Abstract
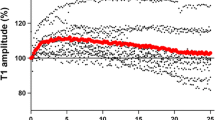
During neuromuscular monitoring, repeated electrical stimulation evokes muscle responses of increasing magnitude (‘staircase phenomenon’, SP). We aimed to evaluate whether SP affects time course and twitches’ values of an acceleromyographic assessed neuromuscular block with or without previous tetanic stimulation. Fifty adult patients were randomized to receive a 50 Hz tetanic stimulus (S group) or not (C group) before monitor calibration. After 20 min of TOF ratio (TOFr) stimulation rocuronium was administered. Onset time of block (primary endpoint), recovery of T1 to 25%, TOFr to 0.9, and recovery index were compared. We also compared T1 and TOFr at baseline, post-stimulation, and during recovery from block. Moreover the correlation between T1 at maximum recovery and (a) baseline T1 and (b) post-stimulation T1 along with T1/TOFr ratio during recovery were evaluated. After stimulation median T1 increased (32%) in group C and decreased (16%) in group S (P = 0.0001). Onset time (Median [IQR] in seconds) was 90 (29–77) vs. 75 (28–60) in C and S group (P = 0.002). Time [Mean (SD) in minutes] to normalized TOFr 0.9 were 70.13 (14.9) vs. 62.1 (21.2) in C and S groups (P = 0.204). TOFr showed no differences between groups at any time point. T1 at maximum recovery showed a stronger correlation with post-stabilization T1 compared to baseline. (ρ = 0.80 and ρ = 0.85, for C and S groups.) Standard calibration does not ensure twitch baseline stabilization and prolongs onset time of neuromuscular block. TOF ratio is not influenced by SP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Neuromuscular blockade (NMB) is an important part of general anesthesia, however, monitoring of NMB is underused for several reasons, including difficulty in interpretating results [1, 2]. Repeated indirect electrical low-frequency stimulation of a motor nerve, even at supramaximal intensity [3], results in evoked muscle responses of increasing amplitude. This is known as the staircase phenomenon (SP) [4, 5]. The SP has been observed with acceleromyography (AMG), mechanomyography or phonomyography, but not with electromyography [6,7,8]. To the best of our knowledge, there are discrepancies regarding the clinical importance of the SP on the onset and offset of neuromuscular block. Moreover, the influence of stimulation frequency and type of muscle stimulated on the magnitude of SP remains to be fully elucidated [9, 10].
While achieving twitch stability for 2–5 min prior to administration of neuromuscular blocking agents (NMBAs) is recommended in research protocols [11], and applying a short high-frequency stimulation [e.g. a 50 Hz tetanic stimulus for 5 s] is advocated to shorten this otherwise time-consuming (up to 20 min) procedure [6, 12, 13], there are no defined strategies for obtaining a stable baseline during calibration of the accelerometer in a clinical setting.
We hypothesized that if the SP is prevented by a prior tetanic stimulation, onset and recovery times of neuromuscular blockade would be shorter than if a tetanic stimulation is not applied. The main objective of our study was to evaluate the time course of rocuronium with our without previous tetanic stimulation. Secondary objectives were to evaluate specific monitor-related aspects such as sensitivity and supramaximal current.
2 Methods
This randomized controlled trial was performed at the Consorcio Hospital General Universitario de Valencia, Valencia (Spain) and Hospital de Manises, Valencia (Spain), between February 2013 and March 2014. Approval for this study was granted by both Institutional Review Boards (protocol number: 86/2009 and 0816/2013 respectively). This trial was registered with ClinicalTrials.gov (Identifier: NCT03146767).
After obtaining written informed consent, patients > 18 year old with an American Society of Anesthesiologists physical status I–II [14], undergoing surgery not involving the airway, and with an expected duration longer than 90 min were recruited. Exclusion criteria included pregnancy, diseases or medications known to interfere with neuromuscular transmission, chronic renal or hepatic failure, allergy to medications used in the study, hemodynamic instability or potentially large intraoperative blood loss.
2.1 Anesthesia management
All patients were monitored with continuous electrocardiography, noninvasive blood pressure, pulse oximetry, capnography, a respiratory gas analyser (Dräger Medical Systems, Lübeck, Germany or GE Healthcare, Chicago, Illinois, USA) and Bispectral index (BIS Vista, Covidien, Dublin, Ireland). After premedication with intravenous (iv) atropine 0.01 mg · kg− 1, fentanyl 0.1 mg, and midazolam 0.03 mg · kg− 1, general anesthesia was induced and maintained with propofol (to achieve a BIS between 40 and 60) and fentanyl boluses as required. No inhalational anesthetic agents were used. Patients lungs were ventilated to maintain an end tidal carbon dioxide (EtCO2) 35–40 mmHg. An upper body forced air warming blanket (Mistral-air plus, The 37° Company, Amersfoort, The Netherlands) was used for normothermia. Skin temperature was recorded and maintained above 32 °C, in the monitored arm on the thenar eminence by a built-in thermometer probe of the neuromuscular monitor.
2.2 Neuromuscular monitoring
Acceleromyography is based of the second law of motion: since force equals acceleration times mass, if mass is constant acceleration is directly proportional to force [15]. An accelerometer (TOF–Watch SX; Organon, Dublin, Ireland) was placed on the volar surface of the thumb distal to the interphalangeal joint to assess the adductor pollicis muscle response to ulnar nerve stimulation. The negative distal stimulating electrode was placed 1 cm proximally to the wrist on the radial side of the flexor carpi ulnari muscle and the positive one 2–3 cm more proximally. A constant preload was applied to the monitored thumb while the other fingers were strapped to the surgical table arm.
2.3 Study design and data collection
Subjects were randomly assigned to two groups with a computer-generated list by a sequence of pseudorandom numbers. For group C (control group), calibration of the AMG was set using the built-in calibration function (CAL 2 [16]) of the monitor, -that automatically determines supramaximal current, sensitivity of the accelerating sensor-, and sets the response of T1 to 100%. Thereafter, 2 Hz TOF stimuli were applied every 15 s for 20 min. Then, rocuronium bromide 0.6 mg kg− 1 was injected iv in 5 s through a fast running infusion of normal saline which was placed on the non-monitored arm.
Subjects in group S (study group) received an initial tetanic stimulation (50 Hz for 5 s, 50 mA current intensity); thereafter, the AMG monitor calibration and TOF stimulation period was the same as in group C.
All calibration procedures were performed immediately after induction of anesthesia.
Acceleromyography data were downloaded to a computer using dedicated software [TOF-Watch SX Monitor program version 2.5 for Windows (Organon, Dublin, Ireland)].
The following variables were recorded (Fig. 1):
Measured twitch values and neuromuscular block time-course as recorded on a patient’s chart from C group. Blue columns: twitch values (scale left Y-axis). Red dots: Train of Four ratio (TOFr) values. Blue continuous line: patient’s temperature (scale left Y-axis). 1: Baseline T1 and TOFr. 2: Post-Stabilization period T1 and TOFr. 3: Maximum T1 depression. 4: 25% T1 recovery. 5: 75% T1 recovery. 6: TOFr 0.9. 7: Maximum T1 recovery. Horizontal square brackets represent time intervals: Onset time (time from rocuronium injection to ≥ 95% T1 depression in seconds, primary endpoint); Time to T1 recovery to 25% (normalized to maximum recovery T1 value, in minutes); Time to TOFr 0.9 (in minutes)
-
Monitor’s sensitivity (adimensional number on a scale from 1 to 512) and supramaximal current (in milliampere, mA) as set by the automated calibration procedure.
-
Immediately after calibration: Baseline T1 and TOFr.
-
After 20 min of TOF stimulation (prior to rocuronium administration): Post-stimulation T1 and TOFr values.
-
Onset and recovery from neuromusclar block times (see Fig. 1 for full details).
-
T1 amplitude at maximum recovery (the highest amplitude reached by T1 during recovery).
In addition two calculated variables were obtained:
-
Normalized TOFr 0.9 and Time to normalized TOFr 0.9 (TOFr0.9N = 0.9 × baseline TOFr).
-
The ratio between T1 and TOFr (T1/TOFr) after calibration, post-stimulation and at T1 maximum recovery.
If the procedure was completed prior to spontaneous recovery of neuromuscular function, a reversal drug was administered, and the data following reversal were excluded from analysis. Patients were blinded to the allocation group. Due to the characteristics of the procedure, no other blinding was possible.
2.4 Statistical analysis
Analysis was performed with SPSS version 19 (IBM, Armonk, New York, USA) and Stata version 13 (Statacorp, College Station, Texas, USA) and R statistical software version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria). A priori sample size calculation determined that 21 patients in each group were required to detect a difference in onset time (primary objective) of 20% with an α error of 5% and β of 10%, with a standard deviation (SD) of 30 s (from a healthy patients’ cohort in a previous study with the same NMBA) [17]. In anticipation of possible losses 50 patients were finally recruited.
Analysis was performed on an intention to treat approach. Parametric and nonparametric data were compared between groups with unpaired Student’s t tests or Mann–Whitney U test respectively. Categorical data were compared with Fisher’s exact test. To assess the relationship between T1 amplitude at maximum recovery and baseline T1 amplitude, and T1 amplitude at maximum recovery and post-stimulation T1 amplitude, Spearman’s rank test was used. Bonferroni–Holm step-down method correction for multiple comparisons was used. For recovery values significance threshold was set at P < 0.05. A posthoc analysis to control for monitor sensitivity was performed by fitting a proportional odds model for T1 amplitude post stabilization period, and T1 amplitude at maximum recovery (in this case significance threshold for the variable in the model was set at 0.01).
3 Results
The flow chart of patient’s enrollment is showed in Fig. 2. There were no differences in demographics between groups as showed in Table 1.
3.1 Clinical results
Onset time [median (25–75 percentile)] was significantly shorter in group S [75 (28–60) s] than in group C [90 (29–77) s], P = 0.002.
Recovery times from neuromuscular block are show in Table 2. There were no differences between groups in time of recovery of T1 to 25%, time to TOFr 0.9, and time to normalized TOFr 0.9.
3.2 Accelerometer registration results
T1 amplitude and TOFr at baseline, 20 min after TOF stimulation (post-stimulation period), and at maximum recovery are summarized in Table 2. T1 was higher in group C than in group S post-stimulation and at T1 maximum recovery while TOFr showed no signifcant differences between groups (Table 2).
AMG sensitivity [mean (SD)] was significantly higher in group C [214.6 (63.2)] than in group S [160.6 (76.1)] (P = 0.014). Supramaximal current was 55 (10) mA and 60 (10) mA in groups C and S, respectively (P = 0.407).
3.3 Calculated parameters results
Proportional odds model with T1 amplitude post-stimulation period as the dependent variable, and study group and sensitivity as independent variables returned a beta coefficient of 0.086 (0.021–0.313, P = 0.001) and 1.007 (1–1.016, P = 0.067) for group S and for sensitivity respectively.
The proportional odds model built with T1 amplitude at maximum recovery as the dependent variable, and study group (with group C as reference category) and sensitivity as independent variables returned a beta coefficient of 0.14 (0.033–0.525, P = 0.005) and 1.009 (1–1.019, P = 0.042) for group S and for sensitivity respectively.
There was a moderate correlation in both groups between baseline T1 amplitude after calibration and T1 amplitude at maximum recovery, and a strong correlation between T1 amplitude post-stimulation period and T1 amplitude at maximum recovery.
T1/TOFr ratio after the stabilization period and at maximum recovery were significantly higher in group C (Table 2).
4 Discussion
The results of this investigation show that, using acceleromyography, the onset of a single bolus of rocuronium is shortened when a tetanic stimulation is used prior to TOF monitoring and calibration. Precise identification of the onset time may help optimize intubating conditions, which are associated with vocal cord injury [18]. In a recently published systematic review on laryngeal morbidity after tracheal intubation, the degree of neuromuscular block was identified as an anesthesia-related risk factor for this adverse event [19].
During recovery from NMB, both the time for T1 to reach 25% and the time to reach a TOFr 0.9 in the recovery phase showed a lag (both in raw and normalized value) in the standard calibration (control) group, albeit not reaching statistical significance. Additionally, we found that T1 amplitude increased significantly during a 20-min stabilization period in the control group and that T1/TOFr ratio was higher in control group both in the post-stabilization time and at recovery from neuromuscular block. Moreover T1 final amplitude showed greater correlation with the post-stimulation value compared to the baseline values in both groups.
The perioperative monitoring responses’ accuracy is crucial since NMB monitoring is intended to be a standard for patient’s safety. In the present study, we observed a progressive increase in evoked muscle responses that reached a plateau approximately after 15–18 min of stimulation; these observations are in agreement with previous reports [4]. It has been shown in humans and animal species that if calibration is performed without allowing twitch stabilization, the magnitude of evoked responses can increase during the recovery phase of neuromuscular block [20, 21]. Under these circumstances, which may resemble routine practice, recovery times might be biased and the displayed AMG values may overestimate the true recovery of neuromuscular function. In the present study, we recorded a difference between groups in the time to reach TOFr 0.9 albeit not statistically different between groups. Several patients were excluded from further analysis of recovery time if NMB was reversed, hence it is possible that the sample size was insufficient to detect differences for this parameter.
Previous reports show that, despite some inter-individual variability, the average twitch amplitude (measured with AMG) remains stable during a period of TOF stimulation if this is preceded by a 5 s 50 Hz tetanus [4]. Our results from patients receiving a tetanic stimulus differ from those mentioned above: twitch amplitudes showed a decrease toward the end of the 20 min of TOF stimulation if this was preceded by a 5 s 50 Hz tetanus. This could be due to a difference in supramaximal stimulation setting (which was not reported in the cited paper) or that a progressive decline in twitch amplitude following a tetanus might be observed once post-tetanic facilitation has ended, or simply by lack of statistical power in the previous report (no sample size calculation nor power analysis were reported). Such a behavior in twitch amplitude during recovery from non-depolarizing neuromuscular block has been previously observed with AMG [12,13,14,15,16,17,18,19,20,21,22] and mechanomyography [23, 24].
In accordance with other reports [12,13,14,15,16,17,18,19,20], we did not observe differences in TOFr values at any time. This observation, and the cited lack of necessity for a control value, highlights the usefulness of TOFr as a clinical monitoring parameter.
The magnitude of T1 at maximal recovery was smaller for group S than for group C. The clinical importance of a low T1 when the TOFr has recovered to 0.9 is unclear, however, a similar observation has been described when the selective reversal binding agent sugammadex was evaluated [25]. We evaluated the ratio between T1 and TOFr as an additional normalization measure, however, its potential role in patients with no or faulty monitor calibration remains to be elucidated.
Monitor characteristics, performance and accuracy are important factors that can determine the assessment that is intended to guide clinical management. Sensitivity is automatically set by the calibration process of the monitor, and ranges between 1 and 512 [26]. Sensitivity is adjusted to amplify the signal, with smaller values meaning less signal amplification. Our results show that the sensitivity of the accelerometer was significantly lower in group S than in group C. It is possible that lower sensitivity (and hence less amplification) was assigned in those patient receiving tetanus, since post-tetanic facilitation produced twitches of larger magnitude. This has also been observed in pediatric patients [13] and proposed as a possible explanation of between group differences. This suggestion raises the question of whether calibration after a tetanus might result in sensitivity values that are inadequately low. With this in mind, we fitted a proportional odds model to control for the potential effect of sensitivity on single twitch amplitude [27]. Our results show that even after controlling for sensitivity, group C has higher twitch amplitudes.
This study has limitations. We included only patients with an ASA physical status I–II without major comorbidities and undergoing elective surgery. We did not recalibrate the monitor after the 20 min stabilization period as research guidelines recommends [11]. This could imply an overestimation of the SP effect. We used standard calibration practices with currents of 55–60 mA and pulse widths (0.2 ms) that, although accepted by current guidelines, cannot absolutely guarantee a supramaximal stimulation [28]. Nevertheless we consider more important to replicate normal practice calibration. As for secondary outcomes, loss of sample size was the crucial limitation.
In conclusion, a tetanic stimulation prior to acceleromyographicmonitoring reduced the onset time of rocuronium as assessed at the adductor pollicis muscle in adult patients, and resulted in lower T1 values both after a period of stimulation and at recovery, compared with patients not receiving a tetanus prior to calibration. Further studies specifically designed to elucidate the impact of monitor sensitivity on neuromuscular block function are warranted.
References
Videira R, Vieira J. What rules of thumb do clinicians use to decide whether to antagonize nondepolarizing neuromuscular blocking drugs? Anesth. Analg. 2011;113(5):1192–6.
Della Rocca G, Iannuccelli F, Pompei L, Pietropaoli P, Reale C, Di Marco P. Neuromuscular block in Italy: a survey of current management. Minerva Anestesiol. 2012;78(7):767–73.
McCoy EP, Mirakhur RK, Connolly FM, Loan PB. The infuence of the duration of control stimulationon the onset and recovery of neuromuscular block. Anesth Analg. 1995;80(2):364–7.
Kopman A, Kumar S, Klewicka M, Neuman G. The staircase phenomenon: implications for monitoring of neuromuscular transmission. Anesthesiology. 2001;95(2):403–7.
Eleveld D, Kopma A, Proost J, Wierda J. Model to describe the degree of twitch potentiation during neuromuscular monitoring. Br J Anaesth. 2004;92(3):373–80.
Hemmerling T, Donati F, Babin D, Beaulieu P. Duration of control stimulation does not affect onset and offset of neuromuscular blockade at the corrugator supercilii muscle measured with phonomyography or acceleromyography. Can J Anaesth. 2002;49(9):913–7.
Engbaek J. Monitoring of neuromuscular transmission by electromyography during anaesthesia. A comparison with mechanomyography in cat and man. Dan Med Bull. 1996;43(4):301–16.
Deschamps S, Trager G, Mathieu PA, Hemmerling TM. The staircase phenomenon at the corrugator supercilii muscle in comparison with the hand muscles. Br J Anaesth. 2005;95(3):372–6.
McCoy E, Mirakhur R, Connolly F, Loan P. The influence of the duration of control stimulation on the onset and recovery of neuromuscular block. Anesth Analg. 1995;80(2):364–7.
Charoensook SN, Williams DJ, Chakraborty S, Leong KW, Vunjak-Novakovic G. Bioreactor model of neuromuscular junction with the electrical stimulation for pharmacological potency testing. Integr Biol (Camb). 2017;9(12):956–67.
Fuchs-Buder TCC, Skovgaard L, Eriksson L, Mirakhur R, Viby-Mogensen J. 8th International Neuromuscular Meeting. Good clinical research practice in pharmacodynamic studies of neuromuscular blocking agents II: the Stockholm revision. Acta Anaesthesiol Scand. 2007;51(7):789–808.
Lee G, Iyengar S, Szenohradsky J, Caldewell J, Wright P, Brown R, et al. Improving the design of muscle relaxant studies. Stabilization period and tetanic recruitment. Anesthesiology. 1997;86(1):48–54.
Carlos R, de Boer H, Torres M, Carmona M. The effect of prior tetanic stimulation on train-of-four monitoring in paediatric patients. Eur J Anaesthesiol. 2017;34(3):163–68.
Dripps RD, Lamont A, Eckenhoff JE. The role of anesthesia in surgical mortality. JAMA. 1961;178(3):261–6.
Viby-Mogensen J, Jensen E, Werner M, Nielsen HK. Measurement of acceleration: a new method of monitoring neuromuscular function. Acta Anaesthesiol Scand. 1988;32:45–8.
Fuchs-Buder T. Acceleromyography in: Neuromuscular monitoring in clincal practice and research. 1st ed. Heidelberg: Springer-Verlag; 2010. pp. 134–35.
Khalil M, D’Honneur G, Duvaldestin P, Slavov V, De Hys C, Gomeni R. Pharmacokynetics and pharmacodynamics of rocuronium in patients with cirrhosis. Anesthesiology. 1994;80:1241–47.
Mencke T, Echternach M, Kleinschmidt S, Lux P, Barth V, Plinkert PK, et al. Laryngeal morbidity and quality of tracheal intubation: a randomized controlled trial. Anesthesiology. 2003;98:1049–56.
Mendels E, Brunings J, Hamaekers A, Stokroos R, Kremer B, Baijens L. Adverse laryngeal effects following short-term general anesthesia. A systematic review. Arch Otolaryngol Head Neck Surg 2012;132:257–264.
Viegas O, Kopman AF, Klewicka MM. An open label, parallel group, comparative randomized multicenter trial to compare the time course of the neuromuscular effects and safety of Raplon (rapacuronium bromide) for injection and mivacurium in adults. Anesth Analg. 2001;92:S211.
