Abstract
Metal nanoparticles are widely applied in various biomedical applications because of their unique physicochemical properties. The new and unique properties of gold nanoparticles (AuNPs) including, biocompatibility, low cytotoxicity, and optical properties, make them valuable for applications of biomedical fields including, biosensing, bioimaging, cancer therapy of cancer, and drug delivery. Utilization of AuNPs in radiotherapy and photothermal therapy has created a novel platform for primary diagnosis and cancer therapy. Owing to AuNPs' large surface area, chemical functional groups or biological molecules like drug molecules can be immobilized on gold surface. Thus, the surface functionalization of AuNPs makes them a good carrier for targeted drug delivery. This review focuses on new progress in processes of the functionalization of AuNPs and their possible biomedical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, metal nanoparticles (MNPs)are broadly applied in biomedicine field because of their unique and novel physicochemical properties: large surface area, small sizes, high reactivity to live cells, and stability over a high-temperature range [1]. These properties make metal nanoparticles applicable in biomedical applications because they are different from the physicochemical properties of their bulk materials [2]. MNPs also possess optical properties different from bulk metals that arise from localized surface plasmon resonance (LSPR) [3]. These properties make MNPs very suitable candidates for bioimaging applications. All optical and physicochemical properties of MNPs are affected by particles size and shape as well as local dielectric environment [4].
Among different MNPs, gold nanoparticles (AuNPs) due to their less cytotoxic nature and distinct optical properties are widely used for different applications of biomedical research, such as biosensor and biomedical imaging [5]. AuNPs also exhibit high potential as carriers for the delivery of genes, proteins, drugs, and small molecules. AuNPs entry into the different cells is simply facilitated by their nano size, this is one of the major challenges in utilizing AuNPs to deliver targeted content to specific tissues [6]. To overcome this challenge and increase the interaction of AuNPs with cells, researchers have been functionalizing the surface of these NPs with different ligands and biomolecules. In recent years, applications of functionalized AuNPs in biomedicine have been reviewed that indicate great development in this field. This review gives a general overview of unique optical and physicochemical properties of AuNPs, their different methods of functionalization, and their various applications in biomedicine.
Gold Nanoparticles
Gold is yellow solid and it is inert in nature while AuNPs are wine red solution and have anti-oxidant effects, thus AuNPs properties are different from bulk gold. AuNPs exhibit different sizes ranging from 1 nm to 8 μm and also they show various shapes including nanosphere, nanorod, nanoshell, nanocube, nanocage, and branched (Table 1) [7]. Recently, the biomedical use of AuNPs has peaked interests as they present obvious advantages, such as:
-
1)
They can be simply synthesized in various sizes and shapes.
-
2)
They can be simply functionalized through types of biological molecules, including drugs, genes, and ligands containing functional groups like amines, thiols, and phosphines because of the large surface area and presence of a negative charge on their surfaces.
-
3)
They are stable against oxidation, great biocompatible, and non-cytotoxic.
-
4)
They have unique optical and electronic characteristics that remarkably relate to shape, size, and aggregation status of nanoparticles.
The AuNPs optical properties are defined through their plasmon resonance, that is related to collective excitation of conduction electrons and concentrated in visible to infrared regions, depending on the structure, shape, and size of particle [8]. High absorption coefficients of AuNPs, permitting greater sensitivity in optical detection techniques than the common dyes [9].
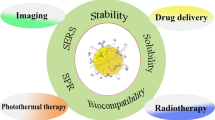
All these specific properties make AuNPs the most potential nanomaterial for different applications in biomolecular ultrasensitive detection, chemical and biological sensing, labeling for proteins and cells, molecular imaging, and delivering drugs, genes, antigens, and antibodies into cells. AuNPs based major potential applications in biomedical engineering are shown in Fig. 1.
AuNPs Synthesis
Generally, there are different techniques to synthesis AuNPs by dividing them into chemical, physical, and biological techniques. The citrate reduction synthesis technique is most common technique used in the synthesis of spherical AuNPs, due to easiness of synthesis, controllable sizes, and stability of produced NPs [10]. This technique that introduced by Turkevitch in 1951, includes chloroauric acid reduction with trisodium citrate, resulting in formation of AuNPs with a size distribution from 10-20 nm. The size of AuNPs can be controlled by varying salt concentration, rate of addition of reactants, and temperature [11]. In this technique, citrate functions as a stabilizing and reducing agent that causes a colloidal suspension which stops aggregation of nanoparticles. The citrate can be replaced by amino acids, ascorbic acid, and UV light [12]. The Brust − Schiffrin method reported in 1994, utilizes toluene using the tetraoctanylammonium bromide as a phase transfer reagent [13]. This method based on a two-phase procedure and can produce particles with high stability and size ranging from 1 to 6 nm [14]. Indeed, the thiol − gold interactions are used in this method for the protection of AuNPs with thiol ligands. AuC14− reduced through sodium borohydride in alkanethiols presence [15].
