Abstract
The objective was to compare the impact of an early goal-directed hemodynamic therapy based on cardiac output monitoring (Endotracheal Cardiac Output Monitor, ECOM) with a standard of care on postoperative outcome following coronary surgery. This prospective, controlled, parallel-arm trial randomized 100 elective primary coronary artery bypass grafting patients to a study group (ECOM; n = 50) or a control group (control; n = 50). In the ECOM group, hemodynamic therapy was guided by respiratory stroke volume variation and cardiac index given by the ECOM system. A standard of care was used in the control. Goal-directed therapy was started immediately after induction of anesthesia and continued until arrival in the intensive care unit (ICU). The primary endpoint was the time when patients fulfilled discharge criteria from hospital (possible hospital discharge). Secondary endpoints were the hospital discharge, the time to reach extubation, the length of stay in ICU, the number of major adverse cardiac events, and in-hospital mortality. Patients in the ECOM group received more often fluid loading and dobutamine. The time to reach extubation was reduced in the ECOM group: 510 min [360–1,110] versus 570 min [320–1,520], P = 0.005. No significant differences were found between both groups for possible hospital discharge [Hazard Ratio = 0.96 (95 % CI 0.64–1.45)] and hospital discharge [Hazard Ratio = 1.20 (95 % CI 0.79–1.81)]. A mini-invasive early goal-directed hemodynamic therapy based on ECOM can reduce the time to reach extubation but fails to significantly reduce the length of stay in hospital and the rate of major cardiac morbidity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Despite important advances in the care of patients leading to a decrease in short-term mortality, the postoperative morbidity remains high following elective cardiac surgery [1]. As a consequence, the lengths of stay in intensive care unit (ICU) and in hospital can be long and are associated with a high healthcare cost [2]. The positive impact of early goal-directed hemodynamic therapy on postoperative outcome has been increasingly investigated for high-risk patients undergoing noncardiac and cardiac surgeries [3–5]. However, these pre-emptive strategies require advanced hemodynamic monitoring to assess cardiac output. The classic available tools (intermittent thermodilution or esophageal Doppler), either invasive or operator-dependent and necessitating a learning curve, are not convenient in routine practice and they remain insufficiently used among North American and European Anesthesiologists [6]. Besides, new mini-invasive cardiac output devices have been developed [7]. More convenient and easy to use, they could help the practitioners in promoting advanced hemodynamic monitoring and early goal-directed therapy at the bedside in high-risk patients, in an attempt to further improve postoperative outcome.
The Endotracheal Cardiac Output Monitor (ECOM Medical, Inc., San Juan Capistrano, CA) is a new Food and Drug Administration-approved device that provides continuous cardiac output measurement via a specifically designed endotracheal tube using three-dimensional bioimpedance in conjunction with an arterial catheter. This new mini-invasive cardiac output monitor was first evaluated in an animal study, which suggested it was both promising and safe [8]. Then, validation studies conducted in the setting of human cardiac surgery have reported contrasting results [9–14]. Data resulting from randomized phase III (clinical utility/outcome) studies are not available. Thus, it is doubtful whether ECOM could help to conduct perioperative goal-directed hemodynamic therapy during cardiac surgery.
The objective of the present randomized controlled trial was to compare the impact of a mini-invasive early goal-directed hemodynamic therapy based on ECOM with a standard of care on postoperative outcome following conventional elective primary coronary surgery. We hypothesized that the use of ECOM would lead to improve intraoperative hemodynamics and result in less postoperative complications and earlier discharge from the hospital in this well-studied and standardized high-risk surgical group of patients.
2 Methods
2.1 Patient population
The study was registered at clinicaltrials.gov (identifier: NCT01535716) and was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) [15]. Institutional approval was obtained from the Ethical Committee (Comité de Protection des Personnes Nord-Ouest III, CHU de Caen, Caen, France) and signed written informed consent was obtained from all participants (Ref. CPP: 2011-A01210-41). It was a single-site, prospective, controlled, randomized, single-blind, parallel-arm trial designed to investigate the potential superiority of an intraoperative hemodynamic treatment algorithm using respiratory stroke volume variation (SVV) and cardiac index given by ECOM. One hundred consecutive adult patients undergoing elective primary coronary artery bypass grafting (CABG) surgery with cardiopulmonary bypass (CPB) were investigated at the Teaching University Hospital of Caen (Caen, France) from January 2012 to February 2013 and randomized into two groups the day before surgery. Allocation to the ECOM group or control group was performed in 1:1 proportion by a random list. Inclusion criteria were an age > 18 year and a primary elective CABG surgery with CPB. Patients with an age < 18 year, pregnant or who refused to give signed written informed consent and patients undergoing emergency surgery (<24 h), reoperation, combined cardiac surgery or off-pump coronary surgery were excluded from the study.