Martin Flores M, Lau E, Erb HGR. Twitch potentiation: a potential source of error during neuromuscular monitoring with accelerometry in anesthesized dogs. Veterinary Anaesth Analg. 2011;38:328–35.
Larsen P, Gatke M, Fredensborg B, Berg H, Engbaek J, Viby-Mogensen J. Acceleromyography of the orbicularis oculi muscle II: comparing the orbicularis oculi and adductor pollicis muscles. Acta Anaesthesiol Scand. 2002;46(9):1131–6.
Harper N, Martlew R, Strang T, Wallace M. Monitoring neuromuscular block by acceleromyography: comparison of the Mini-Accelograph with the Myograph 2000. Br J Anaesth. 1994;72(4):411–4.
Martin-Flores M, Tseng C, Sakai D, M R, Campoy L, Gleed R. Positive and negative staircase effect during single twitch and train-of-four stimulation: a laboratory study in dogs. J Clin Monitor Comput. 2017;31(2):337–42.
Staals L, Driessen J, Van Egmond J, De Boer H, Klimek M, Flockton E, et al. Train-of-four ratio recovery often precedes twitch recovery when neuromuscular block is reversed by sugammadex. Acta Anaesthesiologica Scand. 2011;55(6):700–7.
Organon. (Ireland) Ltd. DRSCDI. TOF-WATCH SX Operator manual 33.512/A.: p. 10.
Harrell FE. Regression modeling strategies with applications to linear models, logistic regression and survival analysis. New York: Springer; 2001.
Brull SJ, Silverman DG. Pulse width, stimulus intensity, elctrode placement, and polarity during assessment of neuromuscular block. Anesthesiology. 1995;83:702–9.
Piotrkiewicz M, Celichowski J. Tetanic potentiation in motor units of rat medial gastrocnemius. Acta Neurobiol Exp (Wars). 2007;67(1):35–42.
Krarup C. Enhancement and diminution of mechanical tension evoked by staircase and by tetanus in rat muscle. J Physiol 1981;311:355–72.
Yamaguchi M, Kimura M, Li ZB, Ohno T, Takemori S, Hoh JF, et al. X-ray diffraction analysis of the effects of myosin regulatory light chain phosphorylation and butanedione monoxime on skinned skeletal muscle fibers. Am J Physiol Cell Physiol. 2016;310(8):C692–700.
Persechini A, Stull J, Cooke R. The effect of myosin phosphorylation on the contractile properties of skinned rabbit skeletal nuscle fibers. J Biol Chem. 1985;260:7951–4.
Lee J, Ho W, Lee S. Post-tetanic increase in the fast-releasing synaptic vesicle pool at the expense of the slowly releasing pool. J Gen Physiol. 2010;136(3):259–72.
Balakrishnan V, Srinivasan G, von Gersdorff H. Post-tetanic potentiation involves the presynaptic binding of calcium to calmodulin. J Gen Physiol. 2010;136(3):243–5.
Funding
No funding was received for the conduct of this study and no sponsor was required.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GM and MMF have no conflict of interest. CLE and OD received payment for educational talks and scientific conferences from MSD (Merck Sharp & Dohme, Inc.). No conflict of interests related with the present work.
Ethical approval
Ethical approval was granted by the applicable Institutional Review Board as detailed in the text.
Additional information
Published in part as a PhD dissertation (GM, available at: https://www.educacion.gob.es/teseo/irGestionarConsulta.do;jsessionid=DBD573CC72EAAC90F8B46CAFA69986F3).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Annex 1
Annex 1
The staircase phenomenon is a phenomenon related to, but not entirely identical to postetanic potentiation [29]. It has been showed in animals that the increment in evoked mechanical response, but not the compound action potential, is associated to the number of indirect stimuli administered (up to a plateau of 250) [30], due to the phosphorilation of the myosin regulatory light chain [31] which enhances cell’s sensitivity to calcium and a subsequent increase in contraction force [32], a process likely mediated by calmodulin [33, 34].
Rights and permissions
About this article
Cite this article
Mazzinari, G., Errando, C.L., Díaz-Cambronero, O. et al. Influence of tetanic stimulation on the staircase phenomenon and the acceleromyographic time-course of neuromuscular block: a randomized controlled trial. J Clin Monit Comput 33, 325–332 (2019). https://doi.org/10.1007/s10877-018-0157-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-018-0157-9