The most common method that is usually used to prepare other shapes of AuNPs such as nanocages, nanocubes, and nanorods, is “growing seed” technique [16]. In the seed-mediated growth method, the first small seeds serve as nucleation centers. In the next step, the very reactive sites on these centers can grow further to achieve a required shape of particles with a predicted size under controlled conditions. Two reducing agents, hydroxylamine and ascorbic acid, are usually used in this technique. The size of the prepared AuNPs can be varied from 5 to 40 nm by control of [HAuCl4]: [seeds] ratio [17].
Methods such as UV radiation, thermolytic, photochemical, microwaves, and sonochemical are categorized as physical techniques. UV radiation and high temperature are major reducing agents in UV radiation and thermolytic techniques. The desired sizes of AuNPs can be obtained by optimizing of synthesis condition [18]. The photochemical process produces gold nanorods that have self-assembly and complex physical properties than spherical AuNPs [19]. In microwave irradiation techniques, to prepare AuNPs, photochemical reduction method or heating are used. The sonochemical technique has the ability to provide AuNPs within pores of the silica and Au/Pd bimetallic nanoparticles [20].
Biological techniques are developing and achieving more interests due to their low-price, eco-friendliness, and high reproducibility. These methods utilized microorganisms and plants as stabilizing and reducing agents [14]. For example, Yu et al. used Citrus maxima fruit extracts as stabilizing and reducing agents to produce environmental friendly AuNPs [21]. Polymeric coating of AuNPs is also a favorite method that is widely used for surface modification (Fig. 2).

Adapted from Ref. [131], 2016, IOP
Methods for synthesis of polymeric AuNPs.
Functionalization of AuNPs
The AuNPs surface functionalization is essential for their functionality, stability, and biological compatibility. AuNPs are easy to functionalize with various types of biological molecules or chemical functional groups to achieve a suitable vehicle for various purposes including diagnostic, cancer therapy, and drug delivery systems [22]. The final goal is to maintain the unique properties of AuNPs and bound molecules, respectively, potent plasmon absorption bands and biorecognition features. Generally, functionalizes AuNPs have the ability to reach the target tissues and perform a therapeutic or detective function. There are two kinds of interaction, physical and chemical, between AuNPs and ligands depending on the type of the ligand and the functionalization techniques [23]. In chemical interaction, covalent bonding is used to modify of AuNPs because of its potent nature and high stability in physiological medium. The utilization of covalent modifications is simply but biocompatible and obtainable ligands for this technique are restricted [24].
Ligand exchange is a fundamental process to conjugate ligands on AuNPs surface, to achieve NPs with desired surface properties. In this process, the first reactive group of functional compound binds to surface of AuNPs, where the other head group of compound functions as a binding site which can react with biomolecules like peptides or antibodies [25]. Trialkoxysilane compound because of the abundance of its coupling sites that have the ability for interaction with vinyl and amino groups, is broadly utilized in this process [26]. Phosphine is a proper candidate to exchange with thiol groups and increase the AuNPs stability in a physiological environment, because of its weak interaction with AuNPs [27]. Physical interactions are relating to noncovalent interactions such as electrostatic interaction between AuNPs with negative charge and molecules with positive charge. Another common technique that is used to functionalize the surface of AuNPs is self-assembly monolayer. This technique based on constituting a particular monolayer on the goal particle, spontaneously [28].
The amides, thiols, and carboxylic acids are proper candidates for AuNPs self-assembly among different molecules. Depending on the required size of NPs, the field of application, and used technique for functionalization, different types of molecules can be selected [29]. Thiol-containing ligands, including α-mercaptoacetate, mercaptoalkanoic, and 3-mercaptopropionate are more proper ligands for functionalization of AuNPs with sulfur − gold interaction [30].
Polymeric coating of AuNPs is one of the most common ways for functionalization. The covalent connection of polymers with AuNPs is primarily in the Au–S form [31]. Polyethylene glycol (PEG) is a biopolymer that usually is used for surface modification of AuNPs. This polymer provides the colloidal stability of AuNPs since PEGylated AuNPs repel each other for steric reasons [32]. PEG is used as alone or in connection with other biomolecules like peptides for the coating of AuNPs. These functionalized AuNPs can serve as suitable drug vehicles because of their ability to link cell membrane and enter target cells [6]. Ruff et al. indicated successful synthesis of hollow gold nanospheres and gold nanorods functionalized with CLPFFD-peptide by two methods, i.e. either direct to the AuNP surface or indirectly to a PEG [33].
The functionalization of AuNPs by amino acids is a useful method to increase the efficacy and specificity of NPs based delivery systems. These types of AuNPs bind DNA with higher efficiency to the delivery of genes without cytotoxicity [34]. Dhanya et al. synthesized AuNPs by using starch-PEI, where starch acts as a stabilizing agent and PEI as a reducing agent, first. Then to increase efficiency the corresponding AuNPs [35].
