6–1.7%) compared to this study (1.74–4.82%) (Table 2).
In soil with vinasse addition, only 0.62% accumulation of 14CO2 was verified up to 35 DAA, with mineralization rates greater than 2% only at 42 DAA and of 7.7% at 112 DAA (Fig. 2). It is known that with the presence of vinasse in the soil there is an increase in the microbiological activity of the soil (Camargo 1954; Santos et al. 2009; Yang et al. 2013). On the other hand, a change or reduction in the diversity of microorganisms may have occurred in the soil, interfering with the mineralization process of the herbicide, reducing its activity during the entire incubation period. Santos et al. (2009) observed this effect of decreased soil microbiota with the addition of sugarcane vinasse, and Tejada et al. (2007) verified the same effect for beet vinasse in the soil.
At 112 DAA, higher amounts of extracted residues of aminocyclopyrachlor from soil were found when vinasse was added (77.31% of 14C), higher than in the other treatments (Table 3 and Fig. 2). The extracted residue is the amount of 14C-aminocyclopyrachlor removed from the soil by chemical extraction. It represents the fraction of the most bioavailable product for the processes of absorption by plants, sorption, leaching and soil degradation. In this sense, the results show that the residues of aminocyclopyrachlor indicated a greater availability in the soil when in the presence of vinasse.
As complementary information for the extracted residue, the bound residue indicates that the amount of herbicide that has not been removed by chemical extraction probably is not available to short-term soil retention, transport and degradation processes. The values of 35.63%, 33.05%, 32.01% and 27.60% of bound residues in the soils with filter cake, straw, vinasse and unamended soil were obtained, respectively, at 112 DAA (Fig. 2). This agrees with the results found by Francisco et al. (2018), with bound residues of aminocyclopyrachlor in the range of 22.5–41.1%, on three distinct soils. From 28 DAA, ~ 20% bound residues of aminocyclopyrachlor had already been formed in all treatments. The formation of bound residues in the soil may be directly associated with chemical reactions between the herbicide and the active surfaces of minerals and soil organic matter (Gevao et al. 2000). In the form of residue bound to soil, the herbicide will be less available for dissipation processes and the molecule will be present in the environment.
The formation of bound residues of aminocyclopyrachlor was little associated with soil sorption of the herbicide, since its sorption is low (Oliveira Jr. et al. 2011, 2013; Francisco et al. 2017). However, Oliveira Jr. et al. (2011) reported a positive correlation of aminocyclopyrachlor sorption with soil OC. In this case, even if low sorption, the OC can act in the formation of bound residues in the presence of organic materials. According to Lehmann and Kleber (2015), bound residues are hardly distinguishable from organic matter. Part of the herbicide and its metabolites can be incorporated into the microbial biomass, and after death, these cells become part of the organic matter of the soil, often in the form of bound residues (Kästner et al. 2014). Further studies regarding the nature of the interaction and the formation of bound residues of aminocyclopyrachlor with organic materials are necessary to understand this.
Aminocyclopyrachlor Biodegradation in Soil Amended with Organic Materials Derived from the Sugarcane System
The biodegradation of aminocyclopyrachlor was verified from the data already reported on the percentage of 14C-extracted residues after 112 DAA. All altered and unchanged soils with organic materials were significant and adequately adjusted to the first-order kinetic model, according to the coefficient of determination (R2 > 0.84) (Table 3).
The DT50 value found for aminocyclopyrachlor was lower for the soil unaltered (187.34 days) than for soils altered with organic materials (247.55–277.26 days) (Table 3). For soils without amendments, Finkelstein et al. (2008) reported DT50 values of 72–128 days for aminocyclopyrachlor. In addition, the DT50 range was 22–126 days (USEPA 2010) for this herbicide in American soils. The increase in the persistence of aminocyclopyrachlor found in soils altered with organic materials indicates the importance that organic matter (OM) has in this process. Soil cover and OC content can also influence this process. This was verified by Finkelstein et al. (2008), who showed that in soil covered with lawn, the DT50 value reached 80–164 days. Moreover, Guerra et al. (2016), by means of a bioassay, observed that the persistence of aminocyclopyrachlor was greater than 150 days in soils with high OM content (12.28–18.42%).
