Abstract
It has been reported that cerebral oxygenation (ScO2) measured by near infrared spectroscopy is maintained or increased by treatment with ephedrine, whereas almost all previous reports demonstrated that phenylephrine reduced ScO2. As the mechanism of the latter, the interference of the extracranial blood flow, that is extracranial contamination, has been suspected. Accordingly, in this prospective observational study, we utilized time-resolved spectroscopy (TRS), in which the effect of extracranial contamination is thought to be minimal, and evaluated whether the same result was obtained. We measured the changes in ScO2 as well as the total cerebral hemoglobin concentration (tHb) after treatment with ephedrine or phenylephrine during laparoscopic surgery by using a tNIRS-1 (Hamamatsu Photonics, Hamamatsu, Japan), which is a commercial instrument utilizing TRS. Based on a mixed-effects model with random intercepts for ScO2 or tHb including mean blood pressure, the mean difference and 95% confidence interval were evaluated as well as the predicted mean difference and its confidence interval using the interquartile range of mean blood pressure. Fifty treatments with ephedrine or phenylephrine were done. The mean differences of ScO2 were less than 0.1% and the predicted mean differences were less than 1.1% for the two drugs. The mean differences of tHb were less than 0.02 μM and the predicted mean differences were less than 0.2 μM for the drugs. The changes in ScO2 and tHb after treatments with ephedrine and phenylephrine were very small and clinically insignificant when measured by TRS. Previous reports about phenylephrine may have been affected by extracranial contamination.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ephedrine and phenylephrine are widely used for the treatment of intraoperative hypotension. It has been reported that cerebral oxygenation (ScO2) measured by near infrared spectroscopy (NIRS) is maintained or increased by treatment with ephedrine [1,2,3,4]. By contrast, almost all the previous reports demonstrated that phenylephrine reduced ScO2 by from 3 to 20% [1,2,3,4,5]. The mechanism of the reduction in ScO2 by phenylephrine has not been fully clarified. The most plausible mechanism for this reduction is a decrease in cardiac output induced by phenylephrine [3,4,5]. It increases sympathetic nerve activity reflexively, which constricts cerebral resistance vessels indirectly [3, 4]. Another possible mechanism for the reduction in the measured values is the interference of the extracranial blood flow with the ScO2 values, that is, extracranial contamination, which is an inherent limitation of NIRS [3, 4].
Indeed, the value of ScO2 measured by near-infrared spectroscopy is affected by scalp blood flow beneath the sensor [6]. In one previous study, ScO2 measured using the INVOS 4100 (Covidien, Mansfield, MA) decreased by 19% after continuous infusion of phenylephrine while mean arterial pressure (MAP) was increased from the baseline by 30 mmHg [7]. External carotid arterial blood flow and skin blood flow of the forehead also decreased by about 25% and there was a significant relationship with the decrease in ScO2. In another study, extracranial hypoxia–ischemia was induced by a circumferential pneumatic head cuff and the changes in ScO2 were compared among the several near infrared spectroscopes that employed different algorithms [8]: the INVOS 5100C and EQUANOX Classic 7600 (Nonin Medical Inc., Plymouth, MN), which employs spatially resolved spectroscopy (SRS), and the FORE-SIGHT (CAS Medical System, Brandford, CT), which employs the modified Beer-Lambert (MBL) law. After inflation of the head cuff, ScO2 measured by the INVOS 5100C, FORE-SIGHT and EQUANOX Classic 7600 decreased by 16.6%, 11.8% and 6.8%, respectively. In another study using the same protocol, ScO2 measured by the INVOS 5100C, FORE-SIGHT and NIRO-TRS (Hamamatsu Photonics, Hamamatsu, Japan), which utilizes time-resolved spectroscopy (TRS), decreased by 21.3%, 14.3% and 3.6%, respectively [9]. Thus, the influence of extracranial contamination depends on the difference of instruments (algorithms) and its effect seems to be minimal with the TRS method.