Over recent years, several research groups have synthesized and used functionalized AuNPs with DNA. Indeed, the binding and optical properties of DNA-AuNPs have been applied for various detection techniques, including scanometric, colorimetric, electronic, and Raman-based strategies [36]. Typically used functionalization strategies are based on the use of any one or a mixture of the groups such as bovine serum albumin (BSA), oligo- orpolyethylene glycol (PEG), oligonucleotides, oligo or polypeptides, antibodies, antisense or sense RNA molecules, cell surface receptors and other similar molecules as presented in Fig. 3.

Adapted from Ref. [6], 2011, MDPI
Types of functionalization of AuNPs and their potential biomedical applications.
Toxicity of AuNPs to Biological Systems
Despite the remarkable capacity to bioconjugate to various molecules, AuNPs are cytotoxic due to their physio-chemical properties. GNP solution can be toxic due to the stabilizing capping agents and chemical remnants used for synthesizing AuNPs [37]. The toxicity can be reduced by the replacement of a toxic capping agent by a suitable biocompatible capping agent or by modifying them to prevent its dissolution [38].In a report on the cytotoxicity of citrate coated AuNPs, it was observed that these particles are non-cytotoxic at low concentration. Toxicity also depends on the size and concentration of AuNPs [39]. The investigation of interactions of AuNPs with the contents of biological medium is important from the perspective of their biomedical engineering applications [38]. AuNPs whose size is < 2 nm are found to cause oxidative damage to the mitochondrial structure and lead to the destruction of biological cells [38]. Toxicity was found to be size and shape dependent. For example, 1.4 nm sized AuNPs were found to be toxic but 15 nm AuNPs were not [40]. Various experimental reports suggested that the toxicity is dose dependent and the safety concentration limit is 1012 particles per mL [41]. The nature of interaction between AuNPs and bio-systems depends upon the synthesis procedure, size/shape, charge on the surface and type of surface coating [42]. In many reports AuNPs have been considered as non-toxic carriers for various drugs, nucleic acids, and gene delivery applications [43]. Interestingly, polyethylene glycol (PEG) and ionic coatings on AuNPs improved their biocompatibility [44]. The synthesis method of AuNPs also affects their toxicity. For example, AuNPs made from plant extracts, algae, and bacteria are less or non-toxic and considered suitable for biomedical applications [45]. Using plant extracts for synthesis of nanoparticles may be better than other biological methods because it eliminates the elaborate preservation of cell cultures, is suitable for large scale synthesis and also can be more affordable. Biosynthesis of gold nanoparticles with extract of plants such as curcumin have been reported in previous studies. Curcumin is a polyphenol derived from turmeric plant that is widely used in food preparation and for medical purposes in south-east Asia, China and India. Investigations on the event of medical purposes for curcumin indicated its anticancer, antimicrobial, antioxidant, and anti-inflammatory properties [46, 47]. Functionalized AuNPs showed almost no toxic effect even for hours under normal body conditions and environment. Explaining the toxic effect of AuNPs on a biological system is very difficult and still more research is required in this research area.
Applications of AuNPs in Biomedical
Functionalized AuNPs have found their route from diagnosis to therapy in biomedicine based on the functional moieties and their abilities. AuNPs possess unique optical, physical, and chemical properties because of their shape and size, also, they can be used in the research area particularly in the field of biosensing, diagnostics, therapy, and drug/gene delivery.
AuNPs in Biosensing
The utilization of AuNPs in biological and chemical sensing is because of their intrinsic properties. AuNPs are applied as sensors for detection of different molecules, including nucleotides, proteins, saccharides, toxins, and analytes like anions, and metal ions, by utilizing various sensing strategies. Biological compatibility and probabilities of proper functionalization of the AuNPs surface with a broad range of ligands for the selective binding of molecules and ions are major advantages of these nanoparticles as sensors [31]. The sensors of AuNPs can be colorimetric, fluorescence, electrochemical, surface plasmon resonance, and surface enhanced Raman scattering (SERS)-based sensors.
Colorimetric Sensing
The colorimetric sensing based on AuNPs supplies a fast, low-price, and simple technique for the detection of various analytes like metal ions, heavy metal ions, alkaline and alkali earth metal ions, and molecules like anions, small organic molecules, proteins, and oligonucleotides [48]. This technique is based on color change of suspension that permits the detection by naked eyes. AuNPs are introduced as interest candidates for colorimetric sensors because of their intense absorption in the visible region and intense dependency of SPR peaks on the immediate environment [49]. In these sensors, AuNPs aggregation causes surface plasmon coupling between nanoparticles leading to a visible color change from red to blue.
