Abstract
Attack by multiple herbivores often leads to modification of induced plant defenses compared to single herbivory, yet little is known about the effects on induced indirect plant defense. Here, we investigated the effect of sequential induction of plant defense by Mamestra brassicae caterpillar oral secretion and an infestation by Tetranychus urticae spider mites on the expression of indirect plant defense in Lima bean plants. The effect on indirect defense was assessed using behavior assays with the specialist predatory mite Phytoseiulus persimilis in an olfactometer, headspace analysis of 11 major herbivore-induced plant volatiles (HIPVs) including (E)-β-ocimene, and transcript levels of the corresponding gene Phaseolus lunatus (E)-β-ocimene synthase (PlOS). Predatory mites were found to distinguish between plants induced by spider mites and caterpillar oral secretion but not between plants with single spider mite infestation and plants induced by caterpillar oral secretion prior to spider mite infestation. Indeed, the volatile blends emitted by plants induced by spider mites only and the sequential induction treatment of caterpillar oral secretion followed by spider mite infestation, were similar. Our results suggest that plant indirect defense is not affected by previous treatment with oral secretion of M. brassicae caterpillars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants that are under attack by herbivores produce and release complex mixtures of volatiles, known as herbivore-induced plant volatiles (HIPVs). Natural enemies of herbivorous arthropods can use these HIPVs to locate their herbivorous prey or host [reviewed by Mumm and Dicke (2010)]. The phenomenon of recruitment of natural enemies via HIPV release is known as indirect plant defense. Phytohormones, such as jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) are involved in generating and modulating induced plant defense responses to herbivory. The phytohormone JA and its volatile derivative methyl jasmonate (MeJA) are the main signalling molecules for induction of plant defense against herbivores, and they play an important role in indirect plant defense [e.g., Dicke et al. (1999); Kessler and Baldwin (2002)]. However, other phytohormones, such as salicylic acid (SA) and ethylene (ET) are known to play modulating roles via pathway cross-talk (Ament et al. 2004; Koornneef and Pieterse 2008; Ozawa et al. 2000; Van Poecke and Dicke 2002). Depending on the signalling pathways induced by an herbivore, the composition of HIPV blends can differ significantly (Zhang et al. 2013), allowing natural enemies to distinguish between plants infested by prey and non-prey herbivores (De Boer et al. 2004; Erb et al. 2010).
In nature, prey and non-prey herbivores can simultaneously feed on the same plant and thereby differentially modulate defense pathways and alter plant defense responses. Multiple herbivory is subject to an increasing number of studies (Stam et al. 2014). However, most of these studies focus on the effect on direct plant defenses and plant-mediated interactions amongst the herbivores (Brunissen et al. 2009; Erb et al. 2011; Kaplan and Denno 2007; Mathur et al. 2013; Rodriguez-Saona et al. 2010). The few studies on indirect plant defense mechanisms (Dicke et al. 2009) show that volatile blends of multiple herbivore-attacked plants can differ quantitatively or qualitatively compared to single induction (Delphia et al. 2007; De Boer et al. 2008; Erb et al. 2010; Rodriguez-Saona et al. 2003; Schwartzberg et al. 2011; Shiojiri et al. 2001; Zhang et al. 2009, 2013) . Multiple herbivory can influence indirect plant defense in a positive, neutral, or negative manner. In Lima bean plants, for example, simultaneous feeding of non-prey caterpillars, Spodoptera exigua Hübner, and prey, the spider mite Tetranychus urticae Koch, results in a synergistic increase in volatile emission and an increased attraction of a natural enemy of the spider mite, i.e., the predatory mite Phytoseiulus persimilis Athias-Henriot (De Boer et al. 2008). However, simultaneous feeding of the cicadellid Euscelidius variegatus Kirshbaum and Spodoptera littoralis Boisduval caterpillars on maize plants did not alter volatile emission differently from S. littoralis-induced volatile emission and did not affect behavior of a parasitoid of S. littoralis (Erb et al. 2010). Whitefly infestation has a negative effect on predator attraction to T. urticae-infested Lima bean plants (Zhang et al. 2009). The underlying mechanism for the differences in the effect of multiple herbivory is likely phytohormone cross-talk (Zhang et al. 2009, 2013).
Phytohormone crosstalk also may be involved in interactions between herbivores that attack a plant in temporally spaced events (Kessler and Baldwin 2004; Poelman et al. 2008). Sequential herbivory can have long-lasting effects on plant defenses (Poelman et al. 2008), whereby prior feeding by one herbivore can result in a kind of “vaccination” that can affect direct and indirect plant defenses against a later-arriving second herbivore (Kessler and Baldwin 2004). Voelckel and Baldwin (2004) suggest that the order/identity of arrival is crucial because some herbivore-induced stress effects on plant defense seem to be more stable than others. This also is likely dependent on the intensity and timing of subsequent defense inductions. In Zea mays seedlings, for example, a low dose of exogenously applied SA increases endogenous JA levels and volatile production upon a second induction by an insect-derived elicitor (Engelberth et al. 2011). However, higher doses resulted in reduced JA responses due to negative cross-talk. Moreover, shorter incubation times than 15 h with the phytohormone did not result in accumulation of JA or enhanced volatile production.
