Abstract
Defensive secretions of adult and larval Suocerathrips linguis (Phlaeothripidae, Thysanoptera) were found to contain a long-chained acetate, (11Z)-11,19-eicosadienyl acetate, that was not previously known to occur naturally. This substance occurred together with octadecyl acetate and other long-chained acetates. The eicosadienyl acetate repels ants and spreads on the surface of such potential predators. The mixture can provide a long-lasting surface coating.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Defensive secretions with low volatility produced by Suocerathrips linguis Mound and Marullo (Thysanoptera, Phlaeothripidae) were investigated in this study. The production of volatile substances by a species of Thysanoptera was noticed long ago by Hodson (1935) as “a pungent and distinctive but not unpleasant odour.” During the last three decades, there have been more than 30 papers dealing with secretions of thrips. Several thrips species, when they are attacked by an enemy, raise and lower their abdomen with a drop of a fluid on the abdominal tip (Lewis, 1973). The secretory reservoir seems to be the hindgut (Howard et al., 1983). Some of the complete secretions, as well as single components, e.g., lactones such as mellein, have been found to be ant repellents (Howard et al., 1983; Blum, 1991; Blum et al., 1992). They may also function as contact irritants, alarm pheromones, or in some cases fumigants.
S. linguis was described from specimens occurring in the Royal Botanical Garden Kew, London, UK, where it was living on cultivated Sansevieria plants (Mound and Marullo, 1994). Despite being restricted to members of this genus, S. linguis feeds on fungi growing on the surface of these plants (Moritz, 2002). Sansevieria is an endemic Afrotropical genus, and presumably S. linguis is also African in origin. Like many other Phlaeothripidae, S. linguis shows aggregation and subsocial behavior (Moritz, 2002; Moritz et al., 2003).
Methods and Materials
Rearing and Sample Collection
S. linguis was reared on Sansevieria trifasciata under a constant light regime of 16:8-hr light/dark (light on at 6:00 am), a temperature of around 23°C, and a relative humidity of 80%. Thrips were stimulated mechanically to produce droplets, and these were collected on the inner wall of a fine capillary glass tube (Minicaps 5 μl, Hirschmann Laborgeräte). The tube was then rinsed with the following solvents: distilled methanol (Aldrich, 49,429-1), hexane (Fluka, 52767), and, for preparation of derivatives, carbon disulfide (Aldrich, 18,017-3). For GC-MS, droplets were collected from 10 to 20 thrips and investigated within 2 hr. For derivative reactions, droplets from 50 thrips were used. For comparison and to examine differences between males and females, whole-body extracts were obtained by soaking 10–20 adults in methanol or hexane for 2 hr. From second instars, 20 droplets were collected and dissolved in methanol.
Chemical Analysis
For GC-MS analyses, samples were dissolved in a small amount (50–100 μl) of methanol or hexane, and then 5 μl were injected into an HP 5890 GC equipped with 5972 MSD and an HP5-MS column (30 m × 0.25 mm ID; stationary phase: 0.25 μm cross-linked 5% phenyl methyl silicone) that used electron impact ionization. The carrier gas was helium at a flow rate of 1 ml/min, the injector temperature was set to 300°C, and the detector temperature to 280°C. Column temperature was maintained at 100°C following the injection and then linearly increased to 300°C at a rate of 7K/min. The NIST database, literature data, and the software AMDIS (NIST, version 2.1) and NIST MS Search (version 1.7) were used to analyze mass spectra and to compare retention times.
To determine position of double-bonds, samples were treated with dimethyl disulfide (DMDS, Aldrich, 52,801-3) as follows. They were dissolved in 50 μl carbon disulfide for 10 min, then 10 μl of a 5% iodine solution (in CS2) and 60 μl DMDS were added and mixed together with the help of a microliter syringe. After 10 hr at 40°C, the reaction was quenched by the addition of 50-μl 5% solution of sodium thiosulfate (Aldrich, 21,726-3) in H2O. The organic phase was evaporated on a slide, and the solid residue was dissolved in methanol and investigated as described above. Pure solvent without thrips secretion was treated and checked for impurities. The resulting mass spectra of the derivatives were evaluated by self-written software ACE_FIND, based on the findings of Buser et al. (1983) and Vincenti et al. (1987). The software calculates the diagnostic mass peaks of DMDS derivatives of aliphatic acetates in a selected chain length range with all possible positions of two double-bonds. It considers aliphatic derivatives, cyclic derivatives, and special properties in conjugated double-bonds as well and compares the resulting mass peaks with up to 16 input values. The output is a list of substances sorted by the number of mass peaks that fit the input data.