MBL and SRS employ continuous wave light and cannot measure the path lengths of the beams. On the other hand, TRS employs short pulse beams and yields a precise path length estimate, which enables measurement of the cerebral hemoglobin concentration [6]. Accordingly, ScO2 was accurately calculated from the percentage of the oxygenated hemoglobin concentration compared with the total hemoglobin concentration. TRS is well-known for its superiority over the SRS and MBL methods with respect to the correctness and reproducibility of the measured data [10]. For example, as to the correctness, good correlation was observed between the hemoglobin concentration in the blood phantom and the total hemoglobin concentration measured by TRS [10]. For the reproducibility, it was demonstrated that standard deviations obtained by repeating measurements at six different positions on the forehead were 2.1% for the TRS method and 5.1% for the SRS method [10]. In clinical settings such as cardiopulmonary bypass surgery [11] and carotid endarterectomy [12], it was reported that good correlation was observed between the ScO2 measured by TRS and the jugular venous oxygen saturation.
In addition, the absolute value of the total cerebral hemoglobin concentration (tHb), reflects cerebral blood volume (CBV) [13, 14]. CBV is another index of cerebral hemodynamics and indicates total arterial and venous blood volume in the brain [14]. As far as we know, there have been no studies to evaluate the changes in ScO2 and tHb after treatment with ephedrine or phenylephrine by using an oximeter which utilized TRS. Accordingly, in this prospective observational study, we measured the changes in these variables after treatment with ephedrine or phenylephrine during laparoscopic surgery by using the tNIRS-1 (Hamamatsu Photonics, Hamamatsu, Japan), which is a commercial instrument using TRS.
2 Materials and methods
This study was a prospective observational study approved by the ethics committee of the Hokkaido University Hospital (No. 017–0490). It was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all patients. It was registered in the UMIN Clinical Trials Registry prior to patient enrollment (UMIN000033310). Patients with ASA physical status classification 1 or 2 who were scheduled to undergo laparoscopic procedures in our university hospital were enrolled. Exclusion criteria were: (1) cardiovascular diseases of NYHA classification 2 or above, (2) cerebral diseases, (3) uncontrolled hypertension with systolic blood pressure of 160 mmHg or more, and (4) uncontrolled diabetes mellitus with HbA1c of 7.0% or more.
General anesthesia was performed using sevoflurane or desflurane with remifentanil or propofol with remifentanil. Neuromuscular blockade was achieved with rocuronium. After the induction of anesthesia, monitoring of invasive blood pressure from the radial artery was started. The sensors of the tNIRS-1 were placed on both sides of the forehead and the absolute values of cerebral oxygenated hemoglobin (O2Hb) and deoxygenated hemoglobin (HHb) concentrations were continuously collected every 5 s. The tHb was obtained by summing O2Hb and HHb. ScO2 was derived from the percentage of O2Hb compared with tHb. Left and right values were averaged.
During surgery, hypotension was treated with 4–8 mg of ephedrine or 0.05–0.2 mg of phenylephrine. The timing of treatment and selection of the drugs were entrusted to the judgement of the anesthesiologists in charge. After the treatment, we observed the changes in blood pressure and indices of tNIRS-1 with the anesthetic management for 10 min. When there were no additional treatments with drugs for this duration and when there were no changes in the concentrations of anesthetics, speed of infusion, setting of mechanical respiration, the patient’s position and conditions of pneumoperitoneum for the same period, we recorded 1-min averages of ScO2 and tHb at two points, just before the injection of ephedrine or phenylephrine and when the MAP maximally increased.
2.1 Statistical analysis
The primary objective was to observe the changes in ScO2 after treatment with ephedrine or phenylephrine. The secondary objective was to evaluate changes in tHb after treatment with ephedrine or phenylephrine. In a previous study using a prototype near infrared TRS instrument, the NIRO-TRS, the standard deviation of ScO2 was 2–5% [9]. For a two-sided 95% confidence interval with a normal mean, assuming a standard deviation of 5%, a sample size of 50 is required to obtain a half-width of at most 1.4%.
Continuous variables were expressed as median values [interquartile range (IQR)s]. We fitted the regression model of the change in ScO2 or tHb including the ephedrine- or phenylephrine-induced change in MAP as an explanatory variable, and a random intercept of patients’ identifiers to account for multiple measurements in the same patients. We presented the mean difference changes by 1 mmHg and changes of the IQR for each explanatory variable with a 95% CI. R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria at https://www.R-project.org/) was used to create charts and summarized the data.