2.2 Perioperative management
General anesthesia, CPB, and postoperative management followed institutional standards. All patients were premedicated with oral lorazepam (1–2.5 mg) and/or hydroxyzine (75–150 mg) the evening before surgery and on the morning of surgery. Betablockers and statins were given until the morning of surgery in chronically treated patients. Oral antiplatelet agents were managed as follows: aspirin was continued and clopidogrel was discontinued 5 days before surgery. Standardized total intravenous anesthesia (i.e. target–control propofol and remifentanil infusion, and atracurium) and monitoring techniques (i.e. five-lead electrocardiogram with computerized analysis of repolarization, invasive arterial blood pressure by means of a radial artery catheter, and central venous pressure by means of a jugular central venous catheter) were used. All patients were intubated with a 7.5 mm endotracheal tube after induction of general anesthesia. In the ECOM group, we used a specially designed endotracheal tube (ECOM-ETT 7.5G, ECOM Medical, Inc., San Juan Capistrano, CA) which contains seven silver electrodes on the cuff and tube that continuously measure the bioimpedance signal from the ascending aorta, in close proximity to the trachea [8]. After processing, it provides real-time continuous stroke volume and cardiac index. The ECOM pressure monitor was connected to the radial arterial line and then to the ECOM endotracheal tube impedance wires. All pressure monitors were zeroed at the mid-axillary line. Antifibrinolytic therapy with tranexamic acid (15 mg/kg twice) was routinely administered. CPB was performed under normothermia (more than 35.5 °C), and myocardial protection was achieved by intermittent warm blood cardioplegia. Boluses of ephedrine and/or phenylephrine were given intraoperatively to maintain mean arterial pressure between 50 and 80 mmHg. The heart was defibrillated after aortic unclamping, if sinus rhythm did not resume spontaneously. After the termination of CPB, norepinephrine was used to maintain the mean arterial pressure above 65 mmHg, and the trigger for transfusion of packed erythrocytes was set to a hematocrit of 21 % in all patients and complied with routine practice at our institution. Hydrxyethylstarch 6 % 130/0.4 (Fresenius Kabi, Sèvres, France) up to a maximum daily dose of 33 mL/kg at day zero was used intraoperatively for volume loading.
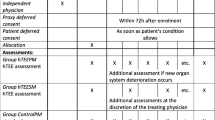
In the control group, intraoperative hemodynamic management was left to the discretion of the attending anesthesiologist and followed institutional standards. Briefly, physicians used usual hemodynamic parameters as blood pressure, heart rate, central venous pressure, urinary output, skin mottling and respiratory variation of arterial pulse pressure to guide fluid loading and inotropic support on an individual basis. Neither advanced hemodynamic monitoring providing stroke volume or cardiac index measurements nor specific algorithm was used in the control group. Furthermore, routine transesophageal echocardiography is not used for elective primary CABG surgery in our institution. In the ECOM group, intraoperative hemodynamic management followed a protocol based on the assessment of SVV and cardiac index given by the ECOM device, as illustrated in Fig. 1. Successive fluid boluses of 100 mL hydrxyethylstarch 6 % 130/0.4 were administered until SVV was less or equal to 11 %. Then, cardiac index was assessed and dobutamine was considered in case of low cardiac index values (less than 2.4 L min−1 m−2). Dobutamine infusion was progressively increased by 2.5 µg kg−1 min−1, if necessary.
All patients were admitted postoperatively to the cardiac surgical ICU intubated, ventilated and sedated with propofol and remifentanil to maintain a Ramsay score above 5. Extubation was performed after completion of the institutional weaning protocol. Hemodynamic parameters given by ECOM were available up to extubation. Postoperative management was left to the discretion of the attending anesthesiologist in both groups. Standard postoperative care included blood glucose control less than 10 mM and a low molecular weight heparin, beginning 6 h after surgery in the absence of significant médiastinal bleeding (more than 50 mL/h). Betablockers and statins were given as soon as possible postoperatively in chronically treated patients. Postoperative care was delivered by anesthesiologists in the ICU and by cardiac surgeons in the ward.
