Abstract
The mycelia of two wood decay basidiomycete fungi were grown opposing each other across a 1-μm pore membrane supported on the surface of malt broth, contained within a sealable reaction vessel. Production of volatiles during the time course of interaction was followed by collecting head space samples by solid phase microextraction (100 μm polydimethylsiloxane fiber) on five occasions over 25 d following coinoculation of the fungi: 1, 3 (i.e., immediately prior to mycelial contact), 9 (1–2 d after initiation of pigment production by Resinicium bicolor), 17, and 25 d. Ten volatiles were produced during interactions that were not detected in single species controls. In general, most (18) fungal volatiles were sesquiterpenes eluted between 12.5 and 21 min, with a further two eluted at 29.1 and 33.9 min; a benzoic acid methyl ester, a benzyl alcohol, and a quinolinium type compound with a distinctive fragmentation pattern at m/z 203, 204, 206, and 207 were also identified; three volatiles with m/z maxima of 163, 159, and 206–208, respectively, remained unidentified. The results are discussed in relation to possible ecological roles of volatiles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During growth through organic resources and soil, basidiomycete mycelia frequently encounter mycelia of other fungi, both of the same and of different species (Rayner and Boddy, 1988; Boddy, 2000). When basidiomycete mycelia meet, recognition as “non-self” (both inter- and intraspecifically) results in antagonistic responses (unless the mycelia are mating compatible) accompanied by changes in morphology in the interaction zone (and often elsewhere) and in production of extracellular enzymes, volatile and diffusible secondary metabolites, often visually manifested as bright pigments in mycelium and culture substratum (Rayner and Boddy, 1988; White and Boddy, 1992; Griffith et al., 1994a,b,c; Score et al., 1997). Mycelial growth is often stopped, reduced, or stimulated, and such responses are often induced prior to contact (Schoeman et al., 1996; Wheatley et al., 1997; Savoie, 2001; Humphris et al., 2002; Wald et al., 2004a,b), but also following contact, and in the case of intraspecific interactions, following hyphal fusion (Rayner and Boddy, 1988; Boddy, 2000). Responses occurring prior to contact are likely to be induced by diffusible and/or volatile organic compounds (VOCs) depending on the combination of interacting species (Rayner et al., 1994; Heilmann-Clausen and Boddy, 2005). In this paper, we concentrate on VOCs.
VOCs have been detected from fungal fruit bodies (Fäldt et al., 1999), fungal-spoiled food (de Lacy Costello et al., 2001; Demyttenaere et al., 2004), and fungi within buildings (Korpi et al., 1999; Ewen et al., 2004). VOCs produced by basidiomycetes and ascomycetes include alcohols, terpenes, aldehydes, ketones, sesquiterpenes, and aromatic compounds (Sunesson et al., 1995; Wheatley et al., 1997; Korpi et al., 1999; Rösecke and König, 2000; Rösecke et al., 2000; Abraham, 2001; Wheatley, 2002). Many sesquiterpenes from plants (e.g., Cakir et al., 2005; Cheng et al., 2005; Wang et al., 2005) and fungi (e.g., Florianowicz, 2000; Abraham, 2001; Roy et al., 2003) appear to have antifungal properties, and VOCs produced by some fungi have been considered as potential biocontrol agents in timber preservation (Schoeman et al., 1996; Wheatley et al., 1997). Some sesquiterpenes may be involved in defense responses against fungivores (Wiens et al., 1991; Stadler and Sterner, 1998).
Most studies have investigated the identities of VOCs produced as a snapshot at a single time point; however, the VOC profile can change over time (Sunesson et al., 1995; Nilsson et al., 1996; Korpi et al., 1999). For example, volatile production by Penicillium growing in pure culture reached a peak after 4–5 d (after normalization for colony size; (Nilsson et al., 1996). When mycelia are interacting, production of VOCs is likely to change considerably during different stages of the interaction. The current study follows VOC production over the time course of mycelial interaction between two wood decay basidiomycetes, Hypholoma fasciculare and Resinicium bicolor. Although the latter is most common in coniferous woodlands, they are both found in angiosperm woodland, where their mycelia could potentially interact while growing across soil or within wood (Kirby et al., 1990; Boddy, 2001).