3 Results
The study was performed in 23 patients. However, 2 patients were excluded due to insufficient acquisition of the tNIRS-1 data. Among the remaining 21 patients, 50 treatments with ephedrine were done in 18 patients and 50 treatments with phenylephrine were done in 14 patients. Patient demographic data are shown in Table 1. There was a trend for older patients to be treated with phenylephrine. Among inhaled anesthetics, desflurane was used for 10 patients and sevoflurane for 8 patients. Desflurane was used for 7 patients treated with ephedrine and 9 patients treated with phenylephrine.
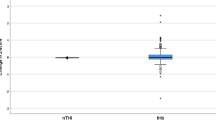
Table 2 shows the intraoperative physiological values and cerebral oxygenation indices. After injection of ephedrine, median MAP increased by about 10 mmHg. Heart rate slightly increased, but there were almost no changes in SpO2, ETCO2 or body temperature, which parameters may affect ScO2 values [15]. Median ScO2 increased by only 0.5% (Fig. 1), while median tHb decreased by only 0.3 μM. After injection of phenylephrine, median MAP increased by about 17 mmHg. Heart rate decreased by 5 bpm, but there were almost no changes in other parameters. Median ScO2 decreased by 1.2% (Fig. 1), while median tHb did not change.
Based on the mixed-effects model with random intercepts for MAP with ephedrine, the mean difference and its 95% confidence interval of changes in ScO2 and tHb were 0.100% [95%CI: 0.039, 0.155] and 0.018 μM [95%CI: − 0.004, 0.040], respectively (Table 3). With phenylephrine, the mean difference by 1 mmHg and its 95% confidence interval of changes in ScO2 and tHb were 0.047% [95%CI: − 0.0003, 0.093] and 0.02 μM [95%CI: 0.001, 0.038] (Table 3), respectively. The mean difference changing by IQR and its confidence interval determined by the interquartile range of MAP in ephedrine were 1.099% [95%CI: 0.427, 1.701] for ScO2 and 0.203 μM [95%CI: − 0.042, 0.435] in tHb (Table 3), respectively The mean difference changes by the IQR and their confidence intervals determined by the interquartile range of MAP for phenylephrine were 0.421% [− 0.003, 0.840] for ScO2 and 0.177 μM [0.01, 0.343] for tHb, respectively (Table 3).
4 Discussion
In this prospective observational study, we observed the changes in ScO2 and tHb after bolus injection of ephedrine or phenylephrine by using near-infrared TRS, which is considered to be the least susceptible to extracranial contamination among those measuring instruments using various algorithms. It is reported that normal tHb is about 85 μM [16]. Therefore, the changes in ScO2 and tHb were very small and seemed to be clinically insignificant, although MAP sufficiently increased.
Several reports have indicated that ScO2 is maintained or increased by treatment with ephedrine [1,2,3,4], which is consistent with our results. Ephedrine increases [1] or preserves [2] cardiac output. The blood flow velocity of the middle cerebral artery measured by transcranial Doppler sonography did not change after the continuous infusion of ephedrine in a healthy volunteer, although MAP increased by about 20 mmHg [17]. Recent studies using positron emission tomography (PET) and magnetic resonance imaging (MRI) have demonstrated that the continuous infusion of ephedrine increases cerebral blood flow in both the peritumoral area and contralateral hemisphere in anesthetized brain tumor patients [18, 19]. These findings support the concept that ScO2 is maintained or increased by treatment with ephedrine. In addition, external carotid artery flow measured by duplex ultrasonography was maintained after bolus injection of ephedrine in healthy volunteers [20]. Accordingly, constant results may be obtained whatever algorithms of NIRS are used.