So far, many studies have been done to develop the colorimetric sensors for selective detection of organic molecules like cholesterol, glucose, and fructose in biological fluids [50]. Since these molecules are characteristic markers for certain diseases, their detection has very significance in clinical recognition and treatments. Recently, Nirala and Prakash developed a one-step synthesis technique of AuNPs decorated over molybdenum disulfide (MoS2) quantum dots (AuNPs@MoS2-QDs). The AuNPs@MoS2-QDs composite catalyzes the peroxidase substrate 3,3_,5,5_-tetramethylbenzidine in H2O2 presence to produce a bluish-green colored product. The developed composite is used for selective detection of glucose using the colorimetric method [51]. Nirala et al. have developed an easy colorimetric method for determining free cholesterol in aqueous solution based on H2O2-induced association of AuNPs via cascade reactions utilizing cholesterol oxidase (ChOx). In order to, they immobilized ChOx over 11- Mercaptoundecanoic acid (MUDA) Fn-AuNPs. The prepared system interacts with free cholesterol to generate H2O2 in proportion to the level of cholesterol being determined [52].
In addition to organic molecules, proteins also are biomarkers for certain diseases and AuNPs have been successful used for their colorimetric detection. C-reactive protein is one of the important proteins that acts as a biomarker for cardiac diseases [53]. A colorimetric biosensor based on functionalized AuNPs with a great degree of sensitivity and selectivity, employed for estimation of C-reactive protein in serum [54].
Fluorescence Based Sensing
Fluorescence resonance energy transfer (FRET) is a process which transfers energy from an excited state donor fluorophore to a ground state acceptor fluorophore. AuNPs have the ability to act as perfect fluorescence quenchers for FRET-based assays because of their broad energy band and remarkable great molar extinction coefficients [55]. The special DNA sequences detection possess significant applications in genetics, clinical diagnosis, and environmental monitoring. Shi and colleagues developed a FRET biosensor based on AuNPs and graphene quantum dots (GQDs) for determining Staphylococcus aureus specific gene. In order to form this biosensor, they conjugated reporter probes onto AuNPs and immobilized capture probes onto GQDs. Then, the sandwich structure built from co-hybridization of target oligos with reporter and capture probes brought AuNPs and GQDs to close proximity to trigger FRET effect [56].
Electrochemical Sensing
AuNPs are perfect candidate for electrochemical detection of a broad range of molecules including, oligonucleotides, proteins, and organic small molecules because they remarkably improve the sensitivity of the electrochemical biosensors [57]. The sequence-specific DNA detection has high importance in early detection of cancers, precise identification of pathogens, and clinical diagnostics of genetic diseases [58]. Bhatnagar and co-workers have developed a sensitive electrochemical nanobiosensor to diagnose invasive Aspergillosis by detection of a virulent glip target gene. The probe of the sensor was prepared by utilizing 1,6-Hexanedithiol and chitosan stabilized AuNP-mediated self-assembly of glip probes on the gold electrode [59].
The electrochemical microRNA biosensors are easy, low-cost, and effective analytical tools for primary detection of cancer biomarkers [60]. Tian and colleagues have presented a sensitive electrochemical microRNA biosensor by utilizing AuNPs superlattice as a backing substance and toluidine blue as a redox indicator. AuNPs coated with polypyrrole was self-assembled to build a superlattice that presented the most close-packed type to produce maximum current and so improving electron transfer efficiency. The proposed electrochemical biosensor can be beneficial in the early diagnosis of cancer through microRNA-21 detection in real serum samples [61]. Several studies have been used AuNPs to detect electrochemically the different types of small molecules. Buk and colleagues designed a sensitive electrochemical biosensor based on a combination of glucose oxidase and AuNPs immobilized onto carbon quantum dots for glucose detection by reductive determination of consumption of oxygen in lack of any mediator [62]. Several researchers have been developed different electrochemical biosensors for the measurement of proteins in biological samples [63]. In one of these works, an activated protein C electrochemical aptasensor was prepared based on AuNPs-chitosan/graphene paste and aptamer probes [64]. Of the various types of DNA sensors, the electrochemical sensors offer sensitivity, selectivity and low cost for the specific DNA sequence detection. Obviously, the DNA electrochemical detection by using AuNPs will have an important impact on the development of specific and sensitive assays for clinical diagnosis, detection of pathogenic microorganisms in foods and the environment as well as for other applications including proteomics. Further efforts should be directed to enhancement strategies so as to avoid efficiently take their advantages.
Surface Plasmon Resonance Sensors
SPR sensors are optical sensors based on the excitation of surface plasmons that used in different fields like environmental monitoring and biomedical diagnostics. The high sensitivity of SPR without any fluorescent or other labeling of the analyte is its main advantage. The introduction of AuNPs onto the sensing surface can remarkably enhance the sensitivity of sensors that are based on the intensive field coupling between AuNPs LSPR and SPR the sensing surface [65]. Recently, Špringer and co-workers developed a new biosensor for determining the tau-Aβ complex, a prospective biomarker of Alzheimer`s disease. The technique incorporates a novel sandwich assay combining functionalized AuNPs with SPR sensor. They indicated that prepared SPR biosensor can detect tau-Aβ complex in cerebrospinal fluid with a detection limit of 1 pM [66]. It is important to note that AuNPs based optical biosensors have shown higher sensitivity. Moreover, combining the advantages of both AuNPs and sensors, increasingly sensitive and diverse AuNPs-based optical biosensors are expected to be developed in near future.