2.3 Endpoints
The main endpoint of the study was the possible hospital discharge, i.e. the time when patients fulfilled discharge criteria. These “fit for hospital discharge” criteria followed institutional standards and were defined as follows: the patient is fully oriented and able to move without support, hemodynamically stable, laboratory parameters with no sign of increasing organ dysfunction or infection, and no clinical signs of active wound infection [16]. This endpoint was considered as a valuable surrogate marker for global postoperative morbidity.
Secondary endpoints were the true hospital discharge, the time to reach extubation, the length of stay in ICU, the number of major adverse cardiac events (MACE), the number of patients having MACE, and in-hospital mortality. The true hospital discharge was the time when patients left the hospital. MACE were defined as at least one of the following: malignant ventricular arrhythmia requiring therapeutic intervention, postoperative myocardial infarction [17], serum postoperative cardiac troponin I (cTnI) above 9 ng/mL [18], inotropic support >24 h, and cardiac death (including sudden death). In all patients, an independent investigator blinded to the patients ‘group assignment assessed predefined postoperative complications and endpoints.
2.4 Statistical analysis
The number of patients was calculated on the basis of our institutional database showing an expected mean hospital stay of 9 ± 4 days. We considered that the possible hospital discharge would be reduced by 20 % (−2 days) in the ECOM group when compared with the control group at a 5 % type I error rate and a 20 % type II error rate. Thus, the sample size calculation revealed 100 patients divided into two groups of equal size. No interim analysis was performed. Variables are expressed as mean ± SD or median [extremes] for nonnormally distributed variables (Kolmogorov–Smirnov test) or number (%), as appropriate. Comparisons between both groups were made by independent samples t test or Mann–Whitney test according to the distribution for continuous variables and Fisher’s exact test or Chi squared test for categorical variables, as appropriate. Possible and true hospital discharges in both groups were depicted using Kaplan–Meier curves and compared with the Log-rank test. Hazard ratios (HR) are given with their 95 % confidence intervals (CI). For all primary and secondary outcome parameters, an intention-to-treat analysis was performed on all randomized patients. For possible and true hospital discharge, a per-protocol analysis was also performed.
A P value of <0.05 was considered as statistically significant and all P values were two-tailed. Statistical analyses were performed using MedCalc® Software bvba version 12.7.0 (Mariakerke, Belgium).
3 Results
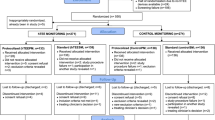
Eight patients were excluded from the analysis because of changes in surgical procedure following randomization: two in ECOM group (one for off-pump CABG surgery and one for aortic valve replacement combined to CABG surgery) and six in control group (one for cancelled surgery, four for off-pump CABG surgery and one for aortic valve replacement combined to CABG surgery). Among the 48 patients included in the ECOM group, 5 patients did not receive the ECOM tube at the time of tracheal intubation for logistic reasons (ECOM endotracheal tube unavailable) and were subsequently managed as patients of the control group. These patients were kept in the ECOM group for the intention-to-treat analysis and switched in the control group for the per-protocol analysis. The complete flow chart of the study is depicted in Fig. 2. All patients tolerated the study regimen well and no complications were noted from the ECOM tube.
Demographic and surgical data in both groups of patients are reported in Table 1. No significant difference was evidenced except for creatinine clearance which was lower in the control group. Absolute values were however in the normal range in the two groups of patients and the number of patients with a normal creatinine clearance was similar in ECOM and control groups. Intraoperative hemodynamic data are reported in Table 2. As a result of application of the algorithm to reach early goal-directed hemodynamic therapy, patients in the ECOM group received more often fluid loading and dobutamine. The total intraoperative amount of fluid loading was however significantly decreased in the ECOM group when compared with the control group while the total intraoperative dobutamine infusion was similar in both groups (Table 2). Primary and secondary outcomes are reported in Table 3. The time to reach extubation was significantly decreased by 60 min on average in the ECOM group when compared with the control group. No significant difference was found between the two groups in the total number of MACE and noncardiac complications, the length of stay in ICU and in-hospital mortality. A trend towards a reduction in the possible hospital discharge by one day was observed in the ECOM group (Table 3). The Kaplan–Meier curves for possible and true hospital discharges are depicted in Figs. 3 and 4, respectively. No significant differences were found between both groups: HR = 0.96 (95 % CI 0.64–1.45) for possible hospital discharge and 1.20 (95 % CI 0.79–1.81) for true hospital discharge, respectively.
