Abstract
An acute leukemia diagnosis can be an extremely stressful experience for most patients. Posttraumatic growth (PTG) is positive psychological change experienced following a struggle with highly challenging life circumstances. The current study is the first longitudinal investigation of predictors of PTG and distress in adult acute leukemia patients undergoing induction chemotherapy. Findings suggest that these patients report PTG, and levels of PTG appear to increase over the weeks following leukemia diagnosis and induction chemotherapy. Variables associated with higher total PTG scores over time included greater number of days from baseline, younger age, and greater challenge to core beliefs. Variables associated with higher distress included greater number of days from baseline, greater perceived cancer threat, higher symptom severity, and lower spiritual well-being. Results underscore the critical role that examination of one’s core beliefs may play in the development of PTG over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diagnosis and treatment of acute leukemia is an extremely stressful experience for most patients (Greenberg et al., 1997; Hjermstad et al., 1999; Molassiotis, van den Akker, Milligan, Goldman, & Boughton, 1996; Montgomery, Pocock, Titley, & Lloyd, 2003; Persson, Hallberg, & Ohlsson, 1997; Sasaki et al., 2000; Zittoun, Achard, &Ruszniewski, 1999). Patients with both acute lymphocytic leukemia (ALL) and acute myelogenous leukemia (AML) usually present with signs and/or symptoms of failure to produce normal blood and bone marrow cells. These symptoms include fever from inability to fight infections, bleeding and bruising from very low platelet counts, weakness/fatigue, and other symptoms of anemia. AML is the most common type of acute leukemia in adults (80%), and ALL accounts for about 20% of acute leukemia cases. The sudden manifestation of leukemia symptoms requiring immediate, intensive treatments, and prolonged hospital stays contribute to the highly stressful nature of this disease (Caudell, 1996; Xuereb & Dunlop, 2003).
Following diagnosis, patients are immediately hospitalized for induction chemotherapy, for a period of 4–6 weeks (and sometimes longer for complications). Induction therapy is the initial phase of treatment with the goal of decreasing the number of leukemia cells to undetectable levels and restoring normal blood cells. Treatment for acute leukemia includes significant risk for neutropenia (a severely low number of white blood cells associated with high risk of infection) and sepsis (the presence of pathogenic infectious organisms or their toxins in the blood and tissues) within the first few months of treatment (Lesko, 1998). Other common side effects of chemotherapy include, but are not limited to, anemia, mucositis (sores in the mouth and gastrointestinal tract), alopecia (hair loss), headaches, nausea, anorexia, fatigue, and increased vulnerability to illness/infection (Caudell, 1996; Persson et al., 2001; Valley & Balmer, 2000; Zittoun et al., 1999). Symptoms and treatment-related side effects are strongly linked to quality of life in acute leukemia patients (Persson et al., 2001).
Patients with acute leukemia also experience psychological difficulties including depressive symptoms (Molassiotis et al., 1996; Montgomery et al., 2003; Sasaki et al., 2000; Zittoun et al., 1999), reduced quality of life (Persson et al., 2001), mood disturbances (Molassiotis et al., 1996), sleep difficulties (Edman, Larsen, Hagglund, & Gardulf, 2001; Sasaki et al., 2000; Zittoun et al., 1999), and fear of recurrence following treatment (Greenberg et al., 1997; Black & White, 2005). Although this diagnosis contributes to a highly stressful experience for most patients with acute leukemia, relatively little research has been conducted during treatment for this patient group.
An increasing amount of research has demonstrated that despite substantial distress associated with the diagnosis of a life-threatening illness, patients commonly report psychological growth and enhanced well-being following cancer diagnosis and treatment (Andrykowski et al., 2005; Bellizzi, 2004; Bellizzi & Blank, 2006; Cordova et al., 2007; Lelorain, Bonnaud-Antignac, & Florin, 2010). Posttraumatic growth (PTG) is defined as “positive psychological change experienced as a result of a struggle with highly challenging life circumstances” (Tedeschi & Calhoun, 2004). These changes have qualities such as improved interpersonal relating, a greater appreciation for life, a sense of personal strength, new life possibilities, and spiritual change (Tedeschi & Calhoun, 1996). In a recent description of the process by which PTG emerges in the aftermath of trauma, Calhoun, Cann, and Tedeschi (2010) emphasize the action of several variables: the extent to which core beliefs or the “assumptive world” (Janoff-Bulman, 1992) is challenged; intrusive/brooding and deliberate/reflective rumination; the ability to manage emotional distress; self-disclosure and social reactions to disclosure; and cultural influences on belief systems. In this model, an event such as a cancer diagnosis can set in motion a questioning of core beliefs that becomes ruminative, with self-disclosure to supportive others making possible more deliberate or reflective rumination, which in turn can allow for changes in core beliefs that yield PTG. A recent study showed core beliefs to be related to both intrusive and deliberate rumination which were, in turn, differentially related to PTG and distress (Triplett, Tedeschi, Calhoun, Cann, & Reeve, 2011). Core belief challenge appears to be more strongly implicated in the process of PTG than other variables are (Lindstrom, Cann, Calhoun, & Tedeschi, 2011).