Surface Enhanced Raman Scattering Based Sensing
Surface-enhanced Raman spectroscopy (SERS) is a potent surface ultrasensitive analytical tool to detect different analytes at very low concentrations. AuNPs due to their size- and shape-dependent physical properties, have been broadly used in SERS [67]. The stable, non-toxicity and efficient SERS-active tags have been developed based on different gold nanostructures [68]. An ultrasensitive platform based on SERS by using peptide-functionalized AuNPs and SERS-active hollow polypyrrole nanohorn established by Choi and co-workers. This sensitive nanohorn platform can be broadly employed to detect proteolytic biomarkers and diagnose several diseases in the early stages [69].
AuNPs in Diagnostics
Visualization Methods
AuNPs are active in the recognition of biological and chemical agents. The visualization techniques by using of AuNP and optical microscopy, particularly, confocal laser microscopy, have reached extending attention in biomedical research. The confocal fluorescence microscopy, two-photon luminescence confocal microscopy, and/or resonance scattering confocal microscopy are the main methods for capturing confocal images. The main advantage of the two-photon luminescence method is that a strong decrease in background signal leads to contrast being increased. The use of two-photon luminescence of AuNPs to visualize the oncomarkers inside a cell or on its surface amongst other objects has been reported successfully [70].
Raman Imaging
Raman spectroscopy is based on the inelastic scattering of a photon and has been explored for biomedical applications like the diagnosis of cancer due to the presentation of precise information about the chemical composition of tissues and cells [71]. AuNPs can be employed for cancer diagnosis by their SERS effect. The enhancement of Raman signal intensity by the SERS effect leads to a sensitivity of detection similar to fluorescence [72]. The diagnostic potential of Raman spectroscopy has been indicated in certain vascular diseases and cancers of different organs. In one research, metal–organic frameworks (MOFs)-based core–shell NPs (Au@Cu3 (BTC)2 NPs) were applied as theranostic agents for Raman imaging and delivery of doxorubicin in the breast, HeLa, and lung cancer cells. They have prepared Au@Cu3 (BTC)2 NPs by chemical encapsulation of Raman reporter molecules between AuNPs and Cu3(1,3,5-benzenetricarboxylate)2. These NPs showed excellent SERS effect that promoted their theranostic application for tracking of cell [73].
Dark-Field Microscopy (DFM)
DFM is another interesting technique in bioimaging and based on light scattering by microscopic samples, including samples with a size under the resolution limit of a light microscope. Upon DFM, only the light scattered by the object can reach the lens; therefore, the scattering object shines brightly against a dark background in images achieved from a DFM [74]. The low accuracy of DFM is a big hindrance to its use in practical applications. To overcome this hindrance, AuNPs, in particular, gold nanorods because of their LSPR, have been introduced for the reduction of fluctuations in scattered light in DFM. These NPs are regarded as non-bleaching labels for DFM imaging of biological samples [75]. Gong et al. developed gold nanorods (AuNRs) and nanospheres (AuNSs) as label-free plasmon scattering probes for multiplex dark-field imaging of cancerous cells. They indicated receptor-mediated delivery of AuNRs and AuNSs conjugated with antibody to EGFR-overexpressing oral squamous cell carcinoma cells by utilizing the DFM method [76]. The oncologists can diagnose cancer cells in early stages by utilizing this technique, leading to the improvement of the current cancer therapy efficacy. Bioimaging of tumor cells, detection of metabolites and microbial cells, and study of endocytosis are other applications of AuNPs in resonance DFM [41].
Photoacoustic Imaging (PAI)
PAI is a rapidly emerging biomedical imaging modality based on the PA effect that was first observed by Alexander G Bell in 1880 [77]. There has been tremendous effort devoted to the development of PAI agents, and gold nanoparticles as exogenous contrast agents have great potential for PA imaging due to their inherent and geometrically induced optical properties. PAI is a proven method for cancerous cells detection with great resolution. In PAI, the energy of light that is absorbed by contrast agents leads to production of waves in an ultrasonic range. The produced waves then convert to electrical signals using a transducer and the image will be processed using a processor [78]. PAI does not have enough contrast to differentiate between anatomical and molecular content of the targeted area. Accordingly, exogenous contrast agents, like AuNPs, are needed to rise absorption and improve PAI efficacy, for imaging of molecular and cellular events [79]. Various types of AuNPs such as nanospheres, nanostars, nanocages, nanorods, and nanoshells due to their SPR effect broadly applied as contrast agents in PAI [77]. Xu et al. prepared a multifunctional theranostic platform based on Au nanocages (AuNCs) modified with hyaluronic acid (HA). AuNCs-HA with great LSPR peak act as contrast agents for increased PAI. Also, AuNCs-HA revealed significantly intensive PA signals to identify the size, location, and border of the tumor, thus facilitating imaging-guided therapy [80]. The AuNPs coated with glycol-chitosan (GC-AuNPs) have used as photoacoustic contrast agents for imaging of cancerous cells. GC-AuNPs compared with PEG-AuNPs indicated increased accumulation in the cancer cells, which leads to intensive photoacoustic signals in the tissue-mimicking cell phantoms. These signals arise from the SPR effect of GC-AuNPs after cellular uptake in cancerous cells [81]. Hence, due to their distinct and tunable absorption spectra in the tissue optical window (650–1100 nm), AuNPs can be used to identify multiple cell types within heterogeneous tissue, indicating their promising application in multiplexed PA imaging in vitro and in vivo.