Bioassay
For the first preliminary bioassay tests, two colonies of Myrmica rubra (Linnaeus, 1758) (about 25 individuals each, obtained from Antstore, Berlin) were reared separately in glass tanks 0.2 × 0.3 × 0.2 m3, filled to a depth of 50 mm with garden soil. In the test, ants had to choose between two pieces of food (ca. 75 mg of turkey meat). The pieces were placed on a sheet of filter paper at a distance of 50 mm, and a circle (radius 20 mm) was drawn in pencil around each. The edge of one circle was soaked with 0.5 μl of test substance dissolved in 50 μl methanol, and the second was treated only with 50 μl methanol as a control. The sheet of filter paper was placed on a glass slide in the tank to prevent contamination from the soil. The number of ants inside the circles was recorded every 10 min until the food was consumed. Test and control were not replaced during each test.
Results
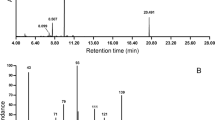
The main components of the droplets were long-chained acetic acid esters (acetates) of one saturated (C18) (Figure 1, peak at 17.53 min retention time) and several unsaturated alcohols (C16,..., C20) (other peaks from 17.11 to 19.82 min retention time). These constituents have a low volatility. In all cases, it was possible to find the peak for the molecular (M+) ion, as well as a fragment at m/z 61 (CH3CHOOH) and a fragment M+-60 because of McLafferty rearrangement in acetates.
No differences were detected between males, females, and second instar larvae. The secretion does not contain any compound from the fungi on which the thrips feed, nor from the host plant (Sansevieria). Sex-specific concentrations of substances with high volatility were found only if the whole thrips were extracted (Tschuch et al., 2002). Thus, the thrips must have additional secretory glands to produce volatile semiochemicals, although these are not the topic of this paper.
The molecular (M+) ion of the main component is 336. A fragment at m/z 61 (CH3COOH+H) and a small fragment at m/z 276 suggested that this compound was an eicosadienyl acetate (C20:2-OAc). The other mass peaks were similar to some octadecadienyl acetates (C18:2-OAc, library data). In the DMDS derivatives of the secretion, one substance had a characteristic mass spectrum with m/z 61 as the highest peak (spectrum measured from m/z 50). There are only four peaks >5% above m/z 120: 121, 169, 217, and 259. With software, all derivatives of acetates between C18:2 and C22:2 were investigated. The only DMDS derivatives fitting all four peaks according to Vincenti et al. (1987) were C20:2-OAc with one double-bond at position 11 and the other double-bond at position 17, 18, or 19. The 11,17-isomer must have a prominent peak at m/z 89, the 11,18-isomer at 75, and the 11,19-isomer at 61. m/z 89 and 75 could not be found as high peaks, so the result must be 11, 19-C20:2-OAc. The mass peaks are as follows: 61 [c]+ together with the 61 from CH3CHOOH (therefore, this m/z has the highest abundance), 121 [bc-3*CH3SH]+, 169 [bc-2*CH3SH]+, 217 [bc-CH3SH]+, and 259 [a]+ (where a = 259 is the part from the acetic acid to former double-bond at position 11, b = 204 is the part between the former two double-bonds, and c = 61 is the remaining part). Small peaks are also visible at 403 and 429 corresponding to [ab-CH3CHOOH]+ and [M-2*CH3SH]+. Thus, the main component is an 11,19-C20-OAc.