Volatile induction by leaf-chewing lepidopterans, and certain cell-content feeding herbivores, such as the spider mite Tetranychus urticae, is regulated primarily by the jasmonic acid pathway, and therefore antagonistic effects on plant defense are not expected between the two (Ozawa et al. 2000). Here, we investigated the effect of sequential induction of plant defense by M. brassicae caterpillar oral secretion and an infestation by T. urticae spider mites, which were temporally separated by a period of 48 h. We hypothesized that sequential induction of JA-induced plant defenses would result in increased attraction of P. persimilis through changes in the HIPV blend. Moreover, we investigated whether transcription levels of the JA-responsive gene Phaseolus lunatus Ocimene Synthase (PlOS), coding for a rate-limiting step in the biosynthesis of the spider-mite inducible plant volatile (E)-β-ocimene, would increase accordingly (Ament et al. 2004). The HIPV (E)-β-ocimene is known to be highly attractive to P. persimilis (Dicke et al. 1990).
Methods and Materials
Plants Lima bean plants (Phaseolus lunatus L., cv. Wonderbush, De Bruyn Seed Company, Michigan, USA) were cultivated in a greenhouse compartment at 23 ± 2 °C, 60 ± 10 % relative humidity (RH) and a 16:8 h L:D photoregime. Plants were grown in 5 × 5 cm plastic pots for gene transcription experiments or 11 × 11 cm for headspace volatile collection and behavorial experiments, respectively. After 12–14 d, plants with two expanded primary leaves were transferred to a climate chamber and incubated at 25 ± 1 °C, 60 ± 10 % R.H. and 16:8 h L:D. In the climate chamber, plants of different treatments were kept separate in plastic cages (90 × 90 × 60 cm) that were connected to house vacuum to prevent volatile transfer between plants of different treatments.
Herbivores and Predatory Mites A colony of two-spotted spider mites, Tetranychus urticae Koch (Acari: Tetranychidae), was maintained on Lima bean plants in a greenhouse compartment at 25 ± 5 °C, 50–70 % R.H., 16:8 h L:D. Adult female spider mites for experiments were selected randomly from the colony.
Cabbage moth, Mamestra brassicae L. (Lepidoptera: Noctuidae) caterpillars were reared on cabbage plants (B. oleracea var. gemmifera cv. Cyrus, Syngenta seeds BV, Enkhuizen, The Netherlands) at 22 ± 1 °C and 50–70 % R.H., under the same photoregime as for plants and mites. Oral secretions were collected from 18–20 caterpillars in the 5th instar, which were randomly selected from the colony.
Predatory mites, Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae), were reared in Petri dishes (diam 9 cm) on detached Lima bean leaves that were heavily infested with T. urticae under the same conditions as the T. urticae colony. Only gravid female predatory mites were used for behavioral experiments, ca. 1–2 d after their final molt. For behavioral experiments, the females were individually confined in 0.5 ml Eppendorf tubes containing a piece of moist cotton wool to avoid dehydration. Predatory mites were starved for 24 h and left in the experimental room prior to the experiment in order to acclimatise.
Plant Treatments
Sequential induction experiments Plant treatments were: i) control, ii) infestation with 20 spider mites (48 h incubation), iii) caterpillar oral secretion (48 h incubation), iv) caterpillar oral secretion (48 h incubation) followed by infestation with 20 mites (48 h incubation), v) caterpillar oral secretion (96 h incubation). Plants treated with caterpillar oral secretion were first artificially damaged with a pattern wheel by drawing 6 lines of ca. 7 cm length on each primary leaf. Then, the damaged leaves were treated with 10 μl per leaf of diluted caterpillar oral secretion using a fine paint brush for application. Oral secretion consisted of a pooled stock, freshly collected from 18 to 20 5th instar M. brassicae caterpillars using a glass Pasteur pipette. The stock was diluted 1:1 with tap water and kept on ice prior to use to avoid degradation of compounds. Upon the application with oral secretion, plants were incubated for 48 h. Control plants and plants receiving single infestation by spider mites did not receive mechanical damage or oral secretion. Plants were kept in a climate chamber and incubated in groups separated by treatment in the cages described above. After 48 h, plants to be analyzed for single induction by oral secretion were sampled for transcriptional, volatile, or behavioral analysis. Plants with dual induction treatment or single infestation by T. urticae received 20 mites per plant, followed by another 48 h of incubation before sample collection.
Time Series Experiment Plant treatments included i) control, ii) infestation with 20 spider mites, and iii) caterpillar oral secretion. Plant treatments were executed the same as in the previous section. Plants were kept in a climate chamber and incubated separately according to treatment in cages. Samples for transcriptional analysis were taken 6, 20, 26, or 46 h following treatment.