Sampling was timed to coincide with different morphological manifestations of interaction: on agar, growth usually slows following hyphal contact, red/brown pigment is produced by R. bicolor in the interaction zone, while H. fasciculare forms cords following mycelial contact that produce a yellow pigmentation approximately 10 d later, ultimately with slow mutual replacement (Fig. 1). The following hypotheses were tested: (1) VOCs are produced prior to mycelial contact, initiating an early defense response; (2) VOC production is linked to the production of pigments during interspecific interaction; (3) VOC production increases over the course of the interaction independently of colony size; and (4) interspecific interactions produce additional VOCs, which are otherwise not produced during self interactions.
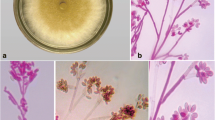
Resinicium bicolor interacting with Hypholoma fasciculare on 2% malt agar in a 9-cm Petri dish, 18 d after mycelial contact at 20°C. Resinicium bicolor is partially replacing H. fasciculare (R rep) and vice versa (H rep). Note that there is dark red/brown pigment production by R. bicolor (R), and yellow pigment production around cords of H. fasciculare (Y). Photograph courtesy of Tim Rotheray
One means of sampling headspace VOCs is to use solid phase microextraction (SPME) fibers. The fiber coatings have different properties for adsorption of different polarity compounds (Nilsson et al., 1996; Jeleń, 2003), and both polydimethylsiloxane (PDMS) and polyacrylate fibers have been used successfully to sample fungal VOCs (Nilsson et al., 1996; Fäldt et al., 1999; Jeleń, 2003; Demyttenaere et al., 2004; Ewen et al., 2004). SPME PDMS sampling, combined with separation and identification by GC/MS, was used to monitor the production of VOCs emitted from interactions of H. fasciculare and R. bicolor grown over 0.5% malt extract broth.
Methods and Materials
Fungi
Cultures of H. fasciculare (HF-DD2) and R. bicolor (RB1), from the Cardiff University Fungal Ecology Group culture collection, were initially grown separately on 2% malt extract agar (Lab M MC23 malt extract, Lab M agar no. 2) in 9-cm nonvented Petri dishes at 20°C to provide fungal inoculum. Inocula comprised plugs of mycelium plus agar (10 mm diameter) cut from the margin of growing cultures with a no. 5 cork borer. Growth rates of the two species were similar; thus, in interaction systems (see below), both plugs were added at the same time.
Interaction Systems
Interactions took place in Reacsyn™ fermentation vessels (BioDiversity, Enfield, UK)—polypropylene bottles containing a porous polyester/polypropylene support at the base of an inner chamber (Fig. 2). The support sat in contact with the surface of the broth medium [30 ml of sterile malt extract broth (0.5%, Lab M MC23 malt extract)] contained in the outer chamber. A Fluropore™ membrane (1 μm pore size; Millipore, Watford, UK) was placed onto the vessel support, and fungal inocula were placed on or near the center (see below). The membrane, although not completely preventing growth into the culture medium, encouraged horizontal growth of the mycelium ensuring a physical interaction (similar to that observed on solid agar plates) and provided a stark white background against which any hyphal pigmentation could clearly be seen. The neck of the inner chamber was stoppered with a gas permeable polyurethane foam bung. The inner/outer chamber design allowed simple sampling of both the headspace (40 ml) and broth (30 ml) within an aseptic system.
Treatments
Five treatments were prepared in triplicate, two single plugs of each isolate, two self pairings, and one interspecific interaction: (1) a single H. fasciculare plug inoculated centrally (HF), (2) two H. fasciculare plugs placed 10 mm apart (HFHF), (3) a single R. bicolor plug inoculated centrally (RB), (4) two R. bicolor plugs placed 10 mm apart (RBRB), and (5) one H. fasciculare plug placed 10 mm away from a single R. bicolor plug (HFRB). The vessels were placed in their commercial racking system and incubated in the dark at 20 ± 0.5°C.