Computed Tomography (CT)
CT is a potent molecular imaging tool for the diagnosis of cancer, that can give precious anatomical information associated with the location and size of tumors [82]. The principle of CT imaging is based on an electron-density difference between targeted tissue and surrounding [24]. Due to the low sensitivity of CT in soft tissues, using contrast agents is needed for precise diagnosis. AuNPs with great electron density and atomic number possess a high X-ray attenuation coefficient and therefore can be applied as CT contrast agents [83]. Keshavarz et al. developed a new theranostic nanocomplex built from alginate hydrogel co-loaded with AuNPs and cisplatin (ACA) for drug delivery monitoring by CT imaging. The study of CT imaging showed which ACA nanocomplex increase the CT images brightness, CT number value, and the contrast-to-noise ratio [84]. Khademi et al. showed that using targeted AuNPs can provide molecular CT imaging of neck and head cancer. They prepared folic acid (FA)-cysteamine (Cys)-AuNPs for targeting the neck and head cancer cells via CT. Their findings demonstrated which a tiny tumor, that is undetectable by CT, is increased through attenuation of X-ray and becomes visible via molecularly targeted AuNPs [85]. Hence, due to longer circulation times and localized agglomeration of AuNPs at the illness site these nanostructures come handy in CT imaging technique. Furthermore, the formation of highly sensitive and selective diagnostic system for target analytes can be attained by engineering their surface monolayer.
AuNPs in Therapy
Photothermal Therapy (PTT)
PTT is a novel type of hyperthermia that induced cell death in cancer cells by generating localized heat, particularly in early tumor stages [86]. PTT is the main application of AuNPs in biomedicine. AuNPs have the ability to absorb light with high efficiency in a specific wavelength (visible or near-infrared (NIR) region) and convert it to heat through phonon-electron interaction [87]. AuNPs are promising candidates for PTT of cancer due to easy conjugation with targeting drugs and molecules, high solubility, and high absorption cross-section [88]. Khiavi et al. engineered a new and efficient tumor therapeutic RnaAuNPs nanobiomedicine prepared of AuNPs conjugated with bovine pancreatic ribonuclease to overcome intracellular ribonuclease inhibitors. They evaluated the photothermal effects of RnaAuNPs nanobiomedicine in the colon cancer cells. Their findings demonstrated that the engineered RnaAuNPs are capable for induction of apoptosis and inhibit metastasis and cell invasion by upregulation of Bax, PTEN, and caspase 3 and downregulation of Bcl-Xl and AKT genes [89].
The shape and size of AuNPs are key factors in PTT. In general, nanorods, nanocages, and nanoshells due to their intensive light absorption in the NIR region are beneficial in PTT [90]. Despite the easy synthesis of nanoshells and their desirable plasmonic properties, these nanoparticles have the larger size and smaller extinction cross-section relative to nanorods and nanocages. The large size of nanoshells may reduce their accumulation in some cancerous tissues [91]. Thus, nanocages and nanorods seem to be the most proper types of AuNPs for PTT. However, the application of nanoshells in PTT evaluated in several studies. In one of these works, hollow gold nanoshells were synthetized and conjugated with aptamers selective to nucleolin (AS1411 aptamer) and then used in skin cancer PTT. According to the obtained results of this study, hollow gold nanoshells can be used as photothermal agents for therapy of skin cancer [92].
Nanorods have advantages, including tunable plasmonic absorption, simple synthesis, and good cell permeability. Moreover, the smaller size of nanorods than other AuNPs, make them promising agents for PTT [93]. Nanorods have been employed as photothermal therapeutic agents to kill human cancerous cells in vivo and in vitro. Pan and coworkers prepared polyacrylic acid (PAA)–coated AuNRs to observe their killing effect on human osteosarcoma cells in vitro and reported AuNRs as promising candidates for phototherapeutic applications in osteosarcoma of human. Their results indicated which AuNRs@PAA induced cell death in human osteosarcoma cells due to damaged-cell membrane and DNA integration [94].
Nanocages are another class of NIR-absorbing AuNPs with tunable plasmonic absorption. Nanocages are smaller in size than the nanoshells that is key for considering tissue accumulation and elimination [95]. Successful use of nanocages as photothermal therapeutic agents have reported both in vitro and in vivo. For example, a NIR-absorbing AuNCs have been synthesized and functionalized with PEG and AS1411 aptamer (AS1411-PEG-AuNCs) to obtain cancer-targeted PTT by Hong and coworkers. They demonstrated that AS1411-PEG-AuNCs selectively kill cancerous cells upon irradiation with light of NIR [96]. Because of its successful documented use in in vitro, pre-clinical, and clinical studies, it has been demonstrated that GNP technology is a promising tool and it is worth investigating future directions that would allow for a further evolution of the use of GNPs for photothermal therapy.