The identified constituents were compared with authentic substances: octadecyl acetate was bought (Sigma, S-5003 stearyl acetate), and (11Z)-11,19-eicosadienyl acetate was synthesized as described in Csuk et al. (2004) because (E) isomers are seldom found in insect allomones or pheromones (with the exception of Lepidoptera sex pheromones). Mixtures of authentic substances with S. linguis secretion were analyzed by GC-MS and yielded only a single peak. Furthermore, the mass spectra of the constituents and authentic compounds matched closely.
Preliminary bioassay tests showed that the (11Z)-C11,19-OAc alone is an effective repellent against M. rubra (Figure 2). For 1 hr, the number of ants inside the acetate ring was at all times lower than inside the control ring. The food in the control ring was removed by the ants in small portions, whereas the food in the acetate ring was left more or less untouched. The test with nest 2 (N2) ended after 40 min because the food in the control (C) was consumed. Only two tests could take place because of the limited amount of substance synthesized.
Discussion
The main constituent of the anal secretion of S. linguis adults and larvae, (11Z)-11,19-eicosadienyl acetate (C20:2-OAc), is a new natural product not previously reported in animals or plants. Secretions of Thysanoptera species consist of different chemical constituents, such as aliphatic hydrocarbons, carboxylic acids, aliphatic esters, cyclic esters of hydroxyl carboxylic acids (lactones), pyranones, naphthoquinones, and terpenes (Terry, 1997). In this discussion, only aliphatic esters of acetic acid, found in 30% of the investigated species, are the focus of attention. The aliphatic ester with the longest chain known previously from Thysanoptera is octadecyl acetate (C18:0-OAc) from Gynaikothrips uzeli (Suzuki et al., 1989: only 2.2% of secretion). Hexadecyl acetate (C16:0-OAc) is reported from Gynaikothrips ficorum (Howard et al., 1987: in 1:1 mixture with pentadecane one of the main constituents), from G. uzeli (Suzuki et al., 1989: in 1:2 mixture with pentadecane one of the main constituents), from Leeuwenia (=Varshneyia) pasaniae, and from Liothrips kuwanai (Suzuki et al., 1988: only 3.0 and 4.7%). A small part of the Li. kuwanai secretion is probably hexadecenyl acetate (C16:1-OAc, double-bond position unknown) (Suzuki et al., 1988: 1.2%). The secretion of L. pasaniae contains mainly tetradecyl acetate (C14:0-OAc: 15.4%), tridecane (51.3%), and pentadecane (12.4%). In contrast to these species of Tubulifera, the only species of Terebrantia from which chemicals have been identified so far, Frankliniella occidentalis, produce a mixture of dodecyl (C12:0-OAc) and decyl acetate (C10:0-OAc) in the anal droplets of second instars, although only in traces in secretions by adults (Teerling et al., 1993a,b; Teerling, 1995; MacDonald et al., 2003). Tubulifera with gregarious and commonly social or gall-dwelling lifestyles may be more prolific producers of defensive secretions than solitary Tubulifera or Terebrantia. To maintain safety within a colony, a strong repellent allomone or alarm pheromone would be effective (Terry, 1997). In solitary Thysanoptera, defensive secretion is more important in larvae because adults are able to fly away if predators are near.
Most species of Tubulifera discharge the fluid from the apex of their tube. This allows them to apply the droplet to a predator, but to resorb the droplet if the application fails (Howard et al., 1987). For this reason, the secretion must be liquid down to the lowest temperature at which predators are active. If the secretion is to disable or irritate potential predators by coating them with the liquid, then a secretion with a low vapor pressure at all possible ambient temperatures would be best. This is necessary to avoid fast evaporation. To fulfill both requirements, the mixture should have a melting point (mp) near 0°C and a boiling point as high as possible. If one of the main constituents is an aliphatic acetate (discussed below), then there are several possible solutions: saturated acetates C12:0-OAc (mp 1°C) or C10:0-OAc (mp −15°C) singly or mixed; longer-chained saturated acetates (e.g., C16:0-OAc, mp 24°C) dissolved in a liquid hydrocarbon or other solvent; and unsaturated acetates with melting points near 0°C.
The first of these is used by larvae of F. occidentalis, and the second by the two species of Gynaikothrips mentioned above. Pentadecane has a melting point of 10°C. The mixture has a melting point below 10°C because the acetate acts as an impurity of the pentadecane.
The third of these methods is used by S. linguis. Because of the lack of experimental data, estimations of melting points are necessary. Unfortunately, software such as MPBPWIN (version 1.41, part of EPI Suite from United States Environmental Protection Agency) is not suitable. For example, it estimates a melting point between 76 and 102°C for eicosyl acetate, whereas the measured melting point is approximately 41°C. Furthermore, this and other software that use the Joback algorithm, the Gold & Ogle algorithm, or similar methods do not consider the position of double-bonds or the stereochemistry. Kobayashi et al. (1978) found the following data for the 11-eicosenyl acetates (C20:1-OAc) and alcohols: (Z)-11-eicosenol (mp 26°C), (E)-11-eicosenol (mp 44°C), (Z)-11-eicosenyl acetate (liquid), and (E)-11-eicosenyl acetate (mp 20°C). The saturated eicosyl acetate has a melting point of 41°C. From data in Beilstein database (BS0402AE, Beilstein GmbH and MDL Information Systems GmbH 2004), it appears that acetates melt 26K (±3K) below the corresponding alcohol (data from nine pairs between C12:0 and C22:1). So the melting point of the (Z)-11-eicosenyl acetate can be estimated as below 0K. The change from single bond to ally group at the end of the alcohol lowers the melting point of fatty alcohols by 0–6K only (Duhamel, 1963; Stubbs and Smith, 1984). Thus, with an error of ±10K, it is possible to estimate the melting point of (11Z)-11,19-C20:2-OAc to be below −3°C and the melting point of (E)-11,19-C20:2-OAc to be around 17°C. The estimation for the Z isomer seems to be good because the synthesized reference substance remains liquid at 0°C in a refrigerator. As a single substance, the Z isomers of 11-C20:1-OAc and 11,19-C20:2-OAc acetate possess an appropriate melting point (around or below 0°C), but the E isomers do not.
The vapor pressure of 11,19-C20:2-OAc is lower than the vapor pressure of C10:0-OAc or C12:0-OAc because the boiling point rises with increasing chain length. In addition, saturated acetates possess lower boiling points than unsaturated acetates. In the secretion of S. linguis, the solid C18:0-OAc (mp 32°C) is dissolved in the main constituent, which leads to an additional vapor pressure lowering according to Raoult’s law. If a potential predator is coated by such a mixture, the C18:0-OAc will remain on the surface after the unsaturated acetate has evaporated. However, it is also conceivable that modifications occur in the unsaturated acetate under the influence of oxygen and ultraviolet light [Panades et al., 1998: (7E,9Z)-7,9-C12:2-OAc] or ozone [Arndt et al., 1996: (Z)-11-C18:1-OAc and (Z)-9-C14:1-OAc]. The reaction products, e.g., epoxides and oligomers, are solid and would also stay for a longer time on the predator.
Acetates with 18, 20, and more C-atoms are well-known sex pheromones in Lepidoptera. In Drosophila species, (Z)-11-C18:1-OAc and (Z)-11-C20:1-OAc act as aggregation pheromones. Acetates with defensive functions can be found in secretions of Coleoptera and Hymenoptera (Wheeler and Duffield, 1988). In Hymenoptera, larvae of a tenthridinid sawfly (Jonsson et al., 1988) and adults of social parasitic bumblebees (Zimma et al., 2003) use acetates such as C18:0-OAc in their defensive secretions. In the parasitic bumblebees, the main component C12:0-OAc is an effective repellent against host bumblebees. (Z)-11-C20:1-OAc can be found in the secretion of some chrysomelid beetles: in larvae of Linaeidea aenea [mixed with (Z)-9-C18:1-OAc and C16:0-OAc] (Sugawara et al., 1979), in adults of Gastrophysa viridula [mixed with (Z)-13-C22:1-OAc] (Eggenberger et al., 1994), and in Gastrophysa atroceanea [mixed with C18:0-OAc] (Sugawara et al., 1978). The last secretion was effective against Lasius niger ants and is similar to the secretion of S. linguis except that it has no allyl group at the end of the alcohol. There is thus strong evidence for long-chained acetates acting as repellents against predators, especially predatory ants.
Some other findings are also relevant. In a primitive Australian ant of the genus Myrmecia, (Z)-11-C20:1-OAc serves, together with (Z)-9-C18:1-OAc and some minor constituents, as an alarm pheromone produced by their Dufour gland (Jackson et al., 1989). C16:0-, C18:0-, C18:1 (9Z), and C20:0-OAc were found in the ovarioles of the weevil Hylobius abietis (Kalo, 1985; Kalo and Nederstrom, 1986), and the acetates may be useful in protecting the eggs against predators. An eicosadienal (double-bond positions unknown) has been described by Attygalle et al. (1998) as a trace component in the trail pheromone of the ant Dolichoderus thoracicus.
In Thysanoptera, Howard et al. (1987) tested the major constituents of G. ficorum secretions, C16:0-OAc and pentadecane, individually and in combination, and found that they were defensive allomones for predatory ants. C16:0-OAc individually is a better repellent than pentadecane, but a 1:1 mixture is more effective. They pointed out that the range of repellency is apparently short but effective. G. ficorum can disable potential predators by coating them with the irritating anal exudates.
Thus, many long-chained acetates serve as repellents, but why did they evolve to be repellents? Ester cocktails similar to the S. linguis defensive secretion are probably optimized to coat predators, especially jamming the chemosensory receptors. Acetates from C14:0 to C18:0 alcohols are known as water-insoluble surfactants able to spread quickly on surfaces (Karkare et al., 1993). In predators such as ants and mites that use mainly the olfactory sense, coating the sensory organs would make the animals “blind.” They would not be able to find the prey again, and ants would have difficulty evaluating their own pheromones, such as trail pheromones to return to the nest and inform nest mates about potential prey. Further studies are needed to prove this and to find out why, in contrast to other insects, the allyl group is necessary in the main constituent of S. linguis secretions.
References
U. Arndt S. Lorenz J. Schachner (1996) ArticleTitleInfluence of ozone on insect pheromones Verh. Ges. Ök. 25 187–194 Occurrence Handle1:CAS:528:DyaK2sXhtFyksL4%3D
A. B. Attygalle A. Mutti W. Rohe U. Maschwitz W. Garbe H. J. Bestmann (1998) ArticleTitleTrail pheromone from the Pavan gland of the ant Dolichoderus thoracicus (Smith). Pheromones, 108 Naturwissenschaften 85 275–277 Occurrence Handle1:CAS:528:DyaK1cXms1yms7k%3D
Blum M. S. 1991. Chemical ecology of the Thysanoptera, pp. 95–112, in B. L. Parker, M. Skinner, and T. Lewis (eds.). Towards Understanding Thysanoptera. USDA Forest Service General Technical Report NE-147.
M. S. Blum R. Footit H. M. Fales (1992) ArticleTitleDefensive chemistry and function of the anal exudate of the thrips Haplothrips leucanthemi Comp. Biochem. Physiol., C 102 209–211
H.-R. Buser H. Arn P. Guerin S. Rauscher (1983) ArticleTitleDetermination of double-bond position in mono-unsaturated acetates by mass spectrometry of dimethyl disulfide adducts Anal. Chem. 55 818–822 Occurrence Handle1:CAS:528:DyaL3sXhs1Shs70%3D
R. Csuk A. Niesen G. Tschuch G. Moritz (2004) ArticleTitleSynthesis of a natural insect repellent isolated from thrips Tetrahedron 60 6001–6004 Occurrence Handle1:CAS:528:DC%2BD2cXkvF2nu7o%3D
L. Duhamel (1963) ArticleTitleÉtudes sur l’acide phellonique Ann. Chim. (Paris) 8 315–346 Occurrence Handle1:CAS:528:DyaF3sXkvVWqtr0%3D
F. Eggenberger S. Heilporn D. Daloze J. M. Pasteels (1994) ArticleTitleSexual dimorphism in the lipid fraction of the defensive secretion of Gastrophysa viridula Experientia (Basel) 50 766–770 Occurrence Handle1:CAS:528:DyaK2cXmt1KrtLY%3D
W. E. H. Hodson (1935) ArticleTitleThe lily thrips (Liothrips vaneeckei Priesner) Bull. Entomol. Res. 26 469–474
D. F. Howard M. S. Blum H. M. Fales (1983) ArticleTitleDefense in thrips: forbidding fruitiness of a lactone Science 220 335–336 Occurrence Handle1:CAS:528:DyaL3sXhslKmt7w%3D
D. F. Howard M. S. Blum T. H. Jones H. M. Fales M. D. Tomalski (1987) ArticleTitleDefensive function and chemistry of the anal exudate of the Cuban laurel thrips Gynaikothrips ficorum (Marchal) Phytophaga 1 163–170
B. D. Jackson J. P. J. Billen E. D. Morgan (1989) ArticleTitleDufour gland contents of three species of Myrmecia (Hymenoptera, Formicidae), primitive ants of Australia J. Chem. Ecol. 15 2191–2205 Occurrence Handle1:CAS:528:DyaL1MXlvFWrtr0%3D
S. Jonsson G. Bergstrom B. S. Lanne U. Stensdotter (1988) ArticleTitleDefensive odor emission from larvae of two sawfly species, Pristiphora erichsonii and P. wasmaeli J. Chem. Ecol. 14 713–721 Occurrence Handle1:CAS:528:DyaL1cXhvVeruro%3D
P. Kalo (1985) ArticleTitleMicroanalytical methods in the structure elucidation of sex-specific components in the large pine weevil Hylobius abietis Coleoptera Curculionidae J. Chromatogr. 323 343–354 Occurrence Handle1:CAS:528:DyaL2MXktF2gurw%3D
P. Kalo A. Nederstrom (1986) ArticleTitleFemale-specific compounds in the ovaries of the large pine weevil Hylobius abietis Coleoptera Curculionidae Ann. Entomol. Fenn. 52 95–101
M. V. Karkare T. L. Hoa T. Fort (1993) ArticleTitleCriteria for effectiveness of surfactants as water-moving agents in “unsaturated” wet sand Langmuir 9 1684–1690 Occurrence Handle1:CAS:528:DyaK3sXktlSnsro%3D
A. Kobayashi F. Sugawara K. Yamashita (1978) ArticleTitleStereoselective synthesis of (Z)-11- and (E)-11-eicosenyl acetate Agric. Biol. Chem. 42 1973–1974 Occurrence Handle1:CAS:528:DyaE1MXlsVCgsw%3D%3D
T. Lewis (1973) Thrips, Their Biology, Ecology and Economic Importance, pp. 348 Academic Press New York
K. M. MacDonald J. G. C. Hamilton R. Jacobson W. D. J. Kirk (2003) ArticleTitleAnalysis of anal droplets of the western flower thrips Frankliniella occidentalis J. Chem. Ecol. 29 2385–2389 Occurrence Handle1:CAS:528:DC%2BD3sXotleju7o%3D Occurrence Handle14682520
Moritz G. 2002. The biology of thrips is not the biology of their adults: A developmental view, pp. 259–267, in R. Marullo, and L. A. Mound (eds.). Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera. Australian National Insect Collection, Canberra.
G. Moritz E. SchÄfer S. Kumm A. Steller G. Tschuch (2003) ArticleTitleDer Alien-Thrips: Suocerathrips linguis-Biologie und Verhalten Mitt. Dtsch. Ges. Allg. Angew. Entomol. 14 177–181
L. A. Mound R. Marullo (1994) ArticleTitleNew thrips on mother-in-law’s tongue Entomol. Mon. Mag. 130 95–98
R. Panades A. Ibarz M. Riba S. Esplugas (1998) ArticleTitlePhotodecomposition of the sex pheromones of Cydia pomonella and Lobesia botrana in aqueous solutions Chemosphere 36 427–434 Occurrence Handle1:CAS:528:DyaK1cXjs1OqtA%3D%3D
C. D. Stubbs A. D. Smith (1984) ArticleTitleThe modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function Biochim. Biophys. Acta 779 89–137 Occurrence Handle1:CAS:528:DyaL2cXhtVSnsL8%3D Occurrence Handle6229284
F. Sugawara A. Kobayashi K. Yamashita K. Matsuda (1978) ArticleTitleIdentification of octadecyl acetate and (Z)-11-eicosenyl acetate, major components of the defensive secretion of Gastrophysa atroceanea Motschulsky Agric. Biol. Chem. 42 687–688 Occurrence Handle1:CAS:528:DyaE1cXktl2ltrk%3D
F. Sugawara K. Matsuda A. Kobayashi K. Yamashita (1979) ArticleTitleDefensive secretion of chrysomelid larvae Linaeidea aenea Linné and Plagiodera versicolora distincta Baly J. Chem. Ecol. 5 929–934 Occurrence Handle1:CAS:528:DyaL3cXhvFCrtbw%3D
T. Suzuki K. Haga S. Kodama K. Watanabe Y. Kuwahara (1988) ArticleTitleSecretions of thrips. II. Secretion of three gall-inhabiting thrips (Thysanoptera: Phlaeothripidae) Appl. Entomol. Zool. 23 291–297 Occurrence Handle1:CAS:528:DyaL1MXkvFej
T. Suzuki K. Haga W. S. Leal S. Kodama Y. Kuwahara (1989) ArticleTitleSecretions of thrips. IV. Identification of β-acaridial from three gall-forming thrips (Thysanoptera: Phlaeothripidae) Appl. Entomol. Zool. 24 222–228 Occurrence Handle1:CAS:528:DyaK3cXkvFWi
C. R. Teerling (1995) Chemical ecology of western flower thrips B. L. Parker M. Skinner T. Lewis (Eds) Thrips Biology and Management. NATO ASI Series A: Life Sciences 276 Plenum Press New York 439–447
C. R. Teerling D. R. Gillespie J. H. Borden (1993a) ArticleTitleUtilization of western flower thrips alarm pheromone as a prey-finding kairomone by predators Can. Entomol. 125 431–437
C. R. Teerling H. D. Pierce J. H. Borden D. R. Gillespie (1993b) ArticleTitleIdentification and bioactivity of alarm pheromone in the western flower thrips, Frankliniella occidentalis J. Chem. Ecol. 19 681–697 Occurrence Handle1:CAS:528:DyaK3sXltFertLc%3D
I. Terry (1997) Host selection, communication and reproductive behavior T. Lewis (Eds) Thrips as Crop Pests CAB International Oxon 65–118
Tschuch G., Lindemann P., and Moritz G. 2002. Chemical defence in thrips, pp. 277–278, in R. Marullo and L. A. Mound (eds.). Thrips and Tospoviruses: Proceedings of the 7th International Symposium on Thysanoptera. Australian National Insect Collection, Canberra.
M. Vincenti G. Guglielmetti G. Cassani C. Tonini (1987) ArticleTitleDetermination of double-bond position in diunsaturated compounds by mass spectrometry of dimethyl disulfide derivatives Anal. Chem. 59 694–699
J. W. Wheeler R. M. Duffield (1988) Pheromones of Hymenoptera and Isoptera E. D. Morgan N. B. Mandava (Eds) Handbook of Natural Pesticides Vol. 4B Pheromones CRC Press Boca Raton, FL 59–206
B. O. Zimma M. Ayasse J. TengÖ F. Ibarra C. Schulz W. Francke (2003) ArticleTitleDo social parasitic bumblebees use chemical weapons? (Hymenoptera, Apidae) J. Comp. Physiol., A 189 769–775
Acknowledgments
We gratefully acknowledge Laurence Mound, CSIRO Canberra, and William Kirk, Keele University, Staffordshire, for valuable discussion. We are also grateful to Phil Griffiths and Alison Scott-Brown, Royal Botanic Garden, Kew, London, for sending us the founders for a stock culture of S. linguis. We thank a diploma student, Gitte Kiessling, for her help in sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tschuch, G., Lindemann, P., Niesen, A. et al. A Novel Long-Chained Acetate in the Defensive Secretion of Thrips. J Chem Ecol 31, 1555–1565 (2005). https://doi.org/10.1007/s10886-005-5797-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-005-5797-9