The per-protocol analysis showed similar results than the intention-to-treat analysis: possible hospital discharge ECOM group 7 [4–57] versus control group 8 [6–22], P = 0.197 and true hospital discharge ECOM group 8 [6–58] versus control group 8 [7–22], P = 0.549.
4 Discussion
The main result of the present randomized controlled study is that an early goal-directed hemodynamic therapy based on endotracheal bioimpedance cardiography significantly reduced the time to reach extubation following elective primary CABG and trended to decrease the time when patients fulfilled discharge criteria from hospital when compared with an usual institutional standard of care.
The ECOM device is a minimally invasive and plug-and-play system easy to use at the bedside. ECOM was first evaluated in an animal study, which suggested this new technology was both promising and safe [8]. Six validation studies have been reported in human [9–14]. These studies were conducted in the setting of cardiac surgery and included only a small number of patients. They compared ECOM with either intermittent pulmonary artery [9, 10, 12, 14] or transpulmonary [11] thermodilution and with pulse contour analysis [13] or transesophageal echocardiography [10] and found a poor correlation and lack of agreement both on absolute values and changes in cardiac output following therapeutic maneuvers, as fluid challenge or phenylephrine administration. However, ECOM was convenient and consistent to monitor cardiac output continuously [10, 12] and seemed able to track the direction of its changes under dynamic loading conditions [13, 14]. Furthermore, SVV given by ECOM had the ability to predict fluid responsiveness with a good accuracy and discrimination [11]. Thus, ECOM could help to conduct perioperative goal-directed hemodynamic therapy during cardiac surgery. Data resulting from randomized phase III (clinical utility/outcome) studies are however not yet available.
In the current study, we found a slight improvement in postoperative outcome following elective primary coronary surgery when using ECOM to conduct intraoperative early goal-directed hemodynamic therapy. The time to reach extubation was decreased by 60 min on average and there was a trend in decreasing the time when patients fulfilled discharge criteria from hospital by 1 day on average. A systematic review and meta-analysis of goal-directed therapy in cardiac surgery has been recently published [5]. This meta-analysis included 5 well-designed randomized clinical trials using either intermittent bolus thermodilution [19, 20], pulse contour analysis [21] or esophageal Doppler [22, 23] and reported a significant decrease in morbidity and hospital length of stay when using pre-emptive goal-directed therapy. Since then, another prospective randomized trial including 100 coronary patients and using transpulmonary thermodilution also demonstrated that individually optimized hemodynamic therapy based on cardiac index, SVV, and global end-diastolic volume index reduced complications and length of ICU stay after cardiac surgery [16]. In these studies, control groups received either standard care or a dedicated protocol based on central venous pressure, mean arterial pressure, heart rate and hematocrit measurement. The advanced hemodynamic monitoring leading to goal-directed therapy in experimental groups was always a reference method, either invasive or necessitating a learning curve and dependent on the operator.