Sampling of Volatiles
Volatiles were collected by SPME (100 μm PDMS fiber, Supelco, Poole, Dorset, UK). Guided by a small preliminary experiment, sampling took place 1, 3, 9, 17, and 25 d after inoculation. The first sample of the volatiles, the baseline, was taken the day after inoculation, allowing the dispersal of any compounds produced or released as a result of mycelial severance. The 3-d sample was prior to mycelial contact with about 1.5–2-mm distance between the colonies. Resinicium bicolor started to produce pigment at 7–8 d; thus, pigment production was initiated 1–2 d prior to sampling at 9 d. The last two time samplings represented later stages of the interactions. The SPME sampling process was as follows: the SPME fiber (preconditioned according to the manufacturers’ instructions) was briefly conditioned at the start of sampling each day (250°C, 15 min) and a fiber blank was then run. The inoculated Reacsyn vessels were flushed to clear any build up of fungal volatiles and plasticizer by placing in a sterile laminar flow system with the foam bung/cap system removed for 10 min (deemed suitable following preliminary trials). The cap was then replaced and the vessel was removed from the flow. The SPME fiber was pushed through the bung and exposed to the headspace of the inner chamber for 1 h. The SPME fiber was withdrawn and immediately injected into the GC/MS system.
GC/MS Analysis
Volatiles were analyzed by GC (GC8000, Thermofinnigan, Hemel Hempstead, UK) and MS (MD 800, Thermofinnigan). Samples were desorbed at 220°C in a split/splitless injection port in splitless mode for 2 min and resolved over 30 m VF23ms (Varian, Palo Alto, CA, USA) polar column (0.25 mm ID, 0.25 μm film thickness), with helium carrier gas (55 KPa). The temperature program was as follows: start 45°C, increasing at 3°C/min to 200°C, then held at 200°C for 5 min. Mass spectra were recorded in electron ionization mode from m/z = 35 to 400, with a source temperature of 200°C and interface at 280°C.
Fiber blanks were used to check for any signs of contamination. Control Reacsyn vessels containing malt broth but no fungus were tested by SPME for any exchange of volatiles between the vessels being incubated together. Putative identifications were made by comparing mass spectra with library entries (National Institute of Standards and Technology v2.1 mass spectral database). An absolute identification would have required preparation and/or provision of individual reference compounds and was beyond the scope of this project. Peaks of interest were integrated by using Masslab v1.4 (Thermofinnigan). Prior to statistical analysis, peak areas for each time point were normalized against dibutylbenzene (plasticizer signal, RT 11.9), which remained constant across all samples throughout the time course of the experiment. Peaks found in only one replicate of a treatment were reported but considered artifacts and were not included in further analysis. The evolution of each volatile (as peak area, in standard equivalent units, over time) was compared by using one-way analysis of variance or two-sample t-test (Minitab v13) by comparison of area under the curve determined by KaleidaGraph software (Synergy Software, Reading, PA, USA).
Results
Visible Changes
Mycelial morphology changed after mycelial contact. Red/brown pigment was produced beneath mycelium of R. bicolor between 3 and 9 d, and yellow pigment developed in H. fasciculare cords between 9 and 17 d.
Between Treatment Differences in Volatiles
Replicates within each treatment were generally consistent (and consistent with the preliminary experiment), although one replicate from the interspecific interaction produced the same volatile peaks as the other two replicates but at lower concentrations. In total, 25 fungal metabolites were detectable in the five treatments (Fig. 3). The interspecific interaction produced 12 volatiles that were not found in the single species samples (peaks 1–4, 6, 7, 9, 14, and 21–23), and additionally three (peaks 8, 12, and 17) were considered to be produced only during interspecific interaction because they were present only in a trace amount in a single replicate of the single-species samples. Four peaks were common to the interspecific interaction and R. bicolor samples (peaks 5, 11, 16, and 19), three to the interspecific interaction and H. fasciculare (peaks 10, 24, and 25), one peak was unique for R. bicolor (peak 13), and three peaks (peaks 15, 18, and 20) were identified in all treatments. Peak 20 was produced in significantly higher concentration by R. bicolor than H. fasciculare (P < 0.05). An additional peak (peak 23), although only present in a single replicate of the interspecific interaction (suggesting it was not a consistently produced volatile), was included in the total peak count, as it was putatively identified as a sesquiterpene. The majority of the metabolites produced were determined to be sesquiterpenes eluting between 12.5 and 21 min (peaks 1–17). Two further sesquiterpenes eluted at 29.1 and 33.9 min (peaks 19 and 23). Two sets of diastereoisomers were putatively identified from mass spectral data, peaks 3, 5, 14, and 16 being γ-muurolene, γ-cadinene, γ-amorphene, and γ-bulgarene, and peaks 4, 11, 15, and 17 being α-muurolene, α-cadinene, α-amorphene, and α-bulgarene, although the order of elution of the isomers could not definitely be assigned due to the similarity of the mass spectra within each set. Peak 12, although a major peak, did not produce any matches in the database, so it was not identified. Of the remaining peaks, peak 18 appeared to be a benzoic acid methyl ester, peak 20 a benzyl alcohol, and peak 24 a quinolinium-type compound with a distinctive fragmentation pattern at m/z 203, 204, 206, and 207. The remaining peaks, peaks 21, 22, and 25, had m/z maxima of 163, 159, and 206–208, respectively, but could not be identified.