DT90 was greater than 600 days in soil without the addition of OM (Table 3). However, with the addition of OM, these values were 300–400 days longer than in soil without change. The persistence of aminocyclopyrachlor was 1.48 times greater than the herbicide in soils altered with OM derived from sugarcane in relation to the soil without change.
Thin Layer Chromatography of Aminocyclopyrachlor in Soil with Organic Materials Derived from the Sugarcane System
Throughout the evaluation period (112 DAA), no metabolite was found in any treatment. In Fig. 3, the chromatograms of the extraction at 112 DAA are presented, and these are representative for all times, due to the non-formation of metabolites at any of these evaluated times. These chromatograms indicated the same retention factor (Rf = 0.6) of aminocyclopyrachlor for soils with and without change. Further research is needed on the formation of metabolites of aminocyclopyrachlor in soil and their availability in the environment.
Conclusion
This was the first study to evaluate the degradation of aminocyclopyrachlor in soils altered with organic materials derived from the sugarcane cultivation system. The addition of organic materials to the soil contributed to the increase in herbicide persistence, with no large increases in herbicide mineralization, but rather with large formation of soil-bound residues. It was verified that the presence of vinasse can be detrimental to the mineralization of aminocyclopyrachlor in the soil. The half-life of the herbicide was prolonged with higher OC content of the soil and with great formation of bound residue. The presence of metabolites in the residues extracted from aminocyclopyrachlor in the soil was not verified. These results emphasize the importance of studies on the degradation of herbicides in the environment, especially those that are directly applied to the soil, and suffer more interferences of the physicochemical properties of this matrix, such as soil OC. With the presence of aminocyclopyrachlor in the soil as a bound residue, part of the herbicide may become unavailable in the soil solution, reducing the effective chemical control of weeds.
References
Albarrán, A., R. Celis, M.C. Hermosín, A. López-Piñeiro, J.J. Ortega-Calvo, and J. Cornejo. 2003. Effects of solid olive-mill waste addition to soil on sorption, degradation and leaching of the herbicide simazine. Soil Management 19 (2): 150–156.
Araújo, A.S.F., S. Cesarz, L.F.C. Leite, C.D. Borges, S.M. Tsai, and N. Eisenhauer. 2013. Soil microbial properties and temporal stability in degraded and restored lands of Northeast Brazil. Soil Biology & Biochemistry 66: 175–181.
Bell, J.L., I.C. Burke, and T.S. Prather. 2011. Uptake, translocation and metabolism of aminocyclopyrachlor in prickly lettuce, rush skeletonweed and yellow starthistle. Pest Management Science 67 (10): 1338–1348.
Cabrera, A., C. Trigo, L. Cox, R. Celis, M.C. Hermosin, J. Cornejo, and W.C. Koskinen. 2012. Sorption of the herbicide aminocyclopyrachlor by cation-modified clay minerals. European Journal of Soil Science 63 (5): 694–700.
Camargo, R. 1954. O desenvolvimento da flora microbiana nos solos tratados com vinhaça. Piracicaba: Instituto Zimotécnico.
Conklin, K.L., and R.G. Lym. 2013. Effect of temperature and moisture on aminocyclopyrachlor soil half-life. Weed Technology 27 (3): 552–556.
Cox, L., R. Celis, M.C. Hermosin, A. Becker, and J. Cornejo. 1997. Porosity and herbicide leaching in soils amended with olive-mill wastewater. Agriculture, Ecosystems & Environment 65 (2): 151–161.
Dias, A.C.R. 2012. Lixiviação, mobilidade, degradação, mineralização e atividade microbiana de herbicidas em função de atributos de cinco tipos de solos. Teses USP. http://www.teses.usp.br/teses/disponiveis/11/11136/tde-17082012-081428/pt-br.php. Accessed 26 January 2019.
Dupont. 2009. Dupont DPX MAT28 herbicide. Technical Bulletin K-15023. Wilmington, DE: Dupont. https://lists.alaska.edu/pipermail/cnipm-l/attachments/20090310/f10dfb94/MAT28TechBulletin.pdf. Accessed 2 February 2019.
Durkin, P.R. 2012. Aminocyclopyrachlor: Human health and ecological risk assessment. Final Report. 226.
Environmental Protection Agency-EPA. 1998. Soil thin layer chromatography. EPA-Fate transport and transformation test guidelines-OPPTS 835.1210. Washington D.C.: EPA.