In our study, the decrease in ScO2 after phenylephrine treatment was small and clinically insignificant, which was inconsistent with previous studies [3,4,5]. First, almost all previous studies except two used INVOS devices [2, 4], in which the influence of extracranial contamination was reported to be more than 15% of the ScO2 values [8, 9]. In the remaining 2 reports, the Oxiplex TS (ISS, Inc., Champaign IL., USA), a quantitative frequency NIRS device [2, 21], was used. There has been no information about extracranial contamination with the use of that device; however, the decrease in ScO2 measured was less than 5% [2, 21]. Second, it was consistently demonstrated that cerebral blood flow increased after treatment with phenylephrine, regardless of whether the methodology was transcranial Doppler sonography [22], duplex ultrasonography [20], PET [18] or MRI [19]. It is reported that cerebral oxygen saturation can be expressed as a function of SaO2, the blood hemoglobin concentration, cerebral blood flow, and the cerebral metabolic rate for oxygen, assuming that the ratio of blood components between the cerebral arterial and cerebral venous vascular beds remains constant [23]. Accordingly, an increase in cerebral blood flow may rather work in the direction of an increase in ScO2. On the other hand, if the ratio of blood components changes, possibly due to cerebral vasoconstriction, there is possibility of a decrease in ScO2 contrary to the increase in cerebral blood flow. However, whether the ratio really changes has not been proven yet. In addition, a recent report using MRI demonstrated that brain tissue oxygen tension was increased to around 1.2 times by treatment with phenylephrine, whereas ScO2 measured by the INVOS decreased by about 4% [19].
CBV was calculated by using a conversion formula with tHb and blood Hb [13, 14].
The formula used was:
where MWHb is the molecular weight of hemoglobin, 64,500; η is the cerebral-to-large-vessel hematocrit ratio, 0.85; and ρ is the density of cerebral tissue, 1.04.
Because it was supposed that blood Hb did not change for less than 5 min during the measurement periods in our study, changes in tHb could reflect changes in CBV. It is reported that CBV, but not cerebral blood flow, influences intracranial pressure and that changes in cerebral blood flow do not reliably predict changes in CBV [15]. In our study, tHb showed almost no changes after bolus injection of ephedrine or phenylephrine. There are few reports about the effects of these drugs on CBV. A study using MRI showed that CBV was increased by ephedrine or phenylephrine in both the peritumoral area and contralateral hemisphere in anesthetized brain tumor patients [19]. However, only one study using NIRS (Oxiplex TS) showed that phenylephrine caused a decrease in CBV of 3% during hypocapnia, although there were no changes during normocapnia or hypercapnia [21]. In our study, the patients were managed by normocapnia and the result was the same as in that study.
As one of the limitations of this study, there were 11 patients who received both of the drugs several times. Accordingly, a crossover effect of two drugs cannot be excluded and it might be that the effect of treatment averaged out. Second, the timing of treatment and selection of the drugs were entrusted to the judgement of the anesthesiologists in charge. Therefore, the choice of ephedrine or phenylephrine may have been subjected to individual bias. Third, no definition or cause of hypotension was provided. In our study, the median MAP at the time of treatment was at the 50 mmHg level in both groups. Accordingly, the degree of hypotension might not have been severe. Fourth, the degree of increase in blood pressure was different for the two drugs. If the increase in blood pressure induced by ephedrine was the same as that induced by phenylephrine, the changes in ScO2 and tHb might be greater. Fifth, we used sevoflurane, desflurane or propofol for the maintenance of anesthesia. Because the effect of cerebral circulation is different among anesthetics [15], the responses of ScO2 and tHb might be different, although the actual changes were small regardless of their type. Finally, we did not strictly fix the PaCO2 values at the time of treatment. However, as shown in Table 2, the median pre and post ETCO2 values were from 38 to 40 mmHg. Accordingly, we consider that the effect of PaCO2 was minimal.
In conclusion, this prospective observational study using near-infrared time-resolved spectroscopy, which is considered to be the least susceptible to extracranial contamination among various algorithms, under the anesthetic management of patients with ASA physical status 1 or 2 showed that the differences in ScO2 and tHb were very small and clinically insignificant after bolus injection of ephedrine or phenylephrine. Previous reports about phenylephrine may have been affected by extracranial contamination. Accordingly, we may be able to choose either one for the maintenance of general circulatory states such as the blood pressure and heart rate during anesthetic management. In the future, it will be necessary to perform a randomized controlled study using only one drug for each patient under the same physiological and anesthetic conditions using this spectroscopy.
References
Nissen P, Brassard P, Jørgensen TB, Secher NH. Phenylephrine but not ephedrine reduces frontal lobe oxygenation following anesthesia-induced hypotension. Neurocrit Care. 2010;12:17–23. https://doi.org/10.1007/s12028-009-9313-x.
Meng L, Cannesson M, Alexander BS, Yu Z, Kain ZN, Cerussi AE, Tromberg BJ, Mantulin WW. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients. Br J Anaesth. 2011;107:209–17. https://doi.org/10.1093/bja/aer150.
Thorup L, Koch KU, Upton RN, Østergaard L, Rasmussen M. Effects of vasopressors on cerebral circulation and oxygenation: a narrative review of pharmacodynamics in health and traumatic brain injury. J Neurosurg Anesthesiol. 2020;32:18–28. https://doi.org/10.1097/ana.0000000000000596.
Bombardieri AM, Singh NP, Yaeger L, Athiraman U, Tsui BCH, Singh PM. The regional cerebral oxygen saturation effect of inotropes/vasopressors administered to treat intraoperative hypotension: a Bayesian network meta-analysis. J Neurosurg Anesthesiol. 2023;35:31–40. https://doi.org/10.1097/ana.0000000000000783.
Larson S, Anderson L, Thomson S. Effect of phenylephrine on cerebral oxygen saturation and cardiac output in adults when used to treat intraoperative hypotension: a systematic review. JBI Evid Synth. 2021;19:34–58. https://doi.org/10.11124/jbisrir-d-19-00352.
Yoshitani K, Kawaguchi M, Ishida K, Maekawa K, Miyawaki H, Tanaka S, Uchino H, Kakinohana M, Koide Y, Yokota M, Okamoto H, Nomura M. Guidelines for the use of cerebral oximetry by near-infrared spectroscopy in cardiovascular anesthesia: a report by the cerebrospinal division of the academic committee of the japanese society of cardiovascular anesthesiologists (JSCVA). J Anesth. 2019;33:167–96. https://doi.org/10.1007/s00540-019-02610-y.
Ogoh S, Sato K, Okazaki K, Miyamoto T, Secher F, Sørensen H, Rasmussen P, Secher NH. A decrease in spatially resolved near-infrared spectroscopy-determined frontal lobe tissue oxygenation by phenylephrine reflects reduced skin blood flow. Anesth Analg. 2014;118:823–9. https://doi.org/10.1213/ane.0000000000000145.
Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology. 2012;116:834–40. https://doi.org/10.1097/ALN.0b013e31824c00d7.
Kato S, Yoshitani K, Kubota Y, Inatomi Y, Ohnishi Y. Effect of posture and extracranial contamination on results of cerebral oximetry by near-infrared spectroscopy. J Anesth. 2017;31:103–10. https://doi.org/10.1007/s00540-016-2275-1.
Fujisaki S, Osaki T, Suzuki T, Kamada T, Kitazawa K, Nishizawa M, Takahashi A, Suzuki S. A clinical tissue oximeter using NIR time-resolved spectroscopy. In: Elwell CE, Leung TS, Harrison DK, editors. Oxygen Transport to Tissue XXXVII, Advances in Experimental Medicine and Biology. 1st ed. New York: Springer; 2016. p. 427–33.
Ohmae E, Oda M, Suzuki T, Yamashita Y, Kakihana Y, Matsunaga A, Kanmura Y, Tamura M. Clinical evaluation of time-resolved spectroscopy by measuring cerebral hemodynamics during cardiopulmonary bypass surgery. J Biomed Opt. 2007;12:062112. https://doi.org/10.1117/1.2804931.
Yoshitani K, Kuwajima K, Irie T, Inatomi Y, Miyazaki A, Iihara K, Ohnishi Y. Clinical validity of cerebral oxygen saturation measured by time-resolved spectroscopy during carotid endarterectomy. J Neurosurg Anesthesiol. 2013;25:248–53. https://doi.org/10.1097/ANA.0b013e31827ee0cf.
Ohmae E, Ouchi Y, Oda M, Suzuki T, Nobesawa S, Kanno T, Yoshikawa E, Futatsubashi M, Ueda Y, Okada H, Yamashita Y. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: correlation with simultaneous positron emission tomography measurements. Neuroimage. 2006;29:697–705. https://doi.org/10.1016/j.neuroimage.2005.08.008.
Tanaka N, Yamamoto M, Abe T, Osawa T, Matsumoto R, Shinohara N, Saito H, Uchida Y, Morimoto Y. Changes of cerebral blood volume during robot-assisted laparoscopic radical prostatectomy: observational prospective study using near-infrared time-resolved spectroscopy. J Endourol. 2019;33:995–1001. https://doi.org/10.1089/end.2019.0217.
Patel PM, Drummond JC, Lemkuil BP. Cerebral physiology and the effets of anesthetic drugs. In: Gropper MA, editor. Miller’s Anesthesia. Philadelphia: Elsevier; 2020. p. 294–332.
Lovell AT, Marshall AC, Elwell CE, Smith M, Goldstone JC. Changes in cerebral blood volume with changes in position in awake and anesthetized subjects. Anesth Analg. 2000;90:372–6. https://doi.org/10.1097/00000539-200002000-00025.
Moppett IK, Wild MJ, Sherman RW, Latter JA, Miller K, Mahajan RP. Effects of ephedrine, dobutamine and dopexamine on cerebral haemodynamics: transcranial Doppler studies in healthy volunteers. Br J Anaesth. 2004;92:39–44. https://doi.org/10.1093/bja/aeh014.
Koch KU, Mikkelsen IK, Aanerud J, Espelund US, Tietze A, Oettingen GV, Juul N, Nikolajsen L, Østergaard L, Rasmussen M. Ephedrine versus phenylephrine effect on cerebral blood flow and oxygen consumption in anesthetized brain tumor patients: a randomized clinical trial. Anesthesiology. 2020;133:304–17. https://doi.org/10.1097/aln.0000000000003377.
Koch KU, Mikkelsen IK, Espelund US, Angleys H, Tietze A, Oettingen GV, Juul N, Østergaard L, Rasmussen M. Cerebral macro- and microcirculation during ephedrine versus phenylephrine treatment in anesthetized brain tumor patients: a randomized clinical trial using magnetic resonance imaging. Anesthesiology. 2021;135:788–803. https://doi.org/10.1097/aln.0000000000003877.
Sørensen H, Rasmussen P, Sato K, Persson S, Olesen ND, Nielsen HB, Olsen NV, Ogoh S, Secher NH. External carotid artery flow maintains near infrared spectroscopy-determined frontal lobe oxygenation during ephedrine administration. Br J Anaesth. 2014;113:452–8. https://doi.org/10.1093/bja/aet481.
Meng L, Gelb AW, Alexander BS, Cerussi AE, Tromberg BJ, Yu Z, Mantulin WW. Impact of phenylephrine administration on cerebral tissue oxygen saturation and blood volume is modulated by carbon dioxide in anaesthetized patients. Br J Anaesth. 2012;108:815–22. https://doi.org/10.1093/bja/aes023.
Soeding PF, Hoy S, Hoy G, Evans M, Royse CF. Effect of phenylephrine on the haemodynamic state and cerebral oxygen saturation during anaesthesia in the upright position. Br J Anaesth. 2013;111:229–34. https://doi.org/10.1093/bja/aet024.
Yagi Y, Yamamoto M, Saito H, Mori T, Morimoto Y, Oyasu T, Tachibana T, Ito YM. Changes of cerebral oxygenation in sequential Glenn and Fontan procedures in the same children. Pediatr Cardiol. 2017;38:1215–9. https://doi.org/10.1007/s00246-017-1647-0.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: [YK, YM]; Methodology: [YK, TK, YM]; Formal analysis and investigation: [IY, TT, Y, YM]; Writing—original draft preparation: [YK, TT]; Writing—review and editing: [YM].
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the ethics committee of the Hokkaido University Hospital (No. 017–0490). This was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and Good Clinical Practice.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kubo, Y., Kubo, T., Toki, T. et al. Effects of ephedrine and phenylephrine on cerebral oxygenation: observational prospective study using near-infrared time-resolved spectroscopy. J Clin Monit Comput 37, 1171–1177 (2023). https://doi.org/10.1007/s10877-023-01036-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-023-01036-y