Representative GC/MS traces indicating fungal metabolites: (a) Hypholoma fasciculare v Resinicium bicolor (HFRB) interaction at 17 d; (b) R. bicolor (RBRB) alone at 17 d indicating position of the peak found in R. bicolor only. Peaks are numbered individually. An additional small peak (25) was found in both treatments at 49.1 min (not shown), BB is dibutylbenzene
In most cases, the production of specific volatiles was not affected by colony size (no significant differences found between single- and double-plug single-species treatments, P > 0.05). Peaks 11 and 16, however, were detected in the double- but not in the single-plug R. bicolor treatment, and peak 19 also indicates some effect of colony size—HFRB being significantly different (P ≤ 0.05) from RB but not RBRB (Fig. 4). Where volatiles were present in both the interspecific interaction and other treatments, up-regulation tended to occur, the area under the volatile evolution curve from the interspecific interaction being greater (P ≤ 0.05) than both single- and double-plug isolate samples for peaks 5 and 11 RBRB (Fig. 4). In the case of peaks 10, 15, and 16, the large amount of variability within the interspecific interaction, which was caused by the outlying response of replicate 1, resulted in a lack of significant difference between some samples (Fig. 4). No up-regulation occurred in the production of the metabolites corresponding to peaks 18, 20, 24, and 25.
Evidence of up-regulation of VOCs during interspecific interaction in those volatiles that were also present in single species inoculations: HF, Hypholoma fasciculare inoculated singly; HFHF, H. fasciculare inoculated as two plugs; HFRB, H. fasciculare v Resinicium bicolor; RB, R. bicolor inoculated singly; RBRB, R. bicolor inoculated as two plugs. Values are means (with standard errors) of three replicates of total area under the curve produced over 25 d. For each peak, the same letter indicates no significant difference (P ≤ 0.05). Log10 transformation of data were required for peaks 10, 11, 15, 16, and 20. One-way ANOVA with Tukey–Kramer a posteriori test performed on 5, 10, 15, 18–20, and 24. Two-sample t-test was carried out on peaks 11 and 16
Production of Volatiles Over Time
All VOCs detected increased over the time course of the experiment and then declined. Of the 25 metabolites, only four were detected at 1 d (Fig. 4, Table 1): peaks 18, 19, 20, and 24 from 4, 1, 4, and 9 samples, respectively; peak 24 being the only metabolite consistently present at 1 d. Production of other metabolites began between 3 and 17 d, but most commonly from 9 d following mycelial contact (Table 1), and increased over time, most reaching a maximum at 17 d and declining by 25 d (Table 1, Fig. 5).
Discussion
There was no evidence of long-distance antagonism (Boddy 2000) in the interaction between H. fasciculare and R. bicolor; morphological responses were not manifest until after mycelial contact, and there was no physiological response (as evidenced by lack of change in VOC profiles) until 8 d after inoculation. By this time, mycelial contact had been made, and R. bicolor had begun to produce red/brown pigments (3–9 d). Those \VOCs emitted before 9 d (peaks 4–6, 8–10, 13–15, 17–20, 22–24, and 25) may relate to R. bicolor pigment production, while those which did not occur until after 9 d (peaks 1–3, 7, 11, 12, 16, and 21) may be associated with the yellow pigment production seen in the mycelium of H. fasciculare at this time. Shifts in metabolism corresponding with pigment production have also been detected in Phlebia radiata (Griffith et al., 1994a; Rayner et al., 1994).
The decline in VOCs concentration towards the end of the experiment may have several causes. First, because there was no overall replacement of one fungus by the other, there was no new mycelial contact to induce fresh VOC production. Continued production of VOCs might be envisaged where one fungus replaces another with the production of successive bands of pigment by either or both the invading and invaded fungus. In other fungal combinations, where pigment production is less, there may be less VOCs produced even during the height of the interaction. Second, the nutrient limitations of the media may have prevented further metabolite production as the number (Sunesson et al., 1995; Korpi et al., 1999) and concentration (Demyttenaere et al., 2004) of VOCs produced can vary depending upon the growth medium.
The outcome of an interaction may depend to some extent on the ability of a fungus to detoxify its opponent’s metabolites. Catabolism or detoxification of VOCs may have occurred during the current study; at least one compound produced by R. bicolor grown on its own was not detected during the interspecific interaction. This may indicate the inhibition or switching off of some metabolites during interaction. Also, there was some evidence that plasticizer (at midretention times) from the Reacsyn vessels decreased with time, perhaps due to fungal metabolism. Evidence that H. fasciculare does breakdown diffusible compounds produced by other fungi was provided by Griffith et al. (1994b): H. fasciculare had a reduced radial extent when inoculated onto a cellulose film overlaid onto agar on which an opposing species had initially been grown; when this cellulose film plus fungus was removed and replaced by a fresh inoculum of H. fasciculare on a fresh cellulose film, the latter grew much more extensively, indicating that some degradation of the inhibitory compounds had occurred during the growth of the initial H. fasciculare inoculum.
Some VOCs were produced as part of the normal metabolism of each fungal isolate, but may still have a role in the defense of territory against invasion by other fungi. Wood previously colonized by decay fungi is inhibitory (and occasionally stimulatory) to other fungi even when the original occupants are dead (Heilmann-Clausen and Boddy, 2005). VOCs may be up-regulated during interaction, and in the current study, two VOCs (peaks 5 and 11) were up-regulated to a statistically significant extent (P ≤ 0.05) during the interaction compared with the fungi growing alone. There was also some indication that peaks 10, 15, 16, and 19, may be up-regulated during interaction. These four peaks, which were not specific to the mixed species interactions, were some of the nonsesquiterpene compounds detected.
Of the 25 VOCs detected when the fungi were growing alone and during interaction, 19 were sesquiterpenes. Sesquiterpenes are widely used for communication among invertebrates, used by invertebrates to locate plant or animal hosts and by plants and fungi to attract insects for propagule dispersal. Many plants (Cakir et al., 2005; Cheng et al., 2005; Wang et al., 2005) and fungi (Florianowicz, 2000; Roy et al., 2003) produce sesquiterpenes with antifungal activity. An increase in sesquiterpene concentration including that of γ-muurolene has been found in response to wounding and fungal inoculation of spruce, suggesting that sesquiterpenes are part of an antifungal response (Viiri et al., 2001). Other sesquiterpene extracts show antifeedant activity or are ultimately toxic to invertebrates (Wiens et al., 1991; Kahlos et al., 1994; Stadler and Sterner, 1998). α- and γ-muurolene and γ-cadinene have been detected in red and white cabbage and nasturtium infested with Pieris brassicae or Pieris rapae larvae, but not in uninfested plants, potentially forming part of an antifeedant plant defense response (Geervliet et al., 1997).
On the other hand, cadinenes, muurolenes, and amorphenes, putatively identified in our samples, are also used in insect communication systems (El-Sayed, 2005); for example, α- and γ-muurolene contribute to the attraction of females of the moth, Helicoverpa armigera, to pigeon-pea plants (Hartlieb and Rembold, 1996). Germacrene D, an important precursor to the cadinenes, muurolenes, and amorphenes (Bulow and König, 2000), also strongly affects insect behavior (Prosser et al., 2004), activating an antennal response (Røstelien et al., 2000; Mozuraitis et al., 2002), and increases attraction to the substrate and oviposition by the tobacco budworm moth Heliothis virescens (Mozuraitis et al., 2002). The precise effect of the volatiles, produced during interspecific fungal interactions, on invertebrate behavior is, however, not yet known.
The two sets of putatively identified diastereoisomers (α- and γ-muurolene, cadinene, amorphene, and bulgarene, peaks 3, 4, 5, 11, 14, 15, 16, and 17) were generally associated with the interspecific interaction, although peaks 5, 11, and 16 were also found in RB treatments. The fact that peaks 11 and 16 occurred in the RBRB interaction but not RB only suggests some interaction response even with self isolates. General inducement or upregulation of compounds during the HFRB interaction suggests that they are part of non-self recognition and/or defense process, which is also visually manifest in the development of pigments from both species and the reduction of growth towards each other. Initial experiments with collembola also indicate attraction to certain regions of fungal growth, and toxicity of the pigmented cords of H. fasciculare (T.D. Rotheray, personal communication).
The preponderance of sesquiterpenes in the VOC profile should, however, be viewed with some caution as SPME fibers are particularly good at absorbing sesquiterpene hydrocarbons (often reported as fungal VOCs), and the PDMS-coated fiber absorbed the highest proportion of sesquiterpenes when compared against three other fiber coatings (Jeleń, 2003). Not all compounds can be picked up by SPME sampling. Sesquiterpene oxygenated compounds were completely missed by SPME sampling, while they were detected by simultaneous distillation extraction. However, other compounds that were detected by SPME were missing from the simultaneous distillation extraction (Jeleń, 2003). Other extraction techniques such as absorption on tenax followed by thermal desorption and solid sample injection (SSI) may offer a more comprehensive extraction. Other extraction techniques also have their disadvantages with respect to, for example, time for preparation and losses by degradation during the introduction of the analyte to the GC column in the case of SSI, and the decomposition of certain compounds on tenax (Ewen et al., 2004). In summary, although SPME sampling by PDMS fiber may provide an incomplete selection of the VOCs released by the fungi, it allows for rapid simple nondestructive sampling, and has provided a sufficient selection of compounds to elucidate the changes in metabolism associated with fungal interactions.
Of the four hypotheses tested, hypothesis 1, that VOCs are produced prior to mycelial contact initiating an early defense response, was not supported. Findings, however, supported the other three hypotheses: VOC production was linked to the timing of pigment production and may even provide a way of separating the metabolites produced by each species where the pigments are produced at distinctly different times (hypothesis 2). Some VOCs produced by the fungi when growing alone and during the interspecific interaction were significantly up-regulated during the interaction (hypothesis 3), while, compared with the individual fungi, a new set of VOCs was produced during the interspecific interaction (hypothesis 4).
Future priorities are further identification of volatiles and diffusibles exuded during fungal interactions. This will also involve determining whether or not the VOCs are attractive and/or toxic to insects and inhibitory to other fungi. We need to link volatiles/diffusibles back to genes and to determine if genes are being up-regulated. The function of the pigmentation and the relationship between pigmentation and VOC production must be determined. The work also needs to be extended to combinations of fungal species beyond those used here. Different species of fungi are likely to produce different sets of metabolites when growing alone and during interaction, although there is some crossover in the metabolites produced.
References
Abraham, W. R. 2001. Bioactive sesquiterpenes produced by fungi: Are they useful for humans as well? Curr. Med. Chem. 8:583–606.
Boddy, L. 2000. Interspecific combative interactions between wood-decaying basidiomycetes. FEMS Microbiol. Ecol. 31:185–194.
Boddy, L. 2001. Fungal community ecology and wood decomposition processes in angiosperms: From standing tree to complete decay of coarse woody debris. Ecol. Bull. 49:43–56.
Bulow, N. and König, W. A. 2000. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 55:141–168.
Cakir, A., Kordali, S., Kilic, H., and Kaya, E. 2005. Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochem. Syst. Ecol. 33:245–256.
Cheng, S. S., Lin, H. Y., and Chang, S. T. 2005. Chemical composition and antifungal activity of essential oils from different tissues of Japanese cedar (Cryptomeria japonica). J. Agric. Food Chem. 53:614–619.
De Lacy Costello, B. P. J., Evans, P., Ewen, R. J., Gunson, H. E., Jones, P. R. H., Ratcliffe, N. M., and Spencer-Phillips, P. T. N. 2001. Gas chromatography–mass spectrometry analysis of volatile organic compounds from potato tubers inoculated with Phytophthora infestans or Fusarium coeruleum. Plant Pathol. 50:489–496.
Demyttenaere, J. C. R., Morina, R. M., De Kimpe, N., and Sandra, P. 2004. Use of headspace solid-phase microextraction and headspace sorptive extraction for the detection of the volatile metabolites produced by toxigenic Fusarium species. J. Chromatogr. A 1027:147–154.
El-Sayed, A. M. 2005. The pherobase: Database of insect pheromones and semiochemicals. <http://www.pherobase.net>. Accessed: November 2005.
Ewen, R. J., Jones, P. R. H., Ratcliffe, N. M., and Spencer-Phillips, P. T. N. 2004. Identification by gas chromatography–mass spectrometry of the volatile organic compounds emitted from the wood-rotting fungi Serpula lacrymans and Coniophora puteana, and from Pinus sylvestris timber. Mycol. Res. 108:806–814.
Fäldt, J., Jonsell, M., Nordlander, G., and Borg-Karlson, A. K. 1999. Volatiles of bracket fungi Fomitopsis pinicola and Fomes fomentarius and their functions as insect attractants. J. Chem. Ecol. 25:567–590.
Florianowicz, T. 2000. Inhibition of growth and sporulation of Penicillium expansum by extracts of selected basidiomycetes. Acta Soc. Botanicorum Pol. 69:263–267.
Geervliet, J. B. F., Posthumus, M. A., Vet, L. E. M., and Dicke, M. 1997. Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J. Chem. Ecol. 23:2935–2954.
Griffith, G. S., Rayner, A. D. M., and Wildman, H. G. 1994a. Extracellular metabolites and mycelial morphogenesis of Hypholoma fasciculare and Phlebia radiata (Hymenomycetes). Nova Hedwigia 59:311–329.
Griffith, G. S., Rayner, A. D. M., and Wildman, H. G. 1994b. Interspecific interactions and mycelial morphogenesis of Hypholoma fasciculare (Agaricaceae). Nova Hedwigia 59:47–75.
Griffith, G. S., Rayner, A. D. M., and Wildman, H. G. 1994c. Interspecific interactions, mycelial morphogenesis and extracellular metabolite production in Phlebia radiata (Aphyllophorales). Nova Hedwigia 59:331–344.
Hartlieb, E. and Rembold, H. 1996. Behavioral response of female Helicoverpa (Heliothis) armigera Hb (Lepidoptera: Noctuidae) moths to synthetic pigeonpea (Cajanus cajan L) kairomone. J. Chem. Ecol. 22:821–837.
Heilmann-Clausen, J. and Boddy, L. 2005. Inhibition and stimulation effects in communities of wood decay fungi: Exudates from colonised wood influence growth by other species. Microb. Ecol. 49:1–8.
Humphris, S. N., Bruce, A., Buultjens, E., and Wheatley, R. E. 2002. The effects of volatile microbial secondary metabolites on protein synthesis in Serpula lacrymans. FEMS Microbiol. Lett. 210:215–219.
Jeleń, H. H. 2003. Use of solid phase microextraction (SPME) for profiling fungal volatile metabolites. Lett. Appl. Microbiol. 36:263–267.
Kahlos, K., Kiviranta, J. L. J., and Hiltunen, R. V. K. 1994. Volatile constituents of wild and in vitro cultivated Gloeophyllum odoratum. Phytochemistry 36:917–922.
Kirby, J. J. H., Stenlid, J., and Holdenrieder, O. 1990. Population-structure and responses to disturbance of the basidiomycete Resinicium bicolor. Oecologia 85:178–184.
Korpi, A., Pasanen, A. L. and Viitanen, H. 1999. Volatile metabolites of Serpula lacrymans, Coniophora puteana, Poria placenta, Stachybotrys chartarum and Chaetomium globosum. Build. Environ. 34:205–211.
Mozuraitis, R., Stranden, M., Ramirez, M. I., Borg-Karlson, A. K., and Mustaparta, H. 2002. (−)-Germacrene D increases attraction and oviposition by the tobacco budworm moth Heliothis virescens. Chem. Senses 27:505–509.
Nilsson, T., Larsen, T. O., Montanarella, L., and Madsen, J. O. 1996. Application of head-space solid-phase microextraction for the analysis of volatile metabolites emitted by Penicillium species. J. Microbiol. Methods 25:245–255.
Prosser, I., Altug, I. G., Phillips, A. L., Konig, W. A., Bouwmeester, H. J., and Beale, M. H. 2004. Enantiospecific (+)- and (−)-germacrene D synthases, cloned from goldenrod, reveal a functionally active variant of the universal isoprenoid-biosynthesis aspartate-rich motif. Arch. Biochem. Biophys. 432:136–144.
Rayner, A. D. M. and Boddy, L. 1988. Fungal Decomposition of Wood: Its Biology and Ecology. Wiley, New York.
Rayner, A. D. M., Griffith, G. S., and Wildman, H. G. 1994. Induction of metabolic and morphogenetic changes during mycelial interactions among species of higher fungi. Biochem. Soc. Trans. 22:389–394.
Rösecke, J. and König, W. A. 2000. Constituents of various wood-rotting basidiomycetes. Phytochemistry 54:603–610.
Rösecke, J., Pietsch, M., and König, W. A. 2000. Volatile constituents of wood-rotting basidiomycetes. Phytochemistry 54:747–750.
Røstelien, T., Borg-Karlson, A. K., Faldt, J., Jacobsson, U., and Mustaparta, H. 2000. The plant sesquiterpene germacrene D specifically activates a major type of antennal receptor neuron of the tobacco budworm moth Heliothis virescens. Chem. Senses 25:141–148.
Roy, G., Laflamme, G., Bussieres, G., and Dessureault, M. 2003. Field tests on biological control of Heterobasidion annosum by Phaeotheca dimorphospora in comparison with Phlebiopsis gigantea. For. Pathol. 33:127–140.
Savoie, J. M. 2001. Variability in brown line formation and extracellular laccase production during interaction between white-rot basidiomycetes and Trichoderma harzianum biotype th2. Mycologia 93:243–248.
Schoeman, M. W., Webber, J. F., and Dickinson, D. J. 1996. The effect of diffusible metabolites of Trichoderma harzianum on in vitro interactions between basidiomycete isolates at two different temperature regimes. Mycol. Res. 100:1454–1458.
Score, A. J., Palfreyman, J. W., and White, N. A. 1997. Extracellular phenoloxidase and peroxidase enzyme production during interspecific fungal interactions. Int. Biodeterior. Biodegrad. 39:225–233.
Stadler, M. and Sterner, O. 1998. Production of bioactive secondary metabolites in the fruit bodies of macrofungi as a response to injury. Phytochemistry 49:1013–1019.
Sunesson, A. L., Vaes, W. H. J., Nilsson, C. A., Blomquist, G., Andersson, B., and Carlson, R. 1995. Identification of volatile metabolites from 5 fungal species cultivated on 2 media. Appl. Environ. Microbiol. 61:2911–2918.
Viiri, H., Annila, E., Kitunen, V., and Niemela, P. 2001. Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus, Ceratocystis polonica. Trees-Struct. Funct. 15:112–122.
Wald, P., Crockatt, M., Gray, V., and Boddy, L. 2004a. Growth and interspecific interactions of the rare oak polypore Piptoporus quercinus. Mycol. Res. 108:189–197.
Wald, P., Pitkanen, S., and Boddy, L. 2004b. Interspecific interactions between the rare tooth fungi Creolophus cirrhatus, Hericium erinaceus and H coralloides and other wood decay species in agar and wood. Mycol. Res. 108:1447–1457.
Wang, S. Y., Wu, C. L., Chug, F. H., Chien, S. C., Kuo, Y. H., Shyur, L. F., and Chang, S. T. 2005. Chemical composition and antifungal activity of essential oil isolated from Chamaecyparis formosensis Matsum. Wood. Holzforschung 59:295–299.
Wheatley, R., Hackett, C., Bruce, A., and Kundzewicz, A. 1997. Effect of substrate composition on production of volatile organic compounds from Trichoderma spp. Inhibitory to wood decay fungi. Int. Biodeterior. Biodegrad. 39:199–205.
Wheatley, R. E. 2002. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 81:357–364.
White, N. A. and Boddy, L. 1992. Extracellular enzyme localization during interspecific fungal interactions. FEMS Microbiol. Lett. 98:75–79.
Wiens, J. A., Cates, R. G., Rotenberry, J. T., Cobb, N., Vanhorne, B., and Redak, R. A. 1991. Arthropod dynamics on sagebrush (Artemisia tridentata)—effects of plant chemistry and avian predation. Ecol. Monogr. 61:299–321.
Acknowledgements
We thank the Natural Environment Research Council for funding (003/00731), John Hedger, the Cardiff University Fungal Ecology Group, and Tim Rotheray for discussion and for Fig. 1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hynes, J., Müller, C.T., Jones, T.H. et al. Changes in Volatile Production During the Course of Fungal Mycelial Interactions Between Hypholoma fasciculare and Resinicium bicolor . J Chem Ecol 33, 43–57 (2007). https://doi.org/10.1007/s10886-006-9209-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-006-9209-6