Fenoll, J., I. Garrido, P. Hellín, P. Flores, N. Vela, and S. Navarro. 2014a. Use of different organic wastes in reducing the potential leaching of propanil, isoxaben, cadusafos and pencycuron through the soil. Journal of Environmental Science and Health Part B 49 (8): 601–608.
Fenoll, J., N. Vela, G. Navarro, G. Pérez-Lucas, and S. Navarro. 2014b. Assessment of agro-industrial and composted organic wastes for reducing the potential leaching of triazine herbicide residues through the soil. Science of the Total Environment 493: 124–132.
Finkelstein, B.L., G.R. Armel, S.A. Bolgunas, D.A. Clark, J.S. Claus, R.J. Crosswicks, C.M. Hirata, G.J. Hollingshaus, M.K. Koeppe, P.L. Rardon, V.A. Wittenbach, and M.D. Woodward. 2008. Discovery of aminocyclopyrachlor (proposed common name) (DPX-MAT28): a new broad-spectrum auxinic herbicide. In Proceedings of the 236th American Chemical Society National Meeting. Philadelphia, PA.
Flessner, M.L., J.S. McElroy, and R. Wehtje. 2011. Quantification of warm-season turfgrass injury from triclopyr and aminocyclopyrachlor. Weed Technology 25 (3): 367–373.
Francisco, J.G., K.F. Mendes, R.F. Pimpinato, V.L. Tornisielo, and A.C.D. Guimarães. 2017. Aminocyclopyrachlor sorption–desorption and leaching from three Brazilian soils. Journal of Environmental Science and Health Part B 52 (7): 470–475.
Francisco, J.G., K.F. Mendes, R.F. Pimpinato, V.L. Tornisielo, and A.C.D. Guimarães. 2018. Soil factors effects on the mineralization, extractable residue, and bound residue formation of aminocyclopyrachlor in three tropical soils. Agronomy 8 (1): 1–10.
Fried, B., and J. Sharma. 1999. Thin-layer chromatography, 4th ed. New York: Basel.
Gevao, B., K.T. Semple, and K.C. Jones. 2000. Bound pesticide residues in soils: A review. Environmental Pollution 108 (1): 3–14.
Gómez, I., B. Rodríguez-Morgado, J. Parrado, C. García, T. Hernández, and M. Tejada. 2014. Behavior of oxyfluorfen in soils amended with different sources of organic matter. Effects on soil biology. Journal of Hazardous Materials 273: 207–214.
Guerra, N., R.S. Oliveira Jr., J. Constantin, A.M. Oliveira Neto, A. Gemelli, D.M. Pereira Jr., and A. Guerra. 2016. Persistence of biological activity and leaching potential of herbicides aminocyclopyrachlor and indaziflam in soils with different textures. Planta Daninha 34 (2): 345–356.
Hall, K.E., C. Ray, K.A. Spokas, and W.C. Koskinen. 2015. Pesticide sorption and leaching potential on three Hawaiian soils. Journal of Environmental Management 159: 227–234.
Kästner, M., K.M. Nowak, A. Miltner, S. Trapp, and A. Schäffer. 2014. Classification and modelling of nonextractable residue (NER) formation of xenobiotics in soil—A synthesis. Critical Reviews in Environmental Science and Technology 44 (19): 2107–2171.
Kniss, A.R., and D.J. Lyon. 2011. Winter wheat response to preplant applications of aminocyclopyrachlor. Weed Technology 25 (1): 51–57.
Leal, M.R.L., M.V. Galdos, F.V. Scarpare, J.E. Seabra, A. Walter, and C.O. Oliveira. 2013. Sugarcane straw availability, quality, recovery and energy use: A literature review. Biomass and Bioenergy 53: 11–19.
Lehmann, J., and M. Kleber. 2015. The contentious nature of soil organic matter. Nature 528: 60–68.
Lewis, D.F., M.D. Jeffries, H.J. Strek, R.J. Richardson, and F.H. Yelverton. 2013. Effect of ambient moisture on aminocyclopyrachlor efficacy. Weed Technology 27 (2): 317–322.
Mendes, K.F., K.E. Hall, V. Takeshita, M.L. Rossi, and V.L. Tornisielo. 2018. Animal bonechar increases sorption and decreases leaching potential of aminocyclopyrachlor and mesotrione in a tropical soil. Geoderma 316: 11–18.
