Abstract
Tcore™ Sensor is a novel zero-heat-flux thermometer that estimates core temperature from skin over forehead. We tested the hypothesis that this system estimates core temperature to an accuracy within 0.5 °C. 40 cardiac surgical patients were enrolled (960 measurements). Reference core temperatures were measured in nasopharynx, pulmonary artery and the arterial branch of the oxygenator of the cardiopulmonary bypass (CPB) circuit. 95% Bland–Altman limits of agreement for repeated measurement data was used to study the agreement between Tcore™ thermometer and the reference methods. The proportion of all differences that were within 0.5 °C and Lin’s concordance correlation coefficient (LCCC) were estimated as well. The mean overall difference between Tcore™ and nasopharyngeal temperature was − 0.2 ± 0.5 °C (95% limits of agreement of ± 1.09). The proportion of differences within 0.5 °C was 68.80% (95% CI 65.70–71.70%) for nasopharyngeal reference. LCCC was 0.84 (95% CI 0.83–0.86). The mean bias between Tcore™ and the temperature measured in the pulmonary artery was − 0.2 ± 0.5 °C (95% limits of agreement of ± 1.16). 55.30% of measurements were ≤ 0.5 °C (95% CI 51.40–59.20%). LCCC was 0.60 (95% CI 0.56–0.64). The average difference between Tcore™ and the temperature measured at the arterial outlet during the CPB period was − 0.1 ± 0.7 °C (95% limits of agreement of ± 1.43). The proportion of differences within 0.5 °C was 54.40% (95% CI 48.80–60.00%). LCCC was 0.74 (0.69–0.79). Cutaneous forehead zero-flux temperatures were not sufficiently accurate for routine clinical use in the cardiac surgical population.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Changes in body temperature are a common occurrence in any patient undergoing surgery and particularly in cardiac surgery. A study showed that 46.7% of patients undergoing coronary artery bypass grafting without cardiopulmonary bypass (CPB) had hypothermia and 5.6% hyperthermia following surgery [1]. Several studies have shown the impact of hypothermia in the prognosis of patients undergoing surgery (increased incidence of coagulopathy and bleeding, infections of the surgical wound and cardiovascular complications such as myocardial infarction, a lengthy stay in the ICU, and increased costs) [2, 3]. On the other hand, hyperthermia has been associated with greater postoperative neurocognitive dysfunction and kidney damage, especially when the temperature in the arterial branch of the CPB circuit exceeds 37 °C during rewarming [4,5,6]. For this reason, it is considered crucial to adequately monitor and manage temperature in patients subjected to CPB in order to minimize the undesirable consequences of temperature changes in a high anaesthetic and surgical risk population.
Eshragi and co-workers [7] defined the characteristics of the ideal core temperature monitoring system: it should be non-invasive, continuous, accurate (even in conditions where large and rapid temperature changes occur), regardless of the technique used and the operator, and easy to use. In cardiac surgery, the most commonly used sites for measuring core temperature are the nasopharynx and oesophagus. Only the blood temperature in the arterial line leading from the membrane oxygenator to the aortic cannula provides a good approximation of brain temperature in cardiac surgery, while the nasopharyngeal and esophageal temperatures are considered acceptable [8]. Temperatures measured using a nasopharyngeal or oesophageal thermistor are accurate and precise, but nasopharyngeal thermistor could cause epistaxis and measures obtained by oesophageal thermistor may be altered by the concomitant use of a transoesophageal ultrasound probe and, in addition, both techniques are usually reserved for the intraoperative period as they are uncomfortable for patients who are conscious. Determining the temperature at the level of the tympanic membrane or pulmonary artery also provides accurate measurements of core temperature, although the pulmonary artery catheter is not useful for the period of CPB because the pulmonary artery catheter thermistor does not produce an accurate estimate of core temperature as there is no pulmonary artery blood flow during most of the bypass period. The axilla, mouth, rectum or bladder can also be used to estimate core temperature but measurements in these sites are considered less accurate. Although skin temperature has been used to estimate the temperature of the core compartment, it is considered to be poorly correlated with it, especially in situations of a rapid increase in temperature, as occurs during the phase of rewarming of the CPB.