Photodynamic Therapy (PDT)
PDT is a therapy based on photochemical generation of cytotoxic ROS upon irradiation of light on photosensitizers and is applied for the treatment of oncological and infectious diseases. ROS generated by the energy of photosensitizers induced necrosis or apoptosis in tumor cells. PDT leads to tumor malnutrition and death because of the damage inflicted on its microvessels [41]. AuNPs have been used in PDT due to their important properties, including SPR absorption and effective fluorescence quenching. In addition to, the AuNPs tendency to bind with amine, disulfides, and thiols, can promote their intracellular penetration into cells [97]. Recently, a new hybrid nanocomposite based on branched copolymers polyacrylamide-dextran in an anionic and nonionic form with combined AuNPs and chlorin e6 photosensitizer has been prepared to evaluate its biological activity as nanocomposite for PDT. The results showed that AuNPs-contained nanocomposite has higher activity in the induction of sensitivity to photodynamic damage in MT-4 cells than chlorin e6 itself [98]. In summary, the area of GNP-based treatment of cancer has attracted extensive research in recent years due to its high selectivity and minimized side effects. Gold nanoparticles have exhibited great promise as light-heat converter, local field enhancement, drug carriers, and radiation sensitizer for cancer treatment to effectively damage cancerous lesions both in vitro and in vivo. Moreover, considerable advances have been made in developing GNP-mediated multifunctional nanoparticles systems which provides great possibility of introducing combination therapy strategy for enhancing cancer therapeutic efficiency in near future.
Radiotherapy (RT)
RT is one of major modes for therapy of cancer so that about 50% of patients receive RT with palliative or curative purposes [99]. The principle of RT is based on ionizing radiation delivery to the tumor to interact with DNA, directly, or indirectly by the generation of free radicals, ultimately resulting in cellular damage [100]. The final purpose of RT is to exactly impart the radiation-induced damage to the tumor while keeping the radiation toxicities to the normal tissue at acceptable levels [101]. RT is insufficient to eradicate radioresistant hypoxic tumors, therefore, there is a need to improve the efficiency of RT. This improvement can be obtained by using the radiation sensitizers that enhance the radiation sensitivity of the tumors and thereby increasing the radiation-induced damage to the tumor [102]. AuNPs have been widely applied as suitable radiosensitizer agents, because of their advantages, including high X-ray absorption, facility of synthetic manipulation, and ease of exact control of physicochemical properties [101]. The interaction of the incident electromagnetic wave with AuNPs leads to secondary electrons emission that can damage internal organs of cell-like mitochondria via direct interaction or free radicals generation like °OH that can result in cell membrane disruption of cancer cells [24]. The successful targeting of tumor and cellular uptake are two key factors for efficient RT. In addition to enhanced permeability and retention (EPR) effect of AuNPs which can promote selective targeting of the tumor with these NPs, their surface also should be coated properly with active targeting ligands to enhance the local concentration in targeted tissue [103]. Zhang et al., have been studied radiosensitization effects of 4.8, 12.1, 27.3, and 46.6 nm AuNPs coated with PEG in vivo and in vitro. In vivo and in vitro radiotherapy results showed which 12.1 and 27.3 nm coated AuNPs coated with PEG have great radiosensitivity, and therefore are noteworthy for potential applications in radiotherapy increase [104]. Other than size, the efficiency of RT also depends on the shape of the AuNPs. Ma et al. reported that spherical AuNPs indicated greater anticancer efficiency upon irradiation of X-ray, that can be because of their greater efficiency of cellular uptake than other AuNPs [105]. The radioresistant tumors are the main reason failure of treatments by traditional radiotherapy. To overcome this problem, modified AuNPs with active targeting ligands were developed. In one study, glutamine, folic acid, and glucose were used to decorate the albumin (BSA)-stabilized AuNPs to target the tumor in breast tumor-bearing mice. BSA-AuNPs decoration with glutamine and folic acid remarkably enhance their tumour -targeting efficacy. They showed the highest radiosensitizing efficacy and results in an approximately 33% reduction in the volume of tumors than to BSA-AuNPs after radiotherapy [106]. It can be concluded that within the next decade to advance the emerging GNP-aided radiotherapy modalities highlighted in this review to clinical trials, and towards their establishment as more effective radiotherapy treatment options for patients with cancer and other diseases.