Y-tube Olfactometer Responses of predatory mites were tested in a Y-tube olfactometer (Takabayashi and Dicke 1992). A Y-shaped metal wire was located in the center of a glass Y-tube, and each arm was connected to a 5-L glass jar. Glass jars containing plants were connected to air inlets providing a 2 L/min charcoal-filtered air influx to carry volatiles into the two arms of the Y-tube. For behavior experiments, plant pots and loose soil were gently removed, and roots with soil were carefully wrapped in aluminium foil. Three plants of a treatment were placed in a glass jar as odor source, and the system was purged for 30–60 min without closing the vessels. Afterwards, glass jars were sealed with viton-lined glass lids, and the whole Y-tube olfactometer setup was flushed with air for 7–10 min before commencing the behavior experiment. Individual predatory mites were placed downwind on the Y-shaped wire and their choice for either odor source was recorded when they passed a line located halfway up one of the two olfactometer arms, or no-choice was recorded when they had not passed the line within 5 min. Sides of treatments were alternated after every five predatory mites to avoid positional bias. Plants were replaced after every 20 predatory mites or approximately 90 min after the first mite was tested, whichever came first. Each comparison was tested on two different days with 40–60 predatory mites per day.
Dynamic Headspace Collection of Plant Volatiles Plants were prepared for volatile collection by gently removing pots and loose soil, and wrapping roots with soil in aluminium foil. Two plants of each treatment were transferred to a 5-L glass jar. Glass jars were sealed with viton-lined glass lids equipped with an air inlet and outlet. The setup was flushed with 100 ml/min synthetic air (Linde Gas Benelux B.V., The Netherlands) filtered by passing through charcoal before entering the glass jar. Glass jars with plant samples were flushed with air for 30–45 min. A stainless steel tube filled with 200 mg Tenax TA (20/35 mesh; CAMSCO, Houston, TX, USA) was connected to the outlet of each glass jar, and volatile collection was done by sucking air out of the jars at 100 ml/min for 2 h. A total of eight replicates of each treatment were sampled over 2 d. Fresh weight of above-ground plants tissue was determined immediately after volatile collection using an analytical balance (NewClassic ML, Mettler Toledo, Greifensee, Switzerland).
Chemical Analysis of Plant Volatiles A Trace Ultra gas chromatograph (GC) coupled with Trace DSQ quadrupole mass spectrometer (MS) both from Thermo (Thermo Fisher Scientific, Waltham, MA, USA) were used for separation and identification of plant volatiles was as described previously (Menzel et al. 2014).
Standards of (Z)-3-hexen-1-ol, (Z)-3-hexen-1-ol, acetate, (Z)-3-hexen-1-ol, butanoate, (Z)-3-hexen-1-ol, isovalerate, linalool, methyl salicylate, indole, (E)-β-ocimene, as well as alloocimene were obtained from Sigma-Aldrich (Saint Louis, MO, USA). Additional standards (E)-4,8-dimethylnona-1,3,7-triene [(E)-DMNT] and (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene [(E-E)-TMTT] were kindly provided by Prof. W. Boland (Max Planck Institute for Chemical Ecology, Jena, Germany). For quantification, calibration lines were constructed for each compound using seven data points at different concentrations and two replicates of each data point.
RNA Extraction and cDNA Synthesis Spider mites, eggs, feces, and webbing were gently removed from plants of the respective treatments using a soft paint brush. Plant material was obtained by cutting 4 leaf discs out of one primary leaf per plant with a cork borer (diam 2 cm). Leaf discs obtained from the primary leaf of 3 plants were pooled to yield one biological replicate. All samples were shock-frozen in liquid nitrogen immediately after collection and then stored at −80 °C until further processing. Frozen leaf material was homogenized with a mortar and pestle while avoiding thawing. Total RNA was isolated and purified using the Qiagen (Hilden, Germany) RNeasy Plant Mini kit with integrated DNAse treatment, according to manufacturer’s instructions. RNA quality was assessed using an Agilent 2100 Bioanalyzer with the RNA 6000 Nano Labchip® kit (all from Agilent Technologies, Santa Clara, CA, USA). RNA quantifications were done using a NanoDrop® ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Only RNA samples with 260/280 wavelength ratio >2 and a RIN value >7 were used for cDNA synthesis. cDNA was generated from total RNA by using the Bio-Rad iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) according to manufacturer’s instructions.
Quantitative RT-PCR A real-time quantitative RT-PCR was used to quantify gene transcript levels of P. lunatus Ocimene synthase (PlOS; GenBank accession EU194553), acidic pathogenesis-related protein 4 (PR-4), and the two reference genes P. lunatus Actin1 (PlACT1; GenBank accession DQ159907) and P. lunatus Nuclear matrix protein 1 (PlNMP1; GenBank accession AF289260.1). Quantitative RT-PCR was performed in a Rotor-Gene 6000 machine (Corbett Research) with a 72-well rotor; for a detailed description see Menzel et al. (2014). PlOS primers were F-PlOS 5′-TGCATGGGTCTCAGTCTCTG-3′ and R-PlOS 5′-TGCTGCTTCCCCTCTCTCTA-3′, PlPR-4 were F-PlPR-4 5′-ACGCTTTCCTCAGTGCTCTC-3′ and R-PlPR-4 5′-TCCTCGTCGTCGCAGTAATCCTT-3′, PlACT1 primers were F-PlACT1 5′-CCAAGGCTAACCGTGAAAAG-3′ and R-PlACT1 5′-AGCCAGATCAAGACGAAGGA-3′, and PlNMP1 primers F-PlNMP1 5′-CCGGAATGGAGTGTTGACGAGCA-3′ and R-PlNMP1 5′-CCAGCT CAGAAACATCTGGCAATGG5-3′.