Temperatures measured both in the nasopharynx and in the arterial branch of the CPB circuit, being closest to the temperature measured in the jugular bulb (average gradient of 1–2 °C), are considered optimal substitutes for cerebral temperature and are therefore the most used for monitoring core temperature in cardiac surgery (Class I, Level C) [9,10,11,12].
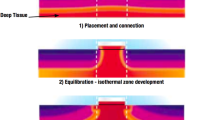
In the early 1970s, Fox and Solman described transcutaneous zero-heat-flux thermometry, a technique that sought to solve the problem of the thermal insulation of cutaneous probes using a servo-controlled system equipped with two thermistors, a heater and a thermal insulator [13]. This technique creates an isothermal tunnel with zero heat flux that enables measuring the temperature between 1 and 2 cm below the surface of the skin [7]. This measurement comes quite close to the temperature of the core compartment in well perfused tissues. There are currently several devices based on zero-heat-flux thermometry that use disposable probes that allow both intra- and postoperative monitoring. One such device is the Tcore™ Sensor (Drägerwerk AG & Co, Lübeck, Germany), which uses a disposable self-adhesive dual sensor placed on the patient’s forehead, and through a specific, battery operated reusable adapter (Tcore™ Adapter), allows its connection to a compatible vital signs monitor and after a short ramp-up time, the system calculates core body temperature continuously.
By means of an observational study, our main goal was to compare the temperature obtained using this novel system with core temperature measured in the nasopharynx in patients undergoing cardiac surgery with CPB. Specifically, we tested the hypothesis that Tcore™ temperatures are sufficiently accurate for routine clinical use. In addition, we performed the same comparison with the temperature measured using a pulmonary artery catheter and that obtained by means of a thermistor in the arterial branch of the oxygenator of the CPB circuit.
2 Methods
Ethical approval for this study was provided by the Salamanca University Hospital Ethics Committee for Research with Medicines (Salamanca, Spain) on 27 April 2016. All patients gave their written consent for the study to be conducted. Data were collected from October 2016 to June 2017. During this period, 277 patients underwent cardiac surgery under CPB at our hospital. We excluded patients with systemic or local infections of the forehead, patients with prior vesical catheterization (without temperature sensor), patients undergoing surgery under deep hypothermic circulatory arrest, as well as those for whom the insertion of a Swan-Ganz catheter was not foreseen. Finally, 40 patients participated in our study.
2.1 Protocol
Anaesthetic techniques as well as the agents used were similar in all patients. During the period prior to CPB, no active warming measures were adopted. Following CPB, active warming was commenced by means of convective air, heating the infusion fluids and those used in the surgical field to 39 °C. Operating room ambient temperature was kept at around 20 °C. At no time was 37 °C exceeded in the arterial blood outlet from the CPB circuit during rewarming.
2.2 Temperature monitoring
Following anesthesia induction, temperatures were measured in the following sites: nasopharynx, via a probe inserted to a depth equal to the distance between the nares and the earlobe (Level 1® Oesophageal Stethoscope Temperature Sensor, Smiths Medical, Kent, UK), pulmonary artery via the thermistor of a continuous cardiac output catheter (Continuous Cardiac Output Pulmonary Artery Catheter, Edwards Lifesciences, Irvine, CA) and the arterial branch of the oxygenator of the CPB circuit via a thermistor (Stöckert S5 Perfusion System, München, Germany). The Tcore™ sensor was carefully placed on the patient’s forehead above the eye and paranasal sinus following the manufacturer’s recommendations (previously removing the fatty layer of the skin and, after drying it, adhering the sensor avoiding the formation of air bubbles) and was connected to a compatible vital signs monitor via the specific adapter. Adequate positioning was confirmed at intervals throughout the study. 24 temperature measures per patient were manually recorded at 5-min intervals throughout surgery (8 readings in the pre-CPB period, 8 during the CPB period and 8 after the weaning of CPB). Thus, we obtained 960 pairs of measurements. The initial 10 min of Tcore™ measurements in each period were discarded, since it takes several minutes for equilibration. We excluded pulmonary artery measurements during the CPB period. For the same period, we obtained the blood temperature in the arterial line leading from the CPB oxygenator.
2.3 Statistics
Bland–Altman analysis with multiple observations per individual was used to evaluate the comparability of the temperature readings between Tcore™ and the other measurement sites [14]. If the observed limits of agreement (± 1.96 SD around the mean difference), within which 95% of the differences are expected to fall, were clinically acceptable, the two methods were considered equivalent. A priori the acceptable limits of agreement were chosen to be ≤ 0.5 °C. These limits can be considered clinically relevant, as a change of > 0.5 °C exceeds the usual temperature cycle variations in humans and such a difference has been associated with clinical complications [15]. Also, a Bland–Altman plot displaying the individual differences between the two measurements versus the average for the two methods was generated to show agreement with the reference method (the smaller the range between these two limits, the better the agreement). We also calculated the proportion of the Tcore™ measurements that were within 0.5 °C of the corresponding method of reference, and the 95% confidence interval for the proportion was estimated using bootstrap percentiles based on 10,000 resamples. Finally, for assessment of reproducibility, the Lin’s concordance correlation coefficient (LCCC) was computed and interpreted using McBride´s strength-of-agreement criteria for continuous variables (almost perfect: > 0.99; substantial: > 0.95–0.99; moderate: 0.90–0.95; poor:< 0.90) [16]. Results are presented as means ± SDs or means (95% limits of agreement).
IBM© SPSS© statistics 21 (IBM Corp, Armonk, NY, USA) was used for Bland–Altman and bootstrap percentile calculation. Finally, LCCC was computed using free Medcalc© software Version 17.7.2 (Medcalc software, Ostend, Belgium) (https://www.medcalc.org/).
3 Results
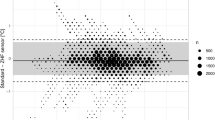
Demographic and surgical characteristics are showed in Table 1. The mean nasopharyngeal temperature after anaesthetic induction was 35.8 ± 0.4 (34.2–36.8), at the start of CPB it was 35.4 ± 0.6 (33.2–36.7) and 37.0 ± 0.6 (34.3–37.9) afterwards. Figure 1 shows the mean temperatures throughout the study period.
The mean overall difference between Tcore™ and nasopharyngeal temperature was − 0.2 ± 0.5 °C (95% limits of agreement of ± 1.09) (Fig. 2a). The proportion of differences within 0.5 °C was 68.80% (95% CI 65.70–71.70%) for nasopharyngeal reference (Table 2). LCCC was 0.84 (95% CI 0.83–0.86).
The mean bias between Tcore™ and the temperature measured in the pulmonary artery was − 0.2 ± 0.5 °C (95% limits of agreement of ± 1.16) (Fig. 2b). 55.30% of measurements were ≤ 0.5 °C (95% CI 51.40–59.20%). LCCC was 0.60 (95% CI 0.56–0.64) (Table 2).
We performed an analysis comparing the agreement between the Tcore™ and the other reference methods (nasopharyngeal and pulmonary artery) during the pre-CPB period. The difference between Tcore™ and nasopharyngeal temperature was − 0.1 ± 0.5 °C (95% limits of agreement of ± 1.09) (Fig. 3a). The proportion of differences within 0.5 °C was 66.90% (95% CI 61.60–71.90%) for the nasopharyngeal reference (Table 2). LCCC was 0.54 (95% CI 0.47–0.61). When compared to pulmonary artery temperature, the Tcore™ thermometer had a bias of − 0.3 ± 0.5 °C, with 95% of the difference expected to fall within ± 1.14 °C (Fig. 3b). Furthermore, 64.40% of measurements were within 0.5 °C (95% CI 59.00–69.00%). LCCC for all patients was 0.44 (95% CI 0.37–0.51).
Similarly, we compared the correlation between Tcore™ and nasopharyngeal temperature during CPB. The Bland–Altman analysis showed an average difference between the Tcore™ and nasopharyngeal probe of − 0.2 ± 0.5 °C (95% limits of agreement of ± 1.05) (Fig. 4a). 73.10% of the measurements were within the preset limit of ≤ 0.5 °C (95% CI 68.10–77.80%). LCCC was 0.85 (95% CI 0.82–0.88). The average difference between Tcore™ and the temperature measured at the arterial outlet during the CPB period was − 0.1 ± 0.7 °C (95% limits of agreement of ± 1.43) (Fig. 4b). The proportion of differences within 0.5 °C was 54.40% (95% CI 48.80–60.00%). LCCC was 0.74 (0.69–0.79) (Table 2).
Finally, we performed a similar analysis after weaning from the CPB. The mean post-CPB difference between Tcore™ and nasopharyngeal temperature was − 0.2 ± 0.5 °C (95% limits of agreement of ± 1.14) (Fig. 5a). 66.30% of the differences were ≤ 0.5 °C (95% CI 60.90–71.30%). LCCC was 0.5 (0.43–0.57) (Table 2). The average difference between temperatures measured by zero-flux thermometer and pulmonary artery thermistor was − 0.2 ± 0.6 °C (95% limits of agreement of ± 1.18) (Fig. 5b); 63.80% of the differences were ≤ 0.5 °C (58.40–69.00%). LCCC was 0.47 (0.40–0.54).
4 Discussion
Temperature monitoring has gained increasing interest in the field of anesthesia and especially in the management of patients undergoing cardiac surgery. This is due to the knowledge that both the anesthesia itself and surgery may cause significant changes in body temperature with deleterious consequences in surgical results. Both hypothermia and hyperthermia increase the incidence of post-surgical complications and adversely affect the patients’ prognosis. For example, mild hyperthermia (1–2 °C) or rapid rewarming have been described to exacerbate ischaemic neuronal damage, accelerate neuronal death and increase the incidence of stroke and cognitive dysfunction [4]. Hyperthermia has also been associated with an increased incidence of postoperative renal dysfunction after cardiac surgery [5]. Close monitoring of temperature during cardiac surgery via a system with adequate accuracy and precision may be crucial for the early detection of such alterations in temperature and prevent the complications resulting therefrom. Currently, temperatures obtained both in the nasopharynx and in the arterial outlet of the CPB circuit are the most used for monitoring core temperature in cardiac surgery because they have a good correlation with cerebral temperature [8,9,10,11].
Given the importance of an adequate temperature monitoring during surgery, and in an attempt of finding a continuous and non-invasive method to monitor core temperature without the disadvantages of the traditional methods, Fox and Solman described the first zero-heat-flux thermometer [13]. This method seeks to overcome one of the drawbacks of measuring skin temperature, the heat dissipation phenomenon, through a system that cancels out or minimizes heat flux through the skin’s surface. This system applied to the forehead creates an isothermal column of tissue just beneath the sensor insulation whose temperature, in theory, would be almost the same as that of the core compartment.
In recent years, several devices have appeared based on this physical principle including the Tcore™ system, developed by Drägerwerk AG & Co. The manufacturer describes a device’s technical accuracy between 25 and 45 °C of ± 0.1 °C and clinical accuracy (within the clinically validated range of 34–39 °C) of ± 0 °C (limits of agreement ± 0.6 °C). The manufacturer does not recommend its use for temperatures below 34 °C due to the lack of scientific literature.
One of the problems with these devices is that they have entered the market without any solid scientific evidence supporting their real usefulness in different clinical situations, including cardiovascular surgery. Hence the need to carry out studies like ours to evaluate the efficacy of the various devices.
Forehead zero-heat-flux measurements using the Tcore™ system reflected core temperature in the nasopharynx and pulmonary artery with a mean bias of − 0.2 °C (95% limits of agreement of ± 1.09) and − 0.2 °C (95% limits of agreement of ± 1.16) respectively. These limits of agreement were greater than our a priori acceptable limit of 0.5 °C, and greater than the previously reported by the manufacturer (± 0.6 °C). Specifically, only in 68 and 55% of the zero-heat-flux temperatures were within 0.5 °C of nasopharynx and pulmonary artery temperature. Lin´s coefficient values were lower than 0.90 (0.84 and 0.60, respectively), indicating that the strength of agreement between the temperatures measured using the Tcore™ system and the reference methods was poor in our study.