Several hypotheses and limitations can be discussed to explain our results. First, ECOM could be unable to provide reliable information on intraoperative SVV and cardiac index helping to conduct effective early goal-directed therapy in the setting of cardiac surgery. Several problems, as the lack of validity of the mathematical model in human, the impossibility to know the precise anatomical position of the cuff and the electrodes for a given patient, the extension of the electric field produced by the electrodes to the superior vena cava, the pulmonary artery and the carotid arteries, and the absence of detection of the coronary blood flow, could significantly alter the accuracy of ECOM. Moreover, ECOM measurements are heavily dependent upon the fidelity of the arterial line tracing and the frequent dampened radial waveform following CPB [24] could also markedly affect the accuracy of the measurement. We reported a good discrimination of SVV to predict fluid responsiveness in closed-chest conditions during the first postoperative hours after cardiac surgery [11]. The use of intraoperative SVV during open-chest conditions could be less straightforward, as found with arterial pulse pressure variation [25]. Second, the intraoperative algorithm based on ECOM we used could be inappropriate or insufficient to significantly improve postoperative outcome and reduce in-hospital stay. The use of the ECOM device is obviously limited to the time of intubation, and then cannot be prolonged beyond the first postoperative hours for the majority of cardiac surgical patients. In contrast, Goepfert et al. [16]. demonstrated that a goal-directed therapy initialized before surgery and continued throughout ICU led to a clinically reduction in postoperative complications in elective cardiac surgical patients. In our study, the postoperative hemodynamic management was left to the discretion of the attending anesthesiologist in the two groups of patients. Thus, the time course of hemodynamic optimization could have been too short. Furthermore, our algorithm did not take into account oxygen parameters, as central venous oxygen saturation, lactate or hematocrit. In contrast, Kapoor et al. [21]. and Smetkin et al. [20]. included minimal values of central venous oxygen saturation in their hemodynamic optimization strategies. While intraoperative hemodynamic optimization is usually based on fluid loading and stroke volume and/or cardiac output maximization, the simultaneous optimization of oxygenation parameters could be of importance in the specific setting of cardiac surgery with CPB. Third, the present study could be underpowered to draw definitive conclusions. Taken into account the excluded patients, we calculated a posteriori that we had a power of 77 % to show an expected difference of 2 days in the mean duration of hospital stay. The possible hospital discharge was 9.8 days on average in the control group and 8.7 days on average in the ECOM group (−1.1 day). Thus, we were unable to conclude for a significant difference. Further larger scale studies conducted in the setting of both cardiac and noncardiac surgery are mandatory to definitely assess the clinical utility of ECOM in improving patients outcome and reducing in-hospital stay. Fourth, it was a single-blind study since patients only were blinded to the study group assignment. We could not use specially designed endotracheal ECOM tubes in both groups of patients for economic reasons. This could have been responsible for partial bias. Furthermore, given the pragmatic nature of the trial protocol, there was no specific hemodynamic algorithm in the control group but individual strategies following an institutional standard of care. This last point could have favored the ECOM group. Other previous pragmatic randomized controlled studies conducted in the setting of cardiac surgery used however standard cares as control [19, 22, 23]. Finally, even if elective primary coronary surgery is a well-studied and standardized high-risk surgical model, more high-risk patients could have a better benefit from our early goal-directed hemodynamic strategy based on ECOM.
In conclusion, this study demonstrated that in elective primary coronary surgery, a mini-invasive early goal-directed hemodynamic therapy algorithm based on ECOM parameters, initialized immediately after induction of anesthesia and continued up to the end of surgery, can reduce the time to reach extubation but fails to significantly reduce the length of stay in hospital and the rate of major cardiac morbidity. Large studies in different subgroups of high-risk patients undergoing cardiac and noncardiac surgery are mandatory to further clarify the clinical interest of ECOM in improving postoperative outcome.
References
Ferguson TB Jr, Hammill BG, Peterson ED, DeLong ER, Grover FL. STS National Database Committee: a decade of change-risk profiles and outcomes for isolated coronary artery bypass grafting procedures, 1990–1999: a report from the STS National Database Committee and the Duke Clinical Research Institute. Society of Thoracic Surgeons. Ann Thorac Surg. 2002;73:480–9 discussion 9–90.
Scott BH, Seifert FC, Grimson R, Glass PS. Octogenarians undergoing coronary artery bypass graft surgery: resource utilization, postoperative mortality, and morbidity. J Cardiothorac Vasc Anesth. 2005;19:583–8.
Phan TD, Ismail H, Herior AG, Ho KM. Improving perioperative outcomes: fluid optimization with the esophageal Doppler monitor, a meta-analysis and review. J Am Coll Surg. 2008;207:935–41.
Hamilton M, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402.
Aya HD, Cecconi M, Hamilton M, Rhodes A. Goal-directed therapy in cardiac surgery: a systematic review and meta-analysis. Br J Anaesth. 2013;110:510–7.
Cannesson M, Pestel G, Ricks C, Hoeft A, Perel A. Hemodynamic monitoring and management in patients undergoing high risk surgery: a survey among North American and European anesthesiologists. Crit Care. 2011;15:R197.
Marik PE. Noninvasive cardiac output monitors: a state-of the-art review. J Cardiothorac Vasc Anesth. 2013;27:121–34.
Wallace AW, Salahieh A, Lawrence A, Spector K, Owens C, Alonso D. Endotracheal cardiac output monitor. Anesthesiology. 2000;92:178–89.
Ball TR, Culp BC, Patel V, Gloyna DF, Ciceri DP, Culp WC Jr. Comparison of the endotracheal cardiac output monitor to thermodilution in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2010;24:762–6.
Maus TM, Reber B, Banks DA, Berry A, Guerrero E, Manecke GR. Cardiac output determination from endotracheally measured impedance cardiography: clinical evaluation of endotracheal cardiac output monitor. J Cardiothorac Vasc Anesth. 2011;25:770–5.
Fellahi JL, Fischer MO, Rebet O, Massetti M, Gerard JL, Hanouz JL. A comparison of endotracheal bioimpedance cardiography and transpulmonary thermodilution in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2012;26:217–22.
Moller-Sorenson H, Hansen KL, Ostergaard M, Andersen LW, Moller K. Lack of agreement and trending ability of the endotracheal cardiac output monitor compared with thermodilution. Acta Anaesthesiol Scand. 2012;56:433–40.
Fellahi JL, Fischer MO, Dalbera A, Massetti M, Gerard JL, Hanouz JL. Can endotracheal bioimpedance cardiography assess hemodynamic response to passive leg raising following cardiac surgery? Ann Intensive Care. 2012;2:26.
Van der Kleij SCJ, Koolen BB, Newhall DA, et al. Clinical evaluation of a new tracheal impedance cardiography method. Anaesthesia. 2012;67:729–33.
Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–4.
Goepfert MS, Richter HP, Eulenburg CZ, et al. Individually optimized hemodynamic therapy reduces complications and length of stay in the intensive care unit: a prospective, randomized controlled study. Anesthesiology. 2013;119:824–36.
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasché P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–35.
Fellahi JL, Hédoire F, Le Manach Y, Monier E, Guillou L, Riou B. Determination of the threshold of cardiac troponin I associated with an adverse postoperative outcome after cardiac surgery: a comparative study between coronary artery bypass graft, valve surgery, and combined cardiac surgery. Crit Care. 2007;11:R106.
Polonen P, Ruokonen E, Hippelainen M, Poyhonen M, Takala J. A prospective, randomized study of goal-oriented hemodynamic therapy in cardiac surgical patients. Anesth Analg. 2000;90:1052–9.
Smetkin AA, Kirov MY, Kuzkov VV, et al. Single transpulmonary thermodilution and continuous monitoring of central venous oxygen saturation during off-pump coronary surgery. Acta Anaesthesiol Scand. 2009;53:505–14.
Kapoor PM, Kakani M, Chowdhury U, Choudhury M, Lakshmy KU. Early goal-directed therapy in moderate to high-risk cardiac surgery patients. Ann Card Anaesth. 2008;11:27–34.
Mythen MG, Webb AR. Perioperative plasma volume expansion reduces the incidence of gut mucosal hypoperfusion during cardiac surgery. Arch Surg. 1995;130:423–9.
McKendry M, McGloin H, Saberi D, Caudwell L, Brady AR, Singer M. Randomized controlled trial assessing the impact of a nurse delivered, flow monitored protocol for optimization of circulatory status after cardiac surgery. BMJ. 2004;329:258.
Mohr R, Lavee J, Goor DA. Inaccuracy of radial artery pressure measurement after cardiac operations. J Thorac Cardiovasc Surg. 1987;94:286–90.
Reuter DA, Goepfert MSG, Goresch T, Schmoeckel M, Kilger E, Goetz E. Assessing fluid responsiveness during open chest conditions. Br J Anaesth. 2005;94:318–23.
Acknowledgments
We thank Sylvain Thuaudet, M.D., (I.S.T. Cardiology, Saint-Contest, France) for kindly providing all the facilities necessary for hemodynamic monitoring with the ECOM device. Support was provided from institutional and departmental sources and from ECOM Medical Corporation (San Juan Capistrano, CA).
Conflict of interest
ECOM Medical Corporation had input neither in the design or conduct of the study nor in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fellahi, JL., Brossier, D., Dechanet, F. et al. Early goal-directed therapy based on endotracheal bioimpedance cardiography: a prospective, randomized controlled study in coronary surgery. J Clin Monit Comput 29, 351–358 (2015). https://doi.org/10.1007/s10877-014-9611-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-014-9611-5