In the cancer literature, PTG has been more strongly related to psychosocial variables than to demographic or medical variables (Lelorain et al., 2010; Stanton, Bower, & Low, 2006), with two notable exceptions being longer time since diagnosis and younger age (Cordova, Cunningham, Carlson, & Andrykowski, 2001; Manne et al., 2004; Ransonm, Sheldon, & Jacobsen, 2008; Sears, Stanton, & Danoff-Burg, 2003; Smith, Dalen, Bernard, & Baumgartner, 2008). Findings from one qualitative study showed personal growth in leukemia patients (Daiter, Larson, Weddington, & Ultmann, 1988), and another study that assessed PTG in patients with hematologic malignancies (including acute leukemia) found similar levels of PTG as in other cancer populations (Carboon, Anderson, Pollard, Szer, & Seymour, 2005). No study to date, however, has examined PTG longitudinally in a sample comprised solely of acute leukemia patients.
The purpose of this study was to document emotional functioning of patients undergoing induction chemotherapy for acute leukemia. Specific goals were to investigate predictors of PTG and distress in adult acute leukemia patients. Clear and present threats to mortality might also lead to attempts to use spiritual coping mechanisms when other ways of coping with such a threat fail. People higher in spirituality may be better able to comfort themselves and therefore engage in the constructive and deliberate cognitive processing that can support PTG. We hypothesized that higher levels of PTG would be associated with greater self-reported levels of spiritual well-being, greater cancer-related rumination, greater perceived threat from cancer, and greater challenge to core belief systems. Following the process described in the model of PTG, scores on the Posttraumatic Growth Inventory were expected to increase with a greater challenge to core beliefs produced by significant levels of threat, and that necessitated a reconsideration through rumination that was initially intrusive and later more deliberate and reflective. Given that PTG has been shown to be relatively orthogonal to measures of distress, it is important to measure distress separately. We also examined the relationship of the same variables in a model using distress as the outcome to examine whether variables associated with distress are similar to those associated with PTG. No directional hypotheses for distress were made.
Method
Design
To address these questions, we conducted a longitudinal study of adult acute leukemia patients (n = 66) hospitalized for induction chemotherapy at Wake Forest Baptist Medical Center (WFBMC). Patients completed up to three packets of questionnaires: Time 1 (T1; week 0 or within 7 days of diagnosis and/or admission), Time 2 (T2; weeks 5–6 or prior to discharge from the hospital if patient was discharged prior to week 5), and Time 3 (T3; approximately weeks 9–13 upon readmission for consolidation chemotherapy (additional treatment to eliminate leukemia cells that cannot be detected and to prevent relapse).
Sample (Eligibility Criteria)
Participants were eligible if they: (1) were adults ≥18 years of age; (2) had a new diagnosis of ALL or AML; (3) were hospitalized for induction chemotherapy; and (4) spoke adequate English to understand informed consent form, complete questionnaires and converse with study staff.
Recruitment
Study participants were approached to participate in the study within 7 days of their admission to the hospital or within 7 days of their diagnosis of acute leukemia, whichever occurred later. (Some patients were admitted following diagnosis at outside hospitals while others were admitted to our medical center and then received an acute leukemia diagnosis.) They were identified by the Clinical Nurse Specialist on the Leukemia Service and then recruited by a member of the research team.
Measures
The following instruments were used to measure basic demographic, clinical, and psychosocial variables at T1, T2, and T3 with the exception of demographic information (collected at T1 only) and chart information (collected at T3 only). Detailed information for each of the study measures is summarized in Table 1. Study measures included:
Outcome Measures
Posttraumatic Growth Inventory (PTGI) (Tedeschi & Calhoun, 1996)
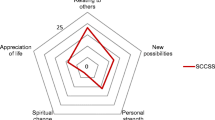
The PTGI is a 21-item scale that measures the degree of reported positive changes experienced in the struggle with major life crises. The scale includes items that assess the degree to which the individual reports specific positive changes attributed to the struggle with trauma. It includes 5 empirically-derived factors of PTG: Relating to Others, New Possibilities, Appreciation of Life, Personal Strength, and Spiritual Change. Cronbach’s alpha for the total score has consistently reported in the high range from α = .91 to 0.93 (Anderson & Lopez-Baez, 2008; Bates, Trajstman, & Jackson, 2004; Brunet, McDonough, Hadd, Crocker, & Sabiston, 2009; Linley, Andrews, & Joseph, 2007; Michael & Snyder, 2005; Morris, Shakespeare-Finch, Rieck, & Newberry, 2005; Taku, Cann, Calhoun, & Tedeschi, 2008) and self-reports of growth tend to be corroborated by others (Moore et al., 2011; Weiss, 2002). Sample items include: “I changed my priorities about what is important in life,” and “I can better appreciate each day.” The total score was used in the current study for all analyses. Analyses were not conducted for individual PTGI factors due to the small sample size.
The Profile of Mood States-Short Form (POMS-SF) (Shacham, 1983)
The POMS-SF is a 37-item adjective checklist to assess for current mood. Participants respond whether they have experienced moods (e.g., unhappy, confused, tense) over the past week. A total mood disturbance (TMD) score was used to measure overall psychological distress. The POMS-SF has sound psychometric qualities (Baker, Denniston, Zabora, Polland, & Dudley, 2002; Curran, Andrykowski, & Studts, 1995).
Predictor Variables
Sociodemographic and Medical Information
The following information was collected at baseline: age, race/ethnicity, marital/partner status, educational history, income, religious affiliation/involvement, and employment status. The patient’s medical record was the source of data for cancer diagnosis.