Statistical Analysis Log transformation was applied to data from gene transcription experiments and volatiles in order to meet assumptions of normality and homogeneity of variances. Data were analyzed using one-way ANOVA or generalized linear model (GLM) followed by Tukey’s Honestly Significant Difference (HSD) post-hoc tests for pair-wise comparisons between treatments in the statistical software SPSS version 19 (SPSS Inc., Chicago, IL, USA). Data that violated assumptions on normality and equal variance after log transformation were analyzed by Kruskal-Wallis tests followed by Mann–Whitney U tests applying the Bonferroni correction for multiple comparisons.
Predator choices in the Y-tube olfactometer experiments were analyzed using a binomial test to examine whether the choice distribution significantly differed from 50:50.
Volatile profiles of plants exposed to different treatments were analyzed using multivariate data analysis. Data were expressed per unit of plant fresh weight, log-transformed, and univariate-scaled. Then, an Orthogonal Projection to Latent Structures-Discriminant Analysis (OPLS-DA) was performed using the software SIMCA P + version 12 (Umetrics, Umeå, Sweden). Pairwise comparisons for individual volatiles among treatments were executed using Mann–Whitney U tests.
Results
Response of Predatory Mites to Single or Multiple Herbivore Infestation The attraction of predatory mites to Lima bean plants exposed to different treatments was tested in a two-choice behavioral assay (Fig. 1). Feeding by T. urticae, with and without prior treatment with M. brassicae oral secretion, was preferred over control plants (binomial test, P < 0.001 in both comparisons). However, predators did not distinguish between control plants and plants treated with M. brassicae oral secretion alone (binomial test, P > 0.05). Moreover, predatory mites showed a preference for odors from plants infested by their prey compared to odors from plants induced by caterpillar oral secretion (binomial test, P < 0.001). The volatile blend of plants induced by the combination of non-prey oral secretion and prey herbivores was more attractive than the volatile blend of plants induced by oral secretion of M. brassicae (binomial test, P < 0.001). Predators did not display a significant preference when they were offered plants infested by prey herbivores vs. volatiles from plants induced by the combination of non-prey oral secretion and prey herbivores (binomial test, P > 0.05).
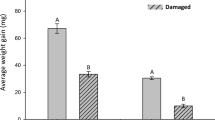
Responses of Phytoseiulus persimilis in a Y-tube olfactometer to volatiles emitted by Lima bean plants induced by mechanical damage and oral secretion of non-prey (Mamestra brassicae; M.b.), prey (Tetranychus urticae; T.u.) infestation, or a combination of the two (M.b. + T.u.). Volatile sources consisted of three Lima bean plants per treatment, i) control plants, or ii) plants infested with 20 T. urticae for 48 h, iii) induction by mechanical damage and M. brassicae oral secretion incubated for 48 h, or iv) induction by mechanical damage and M. brassicae oral secretion incubated for 48 h followed by infestation by 20 T. urticae for 48 h. Bars represent the overall percentages of predators choosing either odor source. Numbers in bars correspond to the number of predators choosing either odor source. For each comparison, the number of mites that did not make a choice within 5 min ranged between 0 and 3. Asterisks indicate significance of predatory mite choices (binomial test; n.s. = not significant, *** P < 0.001)
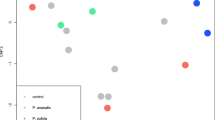
Volatile Analysis Spider mite feeding resulted in higher emission rates of several compounds compared to treatment with M. brassicae oral secretion (Suppl. Information, Figs. S1 and S2). Comparison of volatile profiles consisting of the 11 major HIPV compounds emitted in response to the treatments showed that volatile blends were significantly different between treatments: OPLS-DA resulted in a model with three significant principal components (Fig. 2). Figure 2b shows that the first distinction between treatments was made for treatments including exposure to feeding by T. urticae vs. treatments without T. urticae, which were separated by the first principal component. The second component separated treatments with caterpillar oral secretion from treatments that did not include oral secretion. The volatile blend of the combination treatment of caterpillar oral secretion and T. urticae was most similar to the volatile blend from plants treated with T. urticae infestation alone. Together with the third principal component, 89 % of the total variability of the data could be explained. Moreover, the model showed that four volatile compounds, namely (E,E)-TMTT, (E)-β-ocimene, (Z)-3-hexen-1-ol acetate, and alloocimene, had variable importance in the projection (VIP) values higher than 1, thus contributing most to discrimination between treatments. Pairwise comparison between the two treatments (1) 20 T. urticae (T.u.) and (2) M. brassicae oral secretion followed by infestation with 20 T. urticae (M.b. + T.u.), did not yield significant principal components and thus were not significantly different.
Multivariate data analysis by orthogonal PLS-DA (OPLS-DA) (panel A) and corresponding loading plot (panel B) of volatile blends of Phseolus lunatus plants with i) no treatment (Ctrl), or treated with ii) 20 Tetranychus urticae for 48 h (T.u.), iii) Mamestra brassicae oral secretion 48 h (M.b.), iv) M. brassicae oral secretion 48 h followed by infestation with 20 T. urticae for 48 h (M.b. + T.u.); 8 replicates for each treatment. The first two principal components are depicted (panel A) with the percentage of variation explained in parentheses. Numbers in the loading plot (panel B) represent 1) (Z)-3-hexen-1-ol, 2) (Z)-3-hexen-1-ol, acetate, 3) (E)-β-ocimene, 4) linalool, 5) (E)-4,8-dimethylnona-1,3,7-triene [(E)-DMNT], 6) alloocimene, 7) (Z)-3-hexen-1-ol,butanoate, 8) methyl salicylate, 9) (Z)-3-hexen-1-ol,isovalerate, 10) indole, 11) (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene [(E-E)-TMTT]
Relative Gene Transcription Transcript levels for PlOS, the gene encoding for the enzyme that mediates the rate-limiting step in the biosynthesis of (E)-β-ocimene, which is a principal attractant for P. persimilis (Dicke et al. 1990; Zhang et al. 2009), were compared between treatments. Treatments significantly affected PlOS transcript levels (GLM, P < 0.001; Fig. 3). Plants infested by T. urticae, with or without prior treatment with M. brassicae oral secretion, showed increased levels of PlOS compared to control plants and plants treated with M. brassicae oral secretion alone (post hoc Tukey’s HSD, P < 0.01). Oral secretion from M. brassicae did not increase PlOS transcript levels different from control (post hoc Tukey’s HSD, P > 0.05). Transcript levels of PlPR-4 also were affected by the treatments (ANOVA, F 4,15 = 16.86 P < 0.001). Infestation by T. urticae and treatment with M. brassicae oral secretion both resulted in significantly increased PlPR-4 transcript levels compared to control plants (post hoc Tukey’s HSD, P < 0.001). Sequential treatment induced an increase in transcript levels compared to control plants, and transcript levels were even higher than in response to single treatments (post hoc Tukey’s HSD, P < 0.001).
Relative gene transcript levels of Phaseolus lunatus (E)-β-ocimene synthase (PlOS) and P. lunatus pathogenesis-related protein 4 (PlPR-4) of plants i) without treatment (control), or treated with ii) 20 Tetranychus urticae for 48 h (T.u.), iii) Mamestra brassicae oral secretion for 48 h [M.b. (48 h)], iv) M. brassicae oral secretion for 48 h followed by infestation with 20 T. urticae for 48 h (M.b. + T.u.), v) M. brassicae oral secretion 96 h [M.b. (96 h)]. Values are the mean (±SE) of 10 to 12 biological replicates, pooled from three replications of the same treatment. Different letters above bars indicate significant differences in transcript levels between treatments (Tukey’s HSD tests, P < 0.05). Gene transcript levels were normalized to the normalization factor obtained from geometrically averaging the Ct values of the two reference genes P. lunatus Actin1 (PlACT1) and P. lunatus nuclear matrix protein 1 (PlNMP1) for each sample
Time Series for Relative PlOS Gene Transcription in Response to Single Treatments To investigate PlOS gene transcript levels for plants infested with T. urticae and plants with caterpillar oral secretion treatment, a time series experiment was conducted. The time series of PlOS gene transcription for the single treatment with 20 T. urticae or M. brassicae oral secretion showed clear differences in the gene transcription patterns between the two treatments (Fig. 4). Infestation by 20 T. urticae led to 14 times higher transcript levels than in control plants already after 6 h post treatment (hpt). At 20 hpt, expression levels of both the plants treated with M. brassicae oral secretion and plants exposed to T. urticae feeding were significantly higher than control levels (ANOVA, Tukey’s HSD tests, P < 0.05), whereas at 26 hpt these differences had disappeared. At 46 hpt, the same pattern as at 6 hpt was found, although the degree of upregulation of PlOS was lower. Treatment with caterpillar oral secretion also led to an increase of PlOS transcript levels compared to control levels but only at 20 hpt (ANOVA, Tukey’s HSD tests, P < 0.05). Before and after this time point transcript levels were not different from control levels.
Relative gene transcript levels of PlOS over four time points separated by hours post treatment (hpt). Phaseolus lunatus plants were treated with i) no treatment (control; Ctrl), ii) 20 Tetranychus urticae (T.u.), or iii) Mamestra brassicae oral secretion (M.b.). Values are the mean (±SE) of 4 biological replicates. Different letters above bars indicate significant differences in transcript levels between treatments (Tukey’s HSD tests, P < 0.05) within each time point. Gene transcript levels were normalized to the normalization factor obtained from geometrically averaging the Ct values of the two reference genes P. lunatus Actin1 (PlACT1) and P. lunatus nuclear matrix protein 1 (PlNMP1) for each sample
Discussion
Plants frequently are attacked by multiple herbivores, which may arrive at different moments in time. The resulting sequential herbivory has long-lasting effects on plant resistance against the subsequent herbivores (Mathur et al. 2011; Poelman et al. 2008; Stam et al. 2014; Viswanathan et al. 2007). However, little is known about the effect of sequential herbivory on plant indirect defense (but see e.g., Erb et al. 2010; Zhang et al. 2009, 2013). Here, we investigated the effect of the sequential attack by different herbivore species on plant indirect defense against T. urticae spider mites through the attraction of the specialist predator P. persimilis. Previous studies suggest that plants can form “memories” after stressful events such as herbivory, which enables them to adjust their defense accordingly, thus responding in an enhanced manner to a second stress (Conrath 2009; Frost et al. 2008). Our results show that prior treatment of plants with oral secretions of the generalist caterpillar M. brassicae, as a mimic of caterpillar feeding, does not affect the attraction of P. persimilis to plants infested with its prey T. urticae. The modulating effects caused by interactions with two herbivore species may, thus, depend on several factors such as severity of initial damage or infestation, timing between attacks, identity of the herbivore, and the associated defense pathway induced (Dicke et al. 2009; Viswanathan et al. 2007; Voelckel and Baldwin 2004; Zhang et al. 2009). The defense pathway commonly induced by leaf-chewing insects such as M. brassicae, is the octadecanoid pathway with JA as signalling molecule (Kessler and Baldwin 2002; McConn et al. 1997). The same defense pathway also is induced by feeding by cell-content feeders such as T. urticae (Dicke et al. 1999; Li et al. 2002; Ozawa et al. 2000). Multiple herbivory that occurs simultaneously by herbivores that induce the same pathway may lead to changes in volatile emission that results in increased attraction of predators (De Boer et al. 2008). In our study with sequential herbivory, predators did not distinguish between volatile blends from plants fed upon by T. urticae only and plants pre-treated with caterpillar oral secretions and subsequently exposed to feeding by T. urticae. Headspace analysis demonstrated that the volatile profiles of plants that had been exposed to these two treatments largely overlapped. It thus is possible that the initial defense induction was not strong enough to induce a memory effect or that the memory had decayed at the onset of the second attack by T. urticae. However, the volatile profile from plants with caterpillar oral secretion treatment was notably different compared to plants treated with T. urticae, and predators were significantly more attracted to plants infested with their prey. This is in accordance with data of De Boer and co-workers (2008) who found that the specialist predator P. persimilis can distinguish between volatiles induced by prey and non-prey herbivores. Nevertheless, De Boer et al. (2008) found an increased attraction of P. persimilis to dual-infested Lima bean plants, which were fed upon by T. urticae and Spodoptera exigua caterpillars. In their study, the dual infestation resulted in the emission of increased amounts of a subset of the plant volatiles. In the study by De Boer et al. (2008), the plants were exposed to simultaneous infestation with spider mites and S. exigua caterpillars. Whether the differentiation by the predators in their study and not in ours was due to the different caterpillar species, simultaneous compared to sequential treatments, or to caterpillar feeding instead of the use of oral secretion, remains to be elucidated.
It has been shown that P. persimilis is attracted to five HIPVs, two of which, namely (E,E)-TMTT and (E)-β-ocimene, were indeed found to play a significant role in separating the volatile profiles of Lima bean plants that had received different treatments (De Boer and Dicke 2004; Dicke et al. 1990). Dicke et al. (1999) found that the emission rates of the two homoterpenes (E,E)-TMTT and (E)-DMNT, as well as the phenolic ester MeSA, were involved in the differentiation between JA-treated plants and T. urticae-treated plants, the latter being more attractive to P. persimilis. The two other compounds, namely the terpene alcohol linalool and the monoterpene (E)-β-ocimene also are known to play important roles in the attraction of P. persimilis to T. urticae-infested Lima bean plants (Dicke et al. 1990; Zhang et al. 2009). Arimura et al. (2008) found a close relationship between JA levels and transcription of PlOS, which is the enzyme that leads to the production of the principal predator attractant (E)-β-ocimene in Lima bean. Voelckel and Baldwin (2004) found that simultaneous and sequential herbivory can cause different patterns of gene transcription when compared to individual feeding by the two different herbivores. In our study, treatments with the prey T. urticae and with the prey plus M. brassicae oral secretion resulted in increased PlOS transcript levels in Lima bean plants, but were not different from each other. Treatment with caterpillar oral secretion, which did not result in attraction of P. persimilis, showed PlOS transcript levels comparable to control levels. However, De Boer et al. (2008) found that Lima bean plants infested with S. exigua caterpillars or T. urticae spider mites do both emit large amounts of (E)-β-ocimene. Moreover, it is likely that a time lag exists between gene transcription and metabolite production (Stam et al. 2014). To investigate the temporal effect of caterpillar oral secretion on PlOS transcript levels, a time series experiment was conducted. Results show that treatment with caterpillar oral secretion does increase PlOS transcript levels. Moreover, PlOS transcript levels of plants treated with caterpillar oral secretion peaked at a different time point from in plants infested with T. urticae infestation, and showed different transcriptional patterns. Compared to T. urticae-treated plants, PlOS transcription peaked quite late for plants treated with caterpillar oral secretion. This is in accordance with Arimura et al. (2008) who found that feeding by the generalist caterpillar S. littoralis induced PlOS transcript levels only after 24 hpt but not at 6 hpt, whereas JA treatment and wounding already induced PlOS after 6 h. Continuous infestation, and thus feeding by T. urticae, resulted in elevation of PlOS transcript levels in three out of four time points. Moreover, PlOS transcript levels of plants treated with caterpillar oral secretion declined to control levels within 6 h after the peak. Apart from an effect of M. brassicae oral secretion on transcription of PlOS, transcription of another gene investigated also was affected. Transcription levels of PlPR-4 were upregulated by T. urticae feeding as well as application of M. brassicae oral secretion, and combined application led to higher transcript levels than either single treatment. PlPR-4 is an acidic chitinase, which is MeSA-responsive (Arimura et al. 2000; Margis-Pinheiro et al. 1991). Chitinases commonly are involved in plant direct defense against plant pathogens and arthropods (Kramer and Muthukrishnan 1997; Schlumbaum et al. 1986). Consequently, other defense mechanisms apart from indirect defense could in fact be affected by the combination treatment of T. urticae and M. brassicae oral secretion.
In our study, predatory mite behavior was well reflected in volatile blend analysis. Four compounds, i.e., (E,E)-TMTT, (E)-β-ocimene, (Z)-3-hexen-1-ol acetate, and alloocimene, were found to have an important function in discrimination between treatments. The involvement of (E,E)-TMTT and (E)-β-ocimene in the attraction of P. persimilis to prey-infested Lima bean plants has been described previously (Dicke et al. 1990). Nevertheless, there are other well-known compounds that play an important role in predator attraction (De Boer et al. 2004; Dicke et al. 1990). In fact, volatile blends convey more information than a single compound e.g. on herbivore identity and herbivore developmental stage (De Moraes et al. 1998; Mumm and Dicke 2010; Takabayashi et al. 1995).
In summary, in our study, sequential herbivore treatment involving herbivores that induce the same plant defense pathway did not enhance or interfere with indirect defense against T. urticae. Yet, differences in volatile blends perceived by the predator play a role in distinguishing between prey and non-prey infested plants. Volatiles attractive to natural enemies are perceived in the context of other volatiles thus extracting specific information about the presence of prey. Furthermore, gene transcription is differently induced in terms of timing and magnitude by different herbivore species, and a considerable time lag exists between gene transcription of genes relevant in indirect defense and metabolite emission.
References
Ament K, Kant MR, Sabelis MW, Haring MA, Schuurink RC (2004) Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol 135:2025–2037
Arimura GI, Ozawa R, Shimoda T, Nishloka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defence genes in Lima bean leaves. Nature 406:512–515
Arimura GI, Köpke S, Kunert M, Volpe V, David A, Brand P, Dabrowska P, Maffei ME, Boland W (2008) Effects of feeding Spodoptera littoralis on Lima bean leaves: IV. Diurnal and nocturnal damage differentially initiate plant volatile emission. Plant Physiol 146:965–973
Brunissen L, Cherqui A, Pelletier Y, Vincent C, Giordanengo P (2009) Host-plant mediated interactions between two aphid species. Entomol Exp Appl 132:30–38
Conrath U (2009) Priming of induced plant defense responses. Adv Bot Res 51:361–395
De Boer JG, Dicke M (2004) The role of methyl salicylate in prey searching behavior of the predatory mite Phytoseiulus persimilis. J Chem Ecol 30:255–271
De Boer JG, Posthumus MA, Dicke M (2004) Identification of volatiles that are used in discrimination between plants infested with prey or nonprey herbivores by a predatory mite. J Chem Ecol 30:2215–2230
De Boer JG, Hordijk CA, Posthumus MA, Dicke M (2008) Prey and non-prey arthropods sharing a host plant: effects on induced volatile emission and predator attraction. J Chem Ecol 34:281–290
De Moraes CM, Lewis WJ, Paré PW, Alborn HT, Tumlinson JH (1998) Hervibore-infested plants selectively attract parasitoids. Nature 393:570–573
Delphia CM, Mescher MC, De Moraes CM (2007) Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips. J Chem Ecol 33:997–1012
Dicke M, Van Beek TA, Posthumus MA, Ben Dom N, Van Bokhoven H, De Groot A (1990) Isolation and identification of volatile kairomone that affects acarine predator–prey interactions Involvement of host plant in its production. J Chem Ecol 16:381–396
Dicke M, Gols R, Ludeking D, Posthumus MA (1999) Jasmonic acid and herbivory differentially induce carnivore-attracting plant volatiles in lima bean plants. J Chem Ecol 25:1907–1922
Dicke M, Van Loon JJA, Soler R (2009) Chemical complexity of volatiles from plants induced by multiple attack. Nature Chem Biol 5:317–324
Engelberth J, Viswanathan S, Engelberth MJ (2011) Low concentrations of salicylic acid stimulate insect elicitor responses in Zea mays seedlings. J Chem Ecol 37:263–266
Erb M, Foresti N, Turlings TCJ. 2010. A tritrophic signal that attracts parasitoids to host-damaged plants withstands disruption by non-host herbivores. BMC Plant Biol 10, art. no. 247.
Erb M, Robert CA, Hibbard BE, Turlings TC (2011) Sequence of arrival determines plant-mediated interactions between herbivores. J Ecol 99:7–15
Frost CJ, Mescher MC, Carlson JE, De Moraes CM (2008) Plant defense priming against herbivores: getting ready for a different battle. Plant Physiol 146:818–824
Kaplan I, Denno RF (2007) Interspecific interactions in phytophagous insects revisited: a quantitative assessment of competition theory. Ecology Lett 10:977–994
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328
Kessler A, Baldwin IT (2004) Herbivore-induced plant vaccination. Part I. The orchestration of plant defenses in nature and their fitness consequences in the wild tobacco Nicotiana attenuata. Plant J 38:639–649
Koornneef A, Pieterse CMJ (2008) Cross talk in defense signaling. Plant Physiol 146:839–844
Kramer KJ, Muthukrishnan S (1997) Insect chitinases: molecular biology and potential use as biopesticides. Insect Biochem Molec Biol 27:887–900
Li C, Williams MM, Loh YT, Gyu IL, Howe GA (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol 130:494–503
Margis-Pinheiro M, Metz-Boutigue MH, Awade A, de Tapia M, le Ret M, Burkard G (1991) Isolation of a complementary DNA encoding the bean PR4 chitinase: an acidic enzyme with an amino-terminus cysteine-rich domain. Plant Mol Biol 17:243–253
Mathur V, Ganta S, Raaijmakers CE, Reddy AS, Vet LEM, Van Dam NM (2011) Temporal dynamics of herbivore-induced responses in Brassica juncea and their effect on generalist and specialist herbivores. Entomol Exp Appl 139:215–225
Mathur V, Tytgat TOG, de Graaf RM, Kalia V, Sankara Reddy A, Vet LEM, van Dam NM (2013) Dealing with double trouble: consequences of single and double herbivory in Brassica juncea. Chemoecology 23:71–82
McConn M, Creelman RA, Bell E, Mullet JE, Browse J (1997) Jasmonate is essential for insect defense in Arabidopsis. Proc Natl Acad Sci U S A 94:5473–5477
Menzel TR, Weldegergis BT, David A, Boland W, Gols, R, Van Loon, JJA, Dicke M. 2014. Synergism in the effect of prior jasmonic acid application on herbivore-induced volatile emission by Lima bean plants: transcription of a monoterpene synthase gene and volatile emission. J Exp Bot 65:4821–4831 doi:10.1093/jxb/eru242.
Mumm R, Dicke M (2010) Variation in natural plant products and the attraction of bodyguards involved in indirect plant defense. Canad J Zool 88:628–667
Ozawa R, Arimura GI, Takabayashi J, Shimoda T, Nishioka T (2000) Involvement of jasmonate- and salicylate-related signaling pathways for the production of specific herbivore-induced volatiles in plants. Plant Cell Physiol 41:391–398
Poelman EH, Broekgaarden C, Van Loon JJA, Dicke M (2008) Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol Ecol 17:3352–3365
Rodriguez-Saona C, Crafts-Brandner SJ, Cañas LA (2003) Volatile emissions triggered by multiple herbivore damage: beet armyworm and whitefly feeding on cotton plants. J Chem Ecol 29:2539–2550
Rodriguez-Saona CR, Musser RO, Vogel H, Hum-Musser SM, Thaler JS (2010) Molecular, biochemical, and organismal analyses of tomato plants simultaneously attacked by herbivores from two feeding guilds. J Chem Ecol 36:1043–1057
Schlumbaum A, Mauch F, Vögeli U, Boller T (1986) Plant chitinases are potent inhibitors of fungal growth. Nature 324:365–367
Schwartzberg EG, Böröczky K, Tumlinson JH (2011) Pea aphids, Acyrthosiphon Pisum, suppress induced plant iolatiles in broad bean, Vicia Faba. J Chem Ecol 37:1055–1062
Shiojiri K, Takabayashi J, Yano S, Takafuji A (2001) Infochemically mediated tritrophic interaction webs on cabbage plants. Pop Ecol 43:23–29
Stam JM, Kroes A, Li Y, Gols R, Van Loon JJA, Poelman EH, Dicke M (2014) Plant interactions with multiple insect herbivores: from community to genes. Annu Rev Plant Biol 65:689–713
Takabayashi J, Dicke M (1992) Response of predatory mites with different rearing histories to volatiles of uninfested plants. Entomol Exp Appl 64:187–193
Takabayashi J, Takahashi S, Dicke M, Posthumus MA (1995) Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J Chem Ecol 21:273–287
Van Poecke RMP, Dicke M (2002) Induced parasitoid attraction by Arabidopsis thaliana: involvement of the octadecanoid and the salicylic acid pathway. J Exp Bot 53:1793–1799
Viswanathan DV, Lifchits OA, Thaler JS (2007) Consequences of sequential attack for resistance to herbivores when plants have specific induced responses. Oikos 116:1389–1399
Voelckel C, Baldwin IT (2004) Herbivore-induced plant vaccination. Part II. Array-studies reveal the transience of herbivore-specific transcriptional imprints and a distinct imprint from stress combinations. Plant J 38:650–663
Zhang PJ, Zheng SJ, Van Loon JJA, Boland W, David A, Mumm R, Dicke M (2009) Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc Natl Acad Sci U S A 106:21202–21207
Zhang PJ, Broekgaarden C, Zheng SJ, Snoeren TAL, Van Loon JJA, Gols R, Dicke M (2013) Jasmonate and ethylene signaling mediate whitefly-induced interference with indirect plant defense in Arabidopsis thaliana. New Phytol 197:1291–1299
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 214 kb)
Rights and permissions
About this article
Cite this article
Menzel, T.R., Huang, TY., Weldegergis, B.T. et al. Effect of Sequential Induction by Mamestra brassicae L. and Tetranychus urticae Koch on Lima Bean Plant Indirect Defense. J Chem Ecol 40, 977–985 (2014). https://doi.org/10.1007/s10886-014-0499-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-014-0499-9