Some authors have suggested that temperature measurement by means of a zero-heat-flux technology sensor placed on the forehead would allow reasonably estimating the temperature of the core, including the rapid thermal perturbations that occur during the phases prior to or following CPB during cardiac surgery [7]. Previous studies have shown that deep-forehead temperature correlated well with pulmonary artery temperature, with a bias of 0.0 °C and a determination coefficient (r2) of 0.85 [17]. A recent study [18], conducted in patients undergoing various general, orthopaedic or urological procedures that included 14% of patients undergoing cardiac surgery, found a mean difference (zero-flux minus oesophageal or nasopharyngeal temperatures) of 0 ± 0.29 °C, with 95% of measurements included in the range of ± 0.5 °C. However, other studies show similar results to ours. Thus, Eshraghi et al. [7], in a study conducted in patients undergoing cardiac surgery in which they compared the temperatures measured using a similar technology (SpotOn system) with those obtained with a pulmonary artery catheter, found that the average difference between the two methods was − 0.08 °C (95% limits of agreement of ± 0.88), the proportion of differences within 0.5 °C was 84% (95% CI 80–88%) and LCCC was 0.70 (0.65–0.76). Similarly, in a recent study conducted by Mäkinen et al. [19], the authors found that the agreement between nasopharyngeal and zero-flow temperatures was quite good during the off-CPB period of cardiac surgery, with a 95% limit of agreement of − 0.69 to 0.49; however, throughout cardiac surgery (on- and off-CPB) and during the CPB-period the agreement was worse (95% limits of agreement of − 0.94 to 0.71 °C and − 0.94 to 1.23 respectively), although not as much as in our study. These results (wide limits of agreement and low LCCC value) highlight the fact that, although the accuracy of zero-flux thermometers is suitable, they are not so precise in the field of cardiac surgery.
One common problem in zero-heat-flux systems is that they require some time to achieve equilibrium and reduce heat flux to zero through the surface of the skin. Hence, one limitation of our study is that we do not evaluate the device equilibrium time and we also eliminate measurements in the first 10 min of each surgical period. This need to achieve equilibrium makes some authors consider that zero-heat-flux technology is not a suitable technique for monitoring temperature in situations in which sharp, intense body temperature changes may take place (for example, during cardiac surgery or in malignant hyperthermia) [19]. In our study we analyse each of the periods of surgery separately, since during the period of CPB and after weaning, temperature changes can be more rapid and more intense. The results show that there are no major differences in how the device operates compared with the reference methods throughout the different periods of cardiac surgery. The average duration of CPB-period was 140 min and, in our study, only 8 measurements were made in the first 45 min of it; for this reason, in many of the patients no measurements were taken during the rewarming phase at the end of this period and, therefore, we can suspect that the differences between the methods could be even greater.
One significant detail is that in some patients, Tcore™ underestimated temperature by 2.2 °C and overestimated temperature by 1.9 °C in relation to the reference. This difference is not trivial, especially in patients undergoing cardiac surgery where hyperthermia can exacerbate ischaemic complications of surgery.
During the study we did not apply any measure of external warming to the patient’s forehead to avoid altering the measurements. However, although the operating room climate control was set at 20 °C, we did not measure the ambient temperature of the operating room, and so we cannot rule out any interference in this respect.
Another factor that we have not considered is that some patients needed noradrenaline at some point during the surgery. It is known that vasoconstrictors can alter cutaneous blood flow and make skin temperature measurements less accurate.
Finally, the cited studies in our paper use different commercial devices (Coretemp® [17], SpotOn™ [7, 19],, TempleTouchPro [18]); although all of them are based on zero-heat-flow thermometry, they have small technological differences that make the results not completely comparable with the obtained in our study.
In summary, cutaneous forehead zero-flux temperatures obtained with Tcore™ technology were not sufficiently accurate for routine clinical use in the cardiac surgical population.
References
Hannan EL, Samadashvili Z, Wechsler A, et al. The relationship between perioperative temperature and adverse outcomes after off-pump coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2010;139:1568–75.
Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95:531–43.
Mahoney CB, Odom J. Maintaining intraoperative normothermia: a meta-analysis of outcomes with costs. AANA J. 1999;67:155–63.
Grigore AM, Grocott HP, Mathew JP, et al. The rewarming rate and increased peak temperature alter neurocognitive outcome after cardiac surgery. Anesth Analg. 2002;94:4–10.
Boodhwani M, Rubens FD, Wozny D, et al. Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting. Ann Thorac Surg. 2009;87:489–95.
Shann KG, Likosky DS, Murkin JM, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–90.
Eshraghi Y, Nasr V, Parra-Sanchez I, et al. An evaluation of a zero-heat-flux cutaneous thermometer in cardiac surgical patients. Anesth Analg. 2014;119:543–9.
Nussmeier NA, Cheng W, Marino M, et al. Temperature during cardiopulmonary bypass: the discrepancies between monitored sites. Anesth Analg. 2006;103:1373–9.
Nussmeier NA. Management of temperature during and after cardiac surgery. Tex Heart Inst J. 2005;32:472–6.
Stone JG, Young WL, Smith CR, et al. Do standard monitoring sites reflect true brain temperature when profound hypothermia is rapidly induced and reversed? Anesthesiology. 1995;82:344–51.
Engelman R, Baker RA, Likosky DS, et al. The STS/SCA/AmSECT: clinical practice guidelines for cardiopulmonary bypass-temperature management during cardiopulmonary bypass. Ann Thorac Surg. 2015;100:748–57.
Belway D, Tee R, Nathan HJ, et al. Temperature management and monitoring practices during adult cardiac surgery under cardiopulmonary bypass: results of a Canadian national survey. Perfusion. 2011;26:395–400.
Fox RH, Solman AJ. A new technique for monitoring the deep body temperature in man from the intact skin surface. J Physiol. 1971;212:8P–10P.
Bland JM, Altman DG. Agreement between methods of measurement with multiple observations per individual. J Biopharm Stat. 2007;17:571–82.
Winkler M, Akça O, Birkenberg B, et al. Aggressive warming reduces blood loss during hip arthroplasty. Anesth Analg. 2000;91:978–84.
McBride GB. A proposal for strength-of-agreement criteria for Lin’s Concordance Correlation Coefficient. NIWA Client Report: HAM2005-062; 2005.
Harioka T, Matsukawa T, Ozaki M, et al. “Deep-forehead” temperature correlates well with blood temperature. Can J Anaesth. 2000;47:980–3.
Evron S, Weissman A, Toivis V, et al. Evaluation of the Temple Touch Pro, a novel noninvasive core-temperature monitoring system. Anesth Analg. 2017;125:103–9.
Mäkinen MT, Pesonen A, Jousela I, et al. Novel zero-heat-flux deep body temperature measurement in lower extremity vascular and cardiac surgery. J Cardiothorac Vasc Anesth. 2016;30:973–8.
Funding
Drägerwerk AG & Co (Germany) provided the Tcore™ thermometer used for the study. None of the authors has a personal financial interest in this research.
Author information
Authors and Affiliations
Contributions
JAS: Study design, patient recruitment, data collection, data analysis, drawing up the first draft of the paper and correspondence ; MJP: Study design, drawing up the first draft of the paper; TL: Study design, drawing up the first draft of the paper. All coauthors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr. José A. Sastre has received an honorarium from Smiths Medical for teaching on perioperative temperature management and from 3M for the prize “Temperature monitoring and prevention of hypothermia”, of the Spanish Society of Anesthesiology. Maria J. Pascual and Teresa López have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Sastre, J.A., Pascual, M.J. & López, T. Evaluation of the novel non-invasive zero-heat-flux Tcore™ thermometer in cardiac surgical patients. J Clin Monit Comput 33, 165–172 (2019). https://doi.org/10.1007/s10877-018-0143-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-018-0143-2