M.D. Anderson Symptom Inventory (MDASI) (Cleeland et al., 2000)
The MDASI is a 13-item self-report measure of the severity and impact of cancer-related symptoms such as “Your pain at its worst” and “Your nausea at its worst.” Its core items contain symptoms that account for the majority of symptom distress reported by cancer patients in active treatment; fatigue, pain, nausea, disturbed sleep, distress, shortness of breath, lack of appetite, drowsiness, dry mouth, sadness, emesis, feeling bloated, and numbness/tingling. The MDASI is designed for simplicity, brevity, and acceptability to very ill patients.
Women’s Health Initiative Insomnia Rating Scale (WHIIRS) (Levine et al., 2005)
The WHIIRS was used to measure sleep quality. This 5-item scale is designed to measure sleep initiation and maintenance in the past 4 weeks. The scale assesses sleep problems including, but not limited to the ability to fall asleep, sleep quality, and fatigue and includes items such as “Did you have trouble falling asleep,” and “Did you wake up several times a night?” This scale has acceptable levels of reliability and validity.
Functional Assessment of Chronic Illness Therapy—Spirituality (FACIT-Sp) (Peterman, Fitchett, Brady, Cella, & Hernandez, 2002)
The FACIT-Sp assesses spiritual well-being. It was developed with an ethnically diverse cancer population, contains 12 items and 2 subscales (the role of faith in illness and a sense of meaning/peace), and has good to excellent psychometric properties. One characteristic of this scale is that the wording of items does not assume a belief in God. Therefore, it can be comfortably completed by atheist or agnostic respondents, yet it taps into both traditional religiousness dimensions (faith factor) and spiritual dimensions (meaning and peace factor). Sample items include: “I feel peaceful” and “I have a reason for living.”
Social Constraint Scale (Lepore, Silver, Wortman, & Wayment, 1996)
This 10-item social constraint measure assesses the degree to which cancer patients perceive that others are unwilling or unable to provide support to them in the form of concern and listening (Lepore et al., 1996). A set of five questions is asked twice, once with regard to the “most important person” in the respondent’s life and once with regard to “other people” and includes items such as “How often did you feel as though you had to keep your feelings about your experience with leukemia to yourself because they made (important person/other people) uncomfortable,” and “When you talked about your experience with leukemia, how often did (important person/other people) give you the idea (s)he didn’t want to talk about it?” Higher scores denote greater experience of social constraints. Factor analysis supports a unitary factor for the ten items (Lepore et al., 1996).
Cancer-Related Rumination (Calhoun, Cann, Tedeschi, & McMillan, 2000)
The Cancer-Related Rumination Scale is a 12-item modified version of the Rumination Inventory developed by Calhoun et al. (2000). The items focus on the degree to which the individual reports intrusive thoughts about cancer such as “I have thoughts about cancer and I could not get rid of them.” The scale also measures deliberate thinking about the diagnosis such as, “I have thought about how to best manage the challenges associated with cancer.” A factor analysis of the baseline data identified 2 separate factors: intrusive rumination (6 variables) and deliberate rumination (5 variables), with 1 variable associated with neither. The mean scores of the intrusive rumination and deliberate rumination subscales were used in study analyses.
Perceived Threat from Cancer
The perceived threat measure was developed for this study and includes the following 4 items that assess the degree to which patients perceive that their lives and personal integrity are threatened by their cancer: “How stressful has having cancer been for you?”; “To what extent has cancer been a threat to your life or physical health?”; “To what extent has cancer disrupted your plans for the future?”; and “To what extent has cancer been a threat to your understanding of who you are as an individual?” The four items for this measure are consistent with the focus of previous studies on these aspects of the cancer experience (Horlick-Jones, 2011; Leung & Esplen, 2010; Montgomery & McCrone, 2010; Thombre & Sherman, 2010).
Core Beliefs Inventory (CBI) (Cann et al., 2010)
The CBI includes 9 items originally developed for this study in order to measure the degree of disruption to various aspects of the assumptive world (Janoff-Bulman, 1992). It quantifies the amount an individual examines core beliefs and assumptions about the world when undergoing a significant life experience. Specifically, the amount a person examines his/her beliefs about whether there is order to the world, his/her importance in that world, and that people and the world are generally ‘good’ or ‘fair.’ Participants rate the extent to which the experience with leukemia led them to seriously examine things such as “The degree to which things that happen to people are fair,” and “Your expectations for your future.” Items are summed for a total disruption score where higher indicates greater perceived disruption due to the event.
Clinical Chart Review
Clinical information was obtained from the patient’s medical record. Specifically, this information included leukemia diagnosis, date of diagnosis, date of admission, date of discharge from hospital for induction chemotherapy, previous cancer history, prescribed treatment regimen, performance status, and complications experienced during the hospital stay. Table 2 shows the treatment regimens for study participants. The most common regimen used for AML induction was a standard combination of cytarabine as a continuous infusion for 7 days in conjunction with daunorubicin on days 1–3, with or without etoposide, also on days 1–3; eight patients with acute promyelocytic leukemia, a subset of AML, received cytarabine as a 7-day continuous infusion beginning on day 3, daunorubicin for 4 days beginning on day 3 and all-trans retinoic acid (ATRA) given orally from day 1 until patient achieved remission. The primary regimen for ALL included daunorubicin for 3 days, vincristine weekly for 4 weeks, prednisone for 1 week or longer depending on age, and asparaginase given twice weekly for 6 doses beginning on day 5, with or without cyclophosphamide on day one, again depending on age.