AuNPs in Gene Delivery
In the last decades, gene delivery has drawn significant attention [23].Various nano-scale carriers such as dendrimers, nanotubes, polymer structures, liposomes, nanorods, and nanoparticles have been tried for the delivery of drugs and genes [107]. Among them, AuNPs have been proven to be excellent candidates for drug and gene delivery applications due to their facile attachment, less or no toxicity, high drug/gene loading, abundance of attachment sites, and easily trackable properties [108]. The release of the cargo in AuNPs can be triggered by the internal environment or by external photo-induced stimulation [109]. AuNPs exhibit a large surface to volume ratio and a good amount of drugs, vectors, and genes can be immobilized on the surface of AuNPs and their guided delivery to desired locations can be accomplished [6]. In a study, the tumor necrosis factor was tethered to AuNPs for antitumor activity [110]. The attachment of PEG in addition to the tumor necrosis factor on AuNPs is effective at further increasing the efficacy of the drug conjugate. AuNPs are also examined for the cellular delivery of DNA and small interfering RNA (siRNA) [111, 112]. In the case of nucleic acid conjugation, the electrostatic interactions between the nucleic acid and AuNPs have been explored most [113]. Furthermore, the delivery of nucleic acids via AuNPs can be improved by modification of AuNPs with charge-reversal polymers; the charge reversal of such polymers depends upon the pH value of the surrounding environment [111]. Moreover, permeation and retention both are improved in the case of AuNPs.
AuNPs as Drug Delivery Carriers
Drug delivery system (DDS) is an interesting research field for the delivery of various drugs to the targeted cells [114, 115]. The main goal of a targeted DDS is increasing the drug concentration in the desired tissues while decreasing its relative concentration in other residual tissues. The ideal DDS delivers drugs at a rate dictated through the biological requirement of the body over treatment period [116, 117]. AuNPs can be developed as efficient nanocarriers in DDS because of their physicochemical and optical properties, low toxicity, high biocompatibility, as well as the great surface area that allows to load density of drugs [118]. AuNPs can deliver drugs to their targets and control their release by the external or internal stimuli. There are two types of targeting to deliver the therapeutics agents by AuNPs into the targeted cells or tissues. In passive targeting, NPs or drugs accumulate at a specific site by pharmacological and physiochemical factors, taking benefit of EPR effect. In active targeting, surface of the NPs or drugs is decorated with a specific active molecule that can recognize its receptor on the targeting site [119]. Conjugates of AuNPs with drug molecules provides promising results in the therapy of endocellular diseases [120]. So that, the involvement of AuNPs in antibiotic treatment can clearly enhance the drug delivery efficiency into target cells in several cases [121]. Antibiotics or other drug molecules have the ability to directly conjugate with AuNPs by ionic or covalent bonding, or via physical absorption [119, 122]. For instance, methotrexate, an anticancer drug, has been conjugated to thirteen-nanometer colloidal AuNP [123]. Methotrexate, a folic acid analog, is able to destroy folate metabolism of cells and can bind to the surface of AuNPs by its carboxylic groups [124].
The cytotoxic effect of methotrexate conjugated with AuNPs is greater than that of free methotrexate on several tumor cell lines which could be because of increased accumulation of methotrexate, and thereby greater concentration of drug in tumour cells [123]. The folic acid-modified AuNPs can be recognized and uptake by cancerous cells with overexpression of folic acid receptors [24]. Agabeigi and co-workers investigated the therapeutic efficiency of 25-nm folate/Methotrexate loaded Au@SiO2 NPs against breast cancer. The finding indicated increased cellular uptake of folate/Methotrexate loaded NPs in cell lines of breast cancer because of the overexpression of folate receptors [125]. In other study, Akinyelu and Singh evaluated the cytotoxic efficacy of 5-fluorouracil (5-FU) encapsulated in AuNPs functionalized with chitosan (C) (CAu5-FU-NPs) and a folate (F) linked counterpart (FCAu5-FU-NPs) on liver and breast cancer cells. Their results showed that FCAu5-FU-NPs resulted in greater anticancer effect compared with CAu5-FU-NPs, in folate positive receptor cancer cells because the folate ligand presence, that actively targeted and increased therapeutic efficacy of the nanocarrier through the mediation of receptor proteins [126]. AuNPs functionalized with targeted specific biomolecules can effectively destroy cancer cells or bacteria (Fig. 4).
Functionalized AuNPs for drug delivery: targeting specific cells with higher loading efficiency, targeted delivery and efficient release of drugs Adapted from Ref. [6], 2011, MDPI
The removal of AuNPs trough the reticulo-endothelial system is the main challenge to utilization of these NPs in DDS. Using the polymers for modifying the AuNPs surface strongly enhance the cellular uptake efficiency compared to unmodified AuNPs [30]. A DDS conjugated with doxorubicin (Dox) on AuNPs surface with polyvinylpyrrolidone (Dox@PVP-AuNPs), developed to deliver the dox to lung cancer cells. Dox@PVP-AuNPs can be regarded as a promising DDS for therapy of lung cancer [127]. Dendrimer (DE)-entrapped AuNPs have achieved emerging applications in theranostics, imaging, and therapeutic sciences. DE-AuNPs decorated with a special ligand for cancerous cells can deliver anticancer drugs for anticancer activity. The nonspecific binding of DE-AuNPs because of their free amine groups and toxicity related to DE complex can be minimized by acetylation or PