Abstract
An accurate determination of body core temperature is crucial during surgery in order to avoid and treat hypothermia, which is associated with poor outcome. In a prospective observational study, we evaluated the suitability of the Tcore™ device (Drägerwerk AG & Co. KGaA, Lübeck, Germany)—a non-invasive thermometer—to accurately determine core body temperature. In patients undergoing surgery for ovarian cancer, core body temperature (CBT) was determined with the Tcore™ sensor attached to the forehead and compared with blood temperature (Tblood) as measured within the femoro-iliacal artery. Both temperatures were recorded every 10 s and the measurement error was calculated. 57,302 data pairs of CBT and Tblood were obtained in 22 patients. In a repeated-measurements version of the Bland and Altman test, a bias of − 0.02 °C and 95% limits of agreement of − 0.48 to 0.44 °C were calculated. In a population analysis, a median absolute error of 0 [− 0.1; + 0.1] °C, a bias of 0 [− 0.276; 0.271] % and an inaccuracy of 0.276 [0.274; 0.354] % was determined. Although the Tcore™ sensor was attached to the frontal skin, it provided an accurate measurement of core body temperature in the investigated intraoperative setting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
During anaesthesia, patients become hypothermic due to heat loss to the environment caused by heat radiation and convection. Moreover, both volatile and intravenous anaesthetics impair thermoregulatory control and thus cause vasodilation [1]. As a consequence, heat is redistributed from the core to peripheral tissues, which further aggravates hypothermia. Anaesthesiologists aim at maintaining normothermia, since intraoperative hypothermia is associated with complications such as coagulopathy and increased blood loss [2], as well as with an increased rate of wound infections [3]. Intraoperative temperature management typically comprises active patient warming by forced air and intravenous infusion of warmed fluids. A precise and accurate temperature monitoring is required to detect hypothermia and guide therapy. The gold standard for measuring body core temperature is to determine the blood temperature in the pulmonary artery [4], which is a highly invasive and costly method. Other monitoring locations are the distal oesophagus, nasopharynx, and tympanic membrane [1]. However, these are often not readily available in many patients. Skin temperature is typically well below core temperature[1] and thus deemed unreliable.
Recently, the Tcore™ monitoring system (Drägerwerk AG & Co. KGaA, Lübeck, Germany) has been developed, which is a single device to be attached to the forehead, consisting of two thermometers [5, 6]. One is adjacent to the forehead skin and other faces the environment. Both are separated by a known thermal resistance, which allows to deduce body core temperature [5, 6].
The Tcore™ system has been validated against the gold standard, blood temperature, in cardiac surgery only. However, this is a very special setting since cardiopulmonary bypass can cause rapid changes in body temperature resulting in considerable core temperature gradients, which impedes the transfer of obtained results to other surgeries. Therefore, we performed a study to investigate the accuracy and precision of the Tcore™—thermometer in non-cardiac surgery.
2 Methods
2.1 Study population
Female patients requiring tumour-debulking surgery for ovarian cancer were included in this prospective study after obtaining written informed consent. This study was approved by the ethics committee of the Bonn University Hospital, Germany (approval no. 207/16) and registered at ClinicalTrial.gov (ID: NCT 03368040). Only patients requiring extended haemodynamic monitoring by means of transpulmonary thermodilution were included. Exclusion criteria were an age below 18 years or pregnancy.
2.2 Protocol
In the anaesthesia induction room, standard monitoring including ECG, arterial blood pressure and pulse oximetry monitoring was established. The Tcore™ temperature monitoring system was applied to the forehead as described by the manufacturer. The body core temperature (CBT) is calculated by the monitoring system based on the formula: [7, 6]
where Tskin denotes the skin temperature underneath the sensor, Tenvironment the temperature of the environment above the sensor, and K a given coefficient, determined by the quotient of the heat conduction coefficient of the insulator and the heat transfer coefficient of human tissue.
Anaesthesia induction was performed with propofol, sufentanil, and cis-atracurium, and maintained as balanced anaesthesia with isoflurane and sufentanil. Following endotracheal intubation, a central venous catheter was inserted into the right internal jugular vein and a 5 F arterial catheter with integrated temperature probe (VolumeView™, Edwards Lifesciences LLC, Irvine, CA, USA) was introduced via the right femoral artery into the iliac artery. The patient was transferred to the operating room and the extended haemodynamic monitoring (EV1000, VolumeView™, Edwards Lifesiences LLC, Irvine, CA, USA) was established.
The temperature obtained by the Tcore™ sensor (CBT) was recorded directly by the patient monitor (Infinity® M540 and C500 Acute Care System (IACS), Drägerwerk AG & Co. KGaA, Lübeck, Germany), while the femoro-iliacal blood temperature (Tblood) was displayed on the EV1000 monitoring screen and transferred to the Dräger IACS system via serial communication. Both CBT and Tblood were displayed and recorded with a resolution of 0.1 °C. All Dräger IACS monitored parameters—including CBT and Tblood—were stored on a laptop computer every 10 s using the eDATA-Grabber Software (electronic Dräger Acquisition Tool for Analysis, v2005.10.16, Drägerwerk AG & Co. KGaA, Lübeck, Germany). Temperature was analysed offline using Microsoft Excel (Office Professional Plus 2010, Microsoft, Redmond, WA, USA). Artefacts caused by the injection of cold saline were manually identified and removed from the Tblood-time course by visual inspection.
Surgery for ovarian cancer consisted of extensive tumour debulking including peritonectomy, lymphadenectomy and lasted for several hours [8]. In some patients, cisplatin was administered intraoperatively at 41–43 °C for 90 min by means of a hyperthermic intraoperative intraperitoneal chemoperfusion (HIPEC) [9].
During surgery, patients were heated by forced air warming (Bair Hugger Model 750, Arizant Healthcare Inc., Eden Prairie, MN, USA) and received heated crystalloid fluid infusion (Hotline® Fluid Warmer, Smiths Medical Int. Ltd., Kent, UK). Care was taken to ensure that the warming blanket only covered the arms and upper body, but neither the face nor the forehead. Accordingly, the warming blanket did not cover the Tcore™-sensor. At the end of surgery, temperature monitoring was terminated.
The Tcore™-sensors were stored at room temperature and required some time to adapt to body temperature when attached to the forehead. To investigate this ramp-up independent of perioperative perturbations, four healthy volunteers were studied. In these, only the Tcore™-probe was applied and its temperature values recorded for 25 min. The time from skin attachment until a stable temperature was reached was determined offline. To do so, a temperature measurement remaining constant for more than 5 min was regarded as stable.
2.3 Statistical analysis
For each measurement of blood temperature (Tblood) and core temperature (CBT), the absolute error (AE) was calculated:
With Tblood considered as the gold standard, the percentage prediction error (PE) was determined for each measurement of blood and core temperature as
In a pooled analysis, the bias and limits of agreement were calculated as
where σ2A denotes the variance within subjects and σ2B the variance across subjects (heterogeneity). To do so, the repeated-measurements version of the Bland and Altman test [10] was applied.
Following previous temperature studies [11,12,13], we a priori defined a limit of agreement of ± 0.5 °C as clinical acceptable.
In a population analysis, the following parameters were calculated for each individual patient: The median absolute error (MDAE) was determined as:
where i denotes the i-th patient, in whom ni measurements were obtained.
The bias or median percentage error (MDPE) was calculated as:
Accordingly, the inaccuracy or median absolute percentage error (MDAPE) was determined using the absolute value of PE:
Finally, the population median as well as the 25% and the 75% percentile of the above-mentioned individual time course medians was calculated, e.g.:
where m denotes the number of patients.
Statistical analysis was performed by SigmaPlot (version 14.0, Systat Software, Erkrath/Germany), and statistical significance assumed at a p < 0.05. If not otherwise stated, data are shown as mean ± standard deviation in case of normal distribution and as median [25th percentile, 75th percentile] otherwise. In the pooled analysis, bias and limits of agreement are displayed as value [95% confidence intervals]. Results and statistic measures were rounded to three significant digits.
3 Results
Twenty-two women with an age of 59 ± 9 years, a height of 166 ± 5 cm, a weight of 76 ± 17 kg and a body mass index of 27 ± 6 kg/m2 were included. HIPEC was performed in two patients.
3.1 Pooled analysis
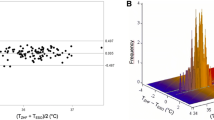
57,302 data pairs of Tblood and CBT were obtained in these 22 patients (Fig. 1). Temperature values in the range from 34.8 to 38.6 °C, and from 34.4 to 38.6 °C were observed for Tblood and CBT, respectively. A mean blood temperature of 36.2 ± 0.4 °C and a mean core temperature of 36.2 ± 0.4 °C was measured in the 22 patients.
Bland–Altman for repeated measures analysis: The difference between blood (Tblood) and core body (CBT) temperature is displayed versus the arithmetic mean of both parameters. The mean difference (bias = − 0.02 °C) as well as the upper and lower limits of agreement (= mean ± 1.96 SD = − 0.48 and 0.44 °C) are shown as blue and red lines, respectively.
The bias was − 0.02 °C and the 95% limits of agreement − 0.48 to 0.44 °C.
3.2 Population analysis
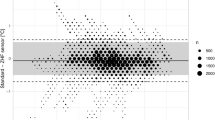
The median of the median absolute error (MDAE) was 0 [− 0.1; 0.1] °C, respectively (Fig. 2). A bias of 0 [− 0.276; 0.271] % and an inaccuracy of 0.276 [0.274; 0.354] % was calculated. Both MDAE and MDPE were statistically not different from zero (Mann–Whitney Rank Sum Test: p = 0.541 and p = 0.542, respectively).
Observed errors of the study population. The median absolute error (MDAE in °C), the median percentage error (bias, MDPE in %), and the median absolute error (inaccuracy, MDAPE in %) of the 22 patients is illustrated. The box plots indicate the median as well as the 25% and 75% percentiles, the whisker caps show the 10 and 90% percentiles. The mean is displayed as a red dashed line
As an example, the time course of CBT and Tblood is shown for a typical patient in Fig. 3 (upper part, MDPE = 0%) as well as for the patient with the worst bias (lower part, MDPE = − 1.37%).
Temperature time course of a typical patient (upper part) as well from the patient with the worst bias (lower part). The temperature obtained with the Tcore™-device (CBT) is shown in blue and the blood temperature (Tblood) in red colour. MDAE = median absolute error, MDPE (bias) = median percentage error, MDAPE (inaccuracy) = median absolute error, n = number of measurements
The two patients that underwent HIPEC did not appear to differ in terms of MDAE (− 0.2 °C and 0.1 °C, respectively), bias (− 0.57% and 0.28%) and inaccuracy (0.57% and 0.28%) from the remaining ones.
3.3 Ramp-up time
The ramp-up of the Tcore™ sensor is shown in Fig. 4. The four volunteers started at a CBT of 35.3 ± 0.6 °C, and it took 11.5 ± 4.7 min until a stable temperature level (37.2 ± 0.2 °C) was attained. A long time to gain a stable temperature correlated significantly (p < 0.05) with a low starting CBT (Pearson r2 = 0.95, Fig. 5 top panel) as well as with a large difference between starting and stable temperature (r2 = 0.97, Fig. 5 bottom panel). Regression analysis revealed that during ramp-up, CBT can be closely approximated by the hyperbolic equation
Initial time course of the Tcore™-device in four healthy volunteers as indicated in blue, red, grey and green colour. The measurements started following the attachment of the sensor to the forehead. The circles indicate the time points, when a stable core body temperature (CBT) was reached. Regression analysis revealed the hyperbolic curve shown in black
with a = 37.4 ± 0.2 °C, b = 5.1 ± 0.9 °C/min, c = 2.3 ± 0.4 min (Fig. 4), yielding a coefficient of determination R2 = 0.95 ± 0.05.
4 Discussion
Our results with the Tcore™ thermometer were within the predefined acceptable limit of agreement of ± 0.5 °C [11,12,13]. Various studies have shown less convincing limits of agreement: Gunga et al. [14] reported a limit of agreement of − 0.72 and + 0.55 °C (Table 1), which is just outside the accepted limits. However, they used rectal temperature as a reference, which is not a site of gold standard core temperature [15, 16]. Kimberger et al. [6] observed a similar limit of agreement (− 0.66 to + 0.50 °C, Table 1), however both authors used pre-production prototypes not comparable to the currently distributed, disposable Tcore™ thermometer used in our study. Another study published by Kimberger et al. [7] revealed a limit of agreement between − 0.65 °C and + 0.59 °C (Table 1). However, in patients receiving regional anaesthesia, bladder temperature was chosen as reference, which is not considered a gold standard of core temperature. We measured the reference temperature Tblood not in the pulmonary but in the femoro-iliacal artery, which has been shown to reflect the gold standard of pulmonary artery temperature most accurately [4].
Sastre et al. [17] as well as Gómez-Romero et al. [18] investigated patients undergoing cardiac surgery. They observed limits of agreement between Tblood and CBT of [− 0.8 °C to + 1.4 °C] and [− 2.0 to + 3.0 °C], respectively, which are outside the predefined acceptable range. Rapid cooling and rewarming during cardiopulmonary bypass may cause temperature gradients even within the core temperature compartment, which are typically in the range of a few tenth of a °C [11]. While temperature changes during cardiopulmonary bypass might have contributed to the poor precision reported by Gómez-Romero et al. [18], this does not explain Sastre et al. [17] results, who report inadequate precision even in the period before cardiopulmonary bypass.
We analysed patients undergoing non-cardiac but still highly invasive surgery, which yielded Tcore™ results that were more precise. Taking into consideration that significant temperature gradients within the core temperature compartment occur in cardiac surgery almost exclusively, our results are suggestive of generalizability to quite generic non-cardiac surgery.
The Tcore™ sensor differs from so-called zero-heat-flux (ZHF) thermometers by the fact that it does not require any active warming of the sensor [5, 6]. Instead it analyses the temperature gradient over a well-defined thermal bridge and calculates body core temperature according to a mathematical model [16]. Therefore, results are not necessarily comparable with ZHF thermometer studies [19, 11].
The manufacturer recommends to wait for 10 min, until reliable CBT-values can be obtained. In our patient study, we focused on monitoring pairs of Tblood and CBT. Since the arterial catheter was inserted only after induction of anaesthesia, we missed the Tcore™ ramp-up since the Tcore™ was attached before induction. Hence, we monitored ramp-up in volunteers to obtain a thorough time course analysis. To our knowledge, this is the first study that experimentally investigated this ramp-up time. We observed a slightly longer time (11.5 ± 4.7 min) until a stable temperature level was achieved than manufacturer recommendation for stabilization. The time course of CBT followed a hyperbolic function that asymptotically approached body temperature.
In studies comparing standard and novel thermometers, measurement pairs are typically pooled across patients, irrespective of the actual number of measurements within a given patient. Hence, patients with a longer duration of temperature measurement contribute more to the overall results than patients with a smaller number of measurements. To avoid this effect, we additionally performed a population analysis calculating bias and inaccuracy for every single patient. Subsequently we determined the median bias and inaccuracy of the study population, so that every patient contributed equally to the final result. Doing so, we obtained a median absolute error (MDAE) of 0 °C, median bias (MDPE) of 0% and median inaccuracy (MDAPE) of 0.3%.
The study is limited by the fact that only female patients were included. Even though the body core temperature is slightly higher in women than in men, there is no reason in principle why the Tcore™-system should work for one sex but not for the other. HIPEC might have affected the temperature in the iliac artery, however HIPEC-patient’s results did not appear to differ from the remaining patients.
In conclusion, this study demonstrated that after a short warm-up time, the non-invasive Tcore™-system enabled an accurate measurement of the body core temperature in the investigated intraoperative setting.
References
Sessler DI. Perioperative thermoregulation and heat balance. Lancet. 2016;387(10038):2655–64.
Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108(1):71–7.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996;334(19):1209–15.
Krizanac D, Stratil P, Hoerburger D, Testori C, Wallmueller C, Schober A, Haugk M, Haller M, Behringer W, Herkner H, Sterz F, Holzer M. Femoro-iliacal artery versus pulmonary artery core temperature measurement during therapeutic hypothermia: an observational study. Resuscitation. 2013;84(6):805–9.
Gunga H-C, Sandsund M, Reinertsen RE, Sattler F, Koch J. A non-invasive device to continuously determine heat strain in humans. J Therm Biol. 2008;33:297–307.
Kimberger O, Thell R, Schuh M, Koch J, Sessler DI, Kurz A. Accuracy and precision of a novel non-invasive core thermometer. Br J Anaesth. 2009;103(2):226–31.
Kimberger O, Saager L, Egan C, Sanchez IP, Dizili S, Koch J, Kurz A. The accuracy of a disposable noninvasive core thermometer. Can J Anaesth. 2013;60(12):1190–6.
Klaschik S, Gehlen J, Neumann C, Keyver-Paik MD, Soehle M, Frede S, Velten M, Hoeft A, Hilbert T. Network of mediators for vascular inflammation and leakage is dysbalanced during cytoreductive surgery for late-stage ovarian cancer. Mediators Inflamm. 2019;2019:5263717.
Zivanovic O, Abramian A, Kullmann M, Fuhrmann C, Coch C, Hoeller T, Ruehs H, Keyver-Paik MD, Rudlowski C, Weber S, Kiefer N, Poelcher ML, Thiesler T, Rostamzadeh B, Mallmann M, Schaefer N, Permantier M, Latten S, Kalff J, Thomale J, Jaehde U, Kuhn WC. HIPEC ROC I: a phase I study of cisplatin administered as hyperthermic intraoperative intraperitoneal chemoperfusion followed by postoperative intravenous platinum-based chemotherapy in patients with platinum-sensitive recurrent epithelial ovarian cancer. Int J Cancer. 2015;136(3):699–708.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17(4):571–82.
Eshraghi Y, Nasr V, Parra-Sanchez I, Van Duren A, Botham M, Santoscoy T, Sessler DI. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Anesth Analg. 2014;119(3):543–9.
Kimberger O, Cohen D, Illievich U, Lenhardt R. Temporal artery versus bladder thermometry during perioperative and intensive care unit monitoring. Anesth Analg. 2007;105(4):1042–7.
Moran JL, Peter JV, Solomon PJ, Grealy B, Smith T, Ashforth W, Wake M, Peake SL, Peisach AR. Tympanic temperature measurements: are they reliable in the critically ill? A clinical study of measures of agreement. Crit Care Med. 2007;35(1):155–64.
Gunga HC, Werner A, Stahn A, Steinach M, Schlabs T, Koralewski E, Kunz D, Belavy DL, Felsenberg D, Sattler F, Koch J. The Double Sensor-A non-invasive device to continuously monitor core temperature in humans on earth and in space. Respir Physiol Neurobiol. 2009;169(Suppl 1):63–8.
Sessler DI. Temperature monitoring and perioperative thermoregulation. Anesthesiology. 2008;109(2):318–38.
Wartzek T, Muhlsteff J, Imhoff M. Temperature measurement. Biomed Tech (Berl). 2011;56(5):241–57.
Sastre JA, Pascual MJ, Lopez T. Evaluation of the novel non-invasive zero-heat-flux Tcore thermometer in cardiac surgical patients. J Clin Monit Comput. 2019;33(1):165–72.
Gomez-Romero FJ, Fernandez-Prada M, Fernandez-Suarez FE, Gutierrez-Gonzalez C, Estrada-Martinez M, Cachero-Martinez D, Suarez-Fernandez S, Garcia-Gonzalez N, Picatto-Hernandez MD, Martinez-Ortega C, Navarro-Gracia JF. Intra-operative temperature monitoring with two non-invasive devices (3M Spoton(R) and Dräger Tcore (R)) in comparison with the Swan--Ganz catheter. Cir Cardiov. 2019;26(4):191–6.
Boisson M, Alaux A, Kerforne T, Mimoz O, Debaene B, Dahyot-Fizelier C, Frasca D. Intra-operative cutaneous temperature monitoring with zero-heat-flux technique (3M SpotOn) in comparison with oesophageal and arterial temperature: a prospective observational study. Eur J Anaesthesiol. 2018;35(11):825–30.
Acknowledgements
The complimentary provision of Tcore™ sensors by the manufacturer, Drägerwerk AG & Co.KGaA, Lübeck, Germany is gratefully acknowledged. The manuscript contains part of the doctoral thesis of HD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SZ has received consultancy fees and travel reimbursement from Drägerwerke AG & Co. KGaA in the past on projects unrelated to the investigated device. This study did not receive any support from the manufacturer other than provision of the device and disposable materials free of charge.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Soehle, M., Dehne, H., Hoeft, A. et al. Accuracy of the non-invasive Tcore™ temperature monitoring system to measure body core temperature in abdominal surgery. J Clin Monit Comput 34, 1361–1367 (2020). https://doi.org/10.1007/s10877-019-00430-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-019-00430-9