Nanita, S.C., A.M. Pentz, J. Grant, E. Vogl, T.J. Devine, and R.M. Henze. 2009. Mass spectrometric assessment and analytical methods for quantitation of the new herbicide aminocyclopyrachlor and its methyl analogue in soil and water. Analytical Chemistry 81 (2): 797–808.
Organisation for Economic Co-operation and Development-OECD. 2002. Guidelines for testing of chemicals: Aerobic and anaerobic transformation in soil. 17 p. (Test 307). Paris: OECD.
Oliveira Jr., R.S., D.G. Alonso, and W.C. Koskinen. 2011. Sorption–desorption of aminocyclopyrachlor in selected Brazilian soils. Journal of Agricultural and Food Chemistry 59 (8): 4045–4050.
Oliveira Jr., R.S., D.G. Alonso, W.C. Koskinen, and S.K. Papiernik. 2013. Comparative sorption, desorption and leaching potential of aminocyclopyrachlor and picloram. Journal of Environmental Science and Health Part B 48 (12): 1049–1057.
Prata, F., and A. Lavorenti. 2000. Comportamento de herbicidas no solo: influência da matéria orgânica. Revista Biociências 6 (2): 17–22.
Raij, B.V., H. Cantarella, J.A. Quaggio, and A.M.C. Furlani. 1996. Recomendações de adubação e calagem para o Estado de São Paulo, 2nd ed. Campinas: Instituto Agronômico e Fundação IAC.
Santos, H.G., P.K.T. Jacomine, L.H.C. Anjos, V.A. Oliveira, J.F. Lumbreras, M.R. Coelho, J.A. Almeida, J.C. Araujo Filho, J.B. Oliveira, and T.F.J. Cunha. 2018. Sistema de Classificação de Solos, 5th ed. Brasília: Embrapa.
Santos, T.M.C., M.A.L. Santos, C.G. Santos, V.R. Santos, and D.S. Pacheco. 2009. Efeito da fertirrigação com vinhaça nos microrganismos do solo. Revista Caatinga 22 (1): 155–160.
Silva, G.S., A.F.M. Silva, K.F. Mendes, R.F. Pimpinato, and V.L. Tornisielo. 2018. Influence of sugarcane straw on aminocyclopyrachlor leaching in a green-cane harvesting system. Water, Air, and Soil pollution 229: 2–7.
Souza, R.A., T.S. Telles, W. Machado, M. Hungria, J. Tavares Filho, and M.F. Guimarães. 2012. Effects of sugarcane harvesting with burning on the chemical and microbiological properties of the soil. Agriculture, Ecosystems & Environment 155: 1–6.
Tejada, M., J.L. Moreno, M.T. Hernandez, and C. Garcia. 2007. Application of two beet vinasse forms in soil restoration: Effects on soil properties in an arid environment in southern Spain. Agriculture, Ecosystems & Environment 119 (3–4): 289–298.
United States Environmental Protection Agency-USEPA. 2010. Registration of the new active ingredient aminocyclopyrachlor for use on non-crop areas, sod farms, turf, and residential laws. Washington, DC: Office of Pesticide Programs, Registration Division. https://www.regulations.gov/document?D=EPA-HQ-OPP-2009-0789-0014. Accessed 25 May 2019.
Westra, P., S. Nissen, T. Gaines, B. Bekun, B. Lindenmayer, and D. Shaner. 2008. Aminocyclopyrachlor for invasive weed management and restoration grass safety in the central great plains. Proceedings North Central Weed Science Society 63: 203.
Yang, S.D., J.X. Liu, J. Wu, H.W. Tan, and Y.R. Li. 2013. Effects of vinasse and press mud application on the biological properties of soils and productivity of sugarcane. Sugar Tech 15 (2): 152–158.
Acknowledgements
The authors would like to thank the Foundation for Research Support of the State of São Paulo (FAPESP), Process 2017/20402-7 and the Coordination of Improvement of Higher Education Personnel—Brazil (CAPES)—Financing Code 001, for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Takeshita, V., Mendes, K.F., Junqueira, L.V. et al. Quantification of the Fate of Aminocyclopyrachlor in Soil Amended with Organic Residues from a Sugarcane System. Sugar Tech 22, 428–436 (2020). https://doi.org/10.1007/s12355-019-00782-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00782-1