Data Analysis
Repeated measures mixed effects models were used to assess the effects of demographic, medical, and psychosocial variables on PTGI and POMS–TMD scores over the course of the study period. Since the study measures were not completed on a pre-determined fixed schedule, a random intercept model was used. The random intercept model fits a separate intercept and regression line for each participant. The repeated measures approach allows the ability to evaluate the effect of each independent measure over the period of observation and estimate its average effect on a participant. Using this approach, a participant’s score at any point during the study interval can be estimated. A complete model was fit, with the independent variables selected through an initial analysis of the Pearson’s correlation coefficient between PTGI and POMS–TMD scores and the broader list of psychosocial measures. The remaining psychosocial variables were most strongly correlated (p < .05) to the PTGI and/or POMS–TMD scores. For consistency and comparability between measures, the same independent measures were used for both the PTGI and POMS–TMD outcomes. Predictor variables included in the full model included demographics (age, education, race, marital status, religious attendance), medical (diagnosis, symptom severity), time (days from completion of baseline measures), and psychosocial (MDASI severity score, FACIT-Sp, social constraints, cancer-related rumination as deliberate and intrusive rumination, perceived threat, WHIIRS, and CBI) variables.
Results
Over the course of three years, 134 potentially eligible patients were approached for study participation. Of these, 78 (58.2% of those approached) provided informed consent, and 66 newly diagnosed acute leukemia patients completed the baseline questionnaire. (See study flow of participants in Fig. 1.) The median age of the sample (N = 66) was 50 years old (range 19–81). Additional descriptive information is available in Table 2.
Of these 66, 40 (60.6%) completed the questionnaire at T2 (mean number of days from baseline = 31.2, SD = 11.0, range 13–63, median 28), and 37 (56.1%) completed the questionnaire at T3 (mean number of days from baseline = 75.1, SD = 32.2; range 40–166; median 69). Of those who refused or could not continue, the main reasons for non-participation were lack of interest (61%), medical issues/feeling too sick (21%), or altered mental status (10%). Participants who completed all study measures were similar to dropouts in terms of age (p = .20), religious attendance (p = .07), education (p = .95), and relationship status (p = .99).
Of the 66 participants, 63 completed the PTGI at baseline (mean score = 58.2; SD = 26.8). The remaining three reported they were either too fatigued to complete the measure or did not find the items applicable so soon after diagnosis. At T2, 40 completed the PTGI (mean score = 66.3; SD = 22.5), and at T3, 37 completed the PTGI (mean score = 73.1; SD = 20.4). The 37 participants who completed the study had mean PTGI scores of 63.4 (SD = 26.1) and 65.3 (SD = 22.5) for T1 and T2, respectively. For distress, 66 completed the POMS–TMD at baseline (mean score = 54.0; SD = 31.2). At T2, 40 completed the POMS–TMD (mean score = 48.7; SD = 32.0), and at T3, 36 completed the POMS–TMD (mean score = 43.0; SD = 28.0). The 36 participants who completed the study had mean POMS–TMD scores of 57.8 (SD = 28.4) and 49.5 (SD = 33.9) for T1 and T2, respectively.
Analysis of covariance models were derived through a process of correlating individual subscales of other psychosocial measures with the total PTGI and POMS–TMD scores, assessing the baseline correlations. (See Table 3 for intercorrelations for all study variables.) Variables that were significant (p < .05) with either outcome were included in the full models. MDASI severity, intrusive rumination, perceived threat, and core beliefs were correlated with both total PTGI and POMS–TMD scores. Deliberate rumination was associated only with PTGI. FACIT-Sp, social constraints, and WHIIRS were correlated only with POMS–TMD. Control measures, such as age and other demographics as noted previously, were added to the models as adjustment factors.
Description of Regression Results
Variables in the full models for total PTGI and POMS–TMD scores were the same for comparability between measures. (See Table 4 for final models.) For total PTGI scores, the significantly associated measures were days from baseline (p = .03), age (p = .03), deliberate rumination (p < .001), and core beliefs (p < .01). These findings indicate greater number of days from baseline, younger age, increased deliberate rumination, and greater challenge to one’s core beliefs were associated with higher PTGI scores over time. For POMS–TMD, days since baseline (p = <.01), perceived threat (p = <.001), deliberate rumination (p < .01), intrusive rumination (p < .01) and FACIT-Sp (p < .0001) were significantly associated with the outcome measure. These findings indicate that fewer days from baseline, greater perceived threat, lower deliberate rumination, higher intrusive rumination, and lower spiritual well-being scores were all associated with increased distress scores. Further, results in Table 4 demonstrate the significant changes in both PTGI and POMS–TMD scores over time. By examining the results for days since baseline, one can see that each additional day during the study period was associated with a 0.11 unit increase (on average) in PTGI score and a 0.12 unit decrease (on average) in POMS–TMD score.
Discussion
The findings of this study suggest that patients newly diagnosed with acute leukemia develop PTG and that levels of PTG appear to increase over the weeks following diagnosis and induction chemotherapy, suggesting that some patients perceive improvements in various aspects of their lives during intensive treatment for acute leukemia. While PTGI scores were increasing, distress decreased with greater number of days from baseline. The mean scores on the total PTGI for the final assessment from this study were noticeably higher than in other cancer patient populations (Stanton et al., 2006). Specifically, our mean PTGI total score for the final assessment was 72.8 while other cancers have shown means scores ranging from the low 40’s to the mid 60’s (Cordova et al., 2001; Oh et al., 2004; Widows, Jacobsen, Booth-Jones, & Fields, 2005).
The results of this study indicated several factors that were associated with higher PTG over time, including greater number of days from baseline, younger age, greater deliberate rumination and greater challenges to core beliefs. These findings support prior cross-sectional research suggesting that greater time since diagnosis (Sears et al., 2003; Smith et al., 2008) is associated with higher PTG. Several factors were also associated with higher levels of distress, including fewer days from baseline, greater perceived threat, lower deliberate rumination, greater intrusive rumination, and lower spiritual well-being. The finding that fewer days from baseline was associated with higher levels of distress coincides with previous research which suggests distress is greatest near diagnosis (Barez, Blasco, Fernandez-Castro, & Viladrich, 2009; Henselmans, Sanderman, Baas, Smink, & Ranchor, 2009; Hoskins, Budin, & Maislin, 1996; Millar, Purushotham, McLatchie, George, & Murray, 2005; Stanton et al., 2002). In terms of perceived threat, it follows that participants whose perceptions that their lives and personal integrity are greatly threatened by cancer would be more distressed than participants who perceive that cancer is less of a threat to their lives. Finally, lower spiritual well-being scores among those reporting the most distress in this study may indicate that either the distress of diagnosis creates some spiritual difficulty or that those with fewer spiritual resources experience greater distress. This finding would support previous research where spirituality has been found to be a moderator of distress (Shapiro et al., 2001).
One of the most novel findings from these data is the association of the degree of challenge to one’s core beliefs with PTG. Tedeschi and Calhoun (2004) theorized that disruptions to the assumptive world/core beliefs are what set the stage for the potential growth that some individuals report following a significant stressor. Specifically, they hypothesized that growth does not occur due to the stressor itself, but from the struggle and re-calibration of the individual’s assumptive world following the stressor (Tedeschi & Calhoun, 2004). Previous research that has attempted to investigate this relationship has been predominantly cross-sectional (Engelkemeyer & Marwit, 2008). The findings of this study provide support for the critical role that examination of one’s core beliefs may play in the development of PTG over time. Specifically, the more an individual reports examining and readjusting his/her core beliefs about such things as his/her importance in the world, whether the world is ‘good’ or ‘fair’ and whether there is meaningfulness in the world over the course of acute leukemia diagnosis and treatment, the more likely (s)he is to report higher levels of PTG.
In accordance with this model of PTG, deliberate rumination also plays a significant role in its development in this sample. People experiencing high levels of PTG report more deliberate rumination, while those who report more distress simultaneously report lower levels of deliberate rumination and greater intrusive rumination. It appears that a capability to reflect on ways to reconfigure one’s core beliefs may be occurring among persons who report more PTG.
This study had several limitations. First, the assessment occurred over a relatively short period of time. Additional follow-up assessments further out with regard to time likely would provide more information regarding the development of PTG. The issue of how long it may take for threat to disrupt core beliefs and processing to produce PTG is an empirical one. The sample here is quite different from those in other studies that focus on retrospective reports of growth. Here we are tracking it as it develops in response to a clear and present threat to mortality. We wished to see if a clear, serious and present threat of mortality hastens this process. The fact that these patients faced a severe threat is supported by the fact that many patients in this sample died. Second, this study had a relatively small sample size, and we lost a considerable number of participants at follow-up. However, given the study sample and the likely complications that these patients experience during treatment for acute leukemia, the number of participants lost was not unexpected for this patient group. Third, the PTGI subscales were not examined separately which limits our understanding of the process of PTG. Given our limited sample size, we were concerned about the potential for Type 1 error and confined our analysis to the PTGI total score. These limitations notwithstanding, this study is the first of its kind to examine PTG in a sample of adult patients with acute leukemia longitudinally. Researchers in the PTG-cancer arena have repeatedly emphasized the need for longitudinal studies of PTG in patients with cancer (Aspinwall & MacNamara, 2005; Bower & Segerstrom, 2004; Stanton et al., 2006; Tartaro et al., 2005).
These data demonstrate the development of PTG over time in a sample of acute leukemia inpatients undergoing highly intensive chemotherapy during a lengthy hospital stay and suggest that, among other variables, challenge to one’s core beliefs related to the cancer experience plays a major role in the development of PTG. The study of PTG points to possibilities for interventions and its potential impact on long-term psychological and physical health. However, as outlined in the debate on PTG (Aspinwall & Tedeschi, 2010; Coyne, Tennen, & Ranchor, 2010; Coyne & Tennen, 2010), we must proceed with caution with designing interventions whose sole purpose is to develop PTG. Instead, integrating an emphasis on discussion of how the cancer experience challenges one’s core beliefs (about oneself, relationships, the future, etc.) into psychosocial interventions may facilitate the development of PTG.
References
Anderson, W. P., Jr, & Lopez-Baez, S. I. (2008). Measuring growth with the Posttraumatic Growth Inventory. Measurement and Evaluation in Counseling and Development, 40, 215–227.
Andrykowski, M. A., Bishop, M. M., Hahn, E. A., Cella, D. F., Beaumont, J. L., Brady, M. J., et al. (2005). Long-term health-related quality of life, growth, and spiritual well-being after hematopoietic stem-cell transplantation. Journal of Clinical Oncology, 23, 599–608.
Aspinwall, L. G., & MacNamara, A. (2005). Taking positive changes seriously. Cancer, 104, 2549–2556.
Aspinwall, L. G., & Tedeschi, R. G. (2010). Of babies and bathwater: A reply to Coyne and Tennen’s views on positive psychology and health. Annals of Behavioral Medicine, 39, 27–34.
Baker, F., Denniston, M., Zabora, J., Polland, A., & Dudley, W. N. (2002). A POMS short form for cancer patients: Psychometric and structural evaluation. Psycho-Oncology, 11, 273–281.
Barez, M., Blasco, T., Fernandez-Castro, J., & Viladrich, C. (2009). Perceived control and psychological distress in women with breast cancer: A longitudinal study. Journal of Behavioral Medicine, 32, 187–196.
Bates, G. W., Trajstman, S. E., & Jackson, C. A. (2004). Internal consistency, test-retest reliability and sex differences on the Posttraumatic Growth Inventory in an Australian sample with trauma. Psychological Reports, 94, 793–794.
Bellizzi, K. M. (2004). Expressions of generativity and posttraumatic growth in adult cancer survivors. International Journal of Aging and Human Development, 58, 267–287.
Bellizzi, K. M., & Blank, T. O. (2006). Predicting posttraumatic growth in breast cancer survivors. Health Psychology, 25, 47–56.
Black, E. K., & White, C. A. (2005). Fear of recurrence, sense of coherence and posttraumatic stress disorder in haematological cancer survivors. Psycho-Oncology, 14, 510–515.
Bower, J. E., & Segerstrom, S. C. (2004). Stress management, finding benefit, and immune function: Positive mechanisms for intervention effects on physiology. Journal of Psychosomatic Research, 56, 9–11.
Brunet, J., McDonough, M. H., Hadd, V., Crocker, P. R., & Sabiston, C. M. (2009). The Posttraumatic Growth Inventory: An examination of the factor structure and invariance among breast cancer survivors. Psycho-Oncology, 19, 830–838.
Calhoun, L. G., Cann, A., & Tedeschi, R. G. (2010). The posttraumatic growth model: Socio-cultural considerations. In T. Weiss & R. Berger (Eds.), Posttraumatic growth and culturally competent practice: Lessons learned from around the globe (pp. 1–14). Hoboken, NJ: Wiley.
Calhoun, L. G., Cann, A., Tedeschi, R. G., & McMillan, J. (2000). A correlational test of the relationship between posttraumatic growth, religion, and cognitive processing. Journal of Traumatic Stress, 13, 521–527.
Cann, A., Calhoun, L. G., Tedeschi, R. G., Kilmer, R. P., Gil-Rivas, V., Vishnevsky, T., et al. (2010). The Core Beliefs Inventory: A brief measure of disruption in the assumptive world. Anxiety, Stress and Coping, 23, 19–34.
Carboon, I., Anderson, V. A., Pollard, A., Szer, J., & Seymour, J. F. (2005). Posttraumatic growth following a cancer diagnosis: Do world assumptions contribute? Traumatology, 11, 269–283.
Caudell, K. A. (1996). Psychoneuroimmunology and innovative behavioral interventions in patients with leukemia. Oncology Nursing Forum, 23, 493–502.
Cleeland, C. S., Mendoza, T. R., Wang, X. S., Chou, C., Harle, M. T., Morrissey, M., et al. (2000). Assessing symptom distress in cancer patients: The M.D. Anderson Symptom Inventory. Cancer, 89, 1634–1646.
Cordova, M. J., Cunningham, L. L., Carlson, C. R., & Andrykowski, M. A. (2001). Posttraumatic growth following breast cancer: A controlled comparison study. Health Psychology, 20, 176–185.
Cordova, M. J., Giese-Davis, J., Golant, M., Kronenwetter, C., Chang, V., & Spiegel, D. (2007). Breast cancer as trauma: Posttraumatic stress and posttraumatic growth. Journal of Clinical Psychology in Medical Settings, 14, 308–319.
Coyne, J. C., & Tennen, H. (2010). Positive psychology in cancer care: Bad science, exaggerated claims, and unproven medicine. Annals of Behavioral Medicine, 39, 16–26.
Coyne, J. C., Tennen, H., & Ranchor, A. V. (2010). Positive psychology in cancer care: A story line resistant to evidence. Annals of Behavioral Medicine, 39, 35–42.
Curran, S. L., Andrykowski, M. A., & Studts, J. L. (1995). Short form of the Profile of Mood States (POMS-SF): Psychometric information. Psychological Assessment, 7, 80–83.
Daiter, S., Larson, R. A., Weddington, W. W., & Ultmann, J. E. (1988). Psychosocial symptomatology, personal growth, and development among young adult patients following the diagnosis of leukemia or lymphoma. Journal of Clinical Oncology, 6, 613–617.
Edman, L., Larsen, J., Hagglund, H., & Gardulf, A. (2001). Health-related quality of life, symptom distress and sense of coherence in adult survivors of allogeneic stem-cell transplantation. European Journal of Cancer Care (England), 10, 124–130.
Engelkemeyer, S. M., & Marwit, S. J. (2008). Posttraumatic growth in bereaved parents. Journal of Traumatic Stress, 21, 344–346.
Greenberg, D. B., Kornblith, A. B., Herndon, J. E., Zuckerman, E., Schiffer, C. A., Weiss, R. B., et al. (1997). Quality of life for adult leukemia survivors treated on clinical trials of Cancer and Leukemia Group B during the period 1971-1988: Predictors for later psychologic distress. Cancer, 80, 1936–1944.
Henselmans, I., Sanderman, R., Baas, P. C., Smink, A., & Ranchor, A. V. (2009). Personal control after a breast cancer diagnosis: Stability and adaptive value. Psycho-Oncology, 18, 104–108.
Hjermstad, M. J., Loge, J. H., Evensen, S. A., Kvaloy, S. O., Fayers, P. M., & Kaasa, S. (1999). The course of anxiety and depression during the first year after allogeneic or autologous stem cell transplantation. Bone Marrow Transplantation, 24, 1219–1228.
Horlick-Jones, T. (2011). Understanding fear of cancer recurrence in terms of damage to ‘everyday health competence’. Sociology of Health & Illness, 33, 884–898.
Hoskins, C. N., Budin, W. C., & Maislin, G. (1996). Medical factors and patterns of adjustment to breast cancer. Psycho-Oncology, 3, 31–44.
Janoff-Bulman, R. (1992). Shattered assumptions: Towards a new psychology of trauma. New York: Free Press.
Lelorain, S., Bonnaud-Antignac, A., & Florin, A. (2010). Long term posttraumatic growth after breast cancer: Prevalence, predictors and relationships with psychological health. Journal of Clinical Psychology in Medical Settings, 17, 14–22.
Lepore, S. J., Silver, R. C., Wortman, C. B., & Wayment, H. A. (1996). Social constraints, intrusive thoughts, and depressive symptoms among bereaved mothers. Journal of Personality and Social Psychology, 70, 271–282.
Lesko, L. M. (1998). Hematopoietic dyscrasias. In J. C. Holland (Ed.), Psycho-Oncology. New York: Oxford University Press.
Leung, D., & Esplen, M. J. (2010). Alleviating existential distress of cancer patients: Can relational ethics guide clinicians? European Journal of Cancer Care, 19, 30–38.
Levine, D. W., Dailey, M. E., Rockhill, B., Tipping, D., Naughton, M. J., & Shumaker, S. A. (2005). Validation of the Women’s Health Initiative Insomnia Rating Scale in a multicenter controlled clinical trial. Psychosomatic Medicine, 67, 98–104.
Lindstrom, C. M., Cann, A., Calhoun, L. G., & Tedeschi, R. G. (2011). The relationship of core belief challenge, rumination, disclosure, and sociocultural elements to posttraumatic growth. Psychological Trauma: Theory, Research, Practice, and Policy. doi:10.1037/a0022030.
Linley, P. A., Andrews, L., & Joseph, S. (2007). Confirmatory factor analysis of the Posttraumatic Growth Inventory. Journal of Loss and Trauma, 12, 321–332.
Manne, S., Ostroff, J., Winkel, G., Fox, K., Grana, G., & Goldstein, L. (2004). Posttraumatic growth after breast cancer: Patient, partner, and couple perspectives. Psychosomatic Medicine, 66, 442–454.
Michael, S. T., & Snyder, C. R. (2005). Getting unstuck: The roles of hope, finding meaning, and rumination in adjustment to bereavement among college students. Death Studies, 29, 435–458.
Millar, K., Purushotham, A. D., McLatchie, E., George, W. D., & Murray, G. D. (2005). A 1-year prospective study of individual variation in distress, and illness perceptions, after treatment for breast cancer. Journal of Psychosomatic Research, 58, 335–342.
Molassiotis, A., van den Akker, O. B. A., Milligan, D. W., Goldman, J. M., & Boughton, B. J. (1996). Psychological adaptation and symptom distress in bone marrow transplant recipients. Psycho-Oncology, 1, 9–22.
Montgomery, M., & McCrone, S. H. (2010). Psychological distress associated with the diagnostic phase for suspected breast cancer: Systematic review. Journal of Advanced Nursing, 66, 2372–2390.
Montgomery, C., Pocock, M., Titley, K., & Lloyd, K. (2003). Predicting psychological distress in patients with leukaemia and lymphoma. Journal of Psychosomatic Research, 54, 289–292.
Moore, A. M., Gamblin, T. C., Geller, D. A., Youseff, M. N., Hoffman, K. E., Gemmell, L., et al. (2011). A prospective study of posttraumatic growth as assessed by self-report and family caregiver in the contet of advanced cancer. Psycho-Oncology, 20, 479–487.
Morris, B. A., Shakespeare-Finch, J., Rieck, M., & Newbery, J. (2005). Multidimensional nature of posttraumatic growth in an Australian population. Journal of Traumatic Stress, 18, 575–585.
Oh, S., Heflin, L., Meyerowitz, B. E., Desmond, K. A., Rowland, J. H., & Ganz, P. A. (2004). Quality of life of breast cancer survivors after a recurrence: A follow-up study. Breast Cancer Research and Treatment, 87, 45–57.
Persson, L., Hallberg, I. R., & Ohlsson, O. (1997). Survivors of acute leukaemia and highly malignant lymphoma–retrospective views of daily life problems during treatment and when in remission. Journal of Advanced Nursing, 25, 68–78.
Persson, L., Larsson, G., Ohlsson, O., & Hallberg, I. R. (2001). Acute leukaemia or highly malignant lymphoma patients’ quality of life over two years: A pilot study. European Journal of Cancer Care (England), 10, 36–47.
Peterman, A. H., Fitchett, G., Brady, M. J., Cella, D., & Hernandez, L. (2002). Measuring spiritual well-being in people with cancer: The Functional Assessment of Chronic Illness Therapy-Spiritual Well-Being Scale (FACIT-Sp). Annals of Behavioral Medicine, 24, 49–58.
Ransom, S., Sheldon, K. M., & Jacobsen, P. B. (2008). Actual change and inaccurate recall contribute to posttraumatic growth following radiotherapy. Journal of Consulting and Clinical Psychology, 76, 811–819.
Sasaki, T., Akaho, R., Sakamaki, H., Akiyama, H., Yoshino, M., Hagiya, K., et al. (2000). Mental disturbances during isolation in bone marrow transplant patients with leukemia. Bone Marrow Transplantation, 25, 315–318.
Sears, S. R., Stanton, A. L., & Danoff-Burg, S. (2003). The yellow brick road and the emerald city: Benefit finding, positive reappraisal coping and posttraumatic growth in women with early-stage breast cancer. Health Psychology, 22, 487–497.
Shacham, S. (1983). A shortened version of the Profile of Mood States. Journal of Personality Assessment, 47, 305–306.
Shapiro, S. L., Lopez, A. M., Schwartz, G. E., Bootzin, R., Figueredo, A. J., et al. (2001). Quality of life and breast cancer: Relationship to psychosocial variables. Journal of Clinical Psychology, 57, 501–519.
Smith, B. W., Dalen, J., Bernard, J. F., & Baumgartner, K. B. (2008). Posttraumatic growth in non-Hispanic White and Hispanic women with cervical cancer. Journal of Psychosocial Oncology, 26, 91–109.
Stanton, A. L., Bower, J. E., & Low, C. A. (2006). Posttraumatic growth after cancer. In L. G. Calhoun & R. G. Tedeschi (Eds.), Handbook of posttraumatic growth: Research, practice, and theory. Mahwah, NJ: Erlbaum.
Stanton, A. L., Danoff-Burg, S., & Huggins, M. E. (2002). The first year after breast cancer diagnosis: Hope and coping strategies as predictors or adjustment. Psycho-Oncology, 11, 93–102.
Taku, K., Cann, A., Calhoun, L. G., & Tedeschi, R. G. (2008). The factor structure of the Posttraumatic Growth Inventory: A comparison of five models using confirmatory factor analysis. Journal of Traumatic Stress, 21, 158–164.
Tartaro, J., Roberts, J., Nosarti, C., Luecken, L., David, A., & Crayford, T. (2005). Who benefits?: Distress, adjustment and benefit-finding among breast cancer survivors. Journal of Psychosocial Oncology, 23, 45–64.
Tedeschi, R. G., & Calhoun, L. G. (1996). The Posttraumatic Growth Inventory: Measuring the positive legacy of trauma. Journal of Traumatic Stress, 9, 455–472.
Tedeschi, R. G., & Calhoun, L. G. (2004). Target Article: ‘Posttraumatic growth: Conceptual foundations and empirical evidence. Psychological Inquiry, 15, 1–18.
Thombre, A., & Sherman, A. C. (2010). Posttraumatic growth among cancer patients in India. Journal of Behavioral Medicine, 33, 15–23.
Triplett, K. N., Tedeschi, R. G., Cann, A., Calhoun, L. G., & Reeve, C. L. (2011). Posttraumatic growth, meaning in life, and life satisfaction in response to trauma. Psychological Trauma: Theory, Research, Practice, and Policy. doi:10.1037/a0024204.
Valley, A. W., & Balmer, C. M. (2000). Cancer treatment and chemotherapy. In J. T. Dipiro, L. R., Talbert, G. C. Yee, G. R. Matzke, B. G. Wells, & L. M. Posey (Eds.), Pharmacotherapy: A pathophysiologic approach 1957–2012 (4th ed). Stamford, CT: Appleton & Lange.
Weiss, T. (2002). Posttraumatic growth in women with breast cancer and their husbands: An intersubjective validation study. Journal of Psychosocial Oncology, 20, 65–80.
Widows, M. R., Jacobsen, P. B., Booth-Jones, M., & Fields, K. K. (2005). Predictors of posttraumatic growth following bone marrow transplantation for cancer. Health Psychology, 24, 266–273.
Xuereb, M. C., & Dunlop, R. (2003). The experience of leukaemia and bone marrow transplant: Searching for meaning and agency. Psycho-Oncology, 12, 397–409.
Zittoun, R., Achard, S., & Ruszniewski, M. (1999). Assessment of quality of life during intensive chemotherapy or bone marrow transplantation. Psycho-Oncology, 8, 64–73.
Acknowledgements
The authors thank the physicians, physician assistants, nurses, and most of all, the patients and their families, who supported this work. Funding was provided by the Higginbotham Memorial Cancer Patient Support Fund.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danhauer, S.C., Russell, G.B., Tedeschi, R.G. et al. A Longitudinal Investigation of Posttraumatic Growth in Adult Patients Undergoing Treatment for Acute Leukemia. J Clin Psychol Med Settings 20, 13–24 (2013). https://doi.org/10.1007/s10880-012-9304-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10880-012-9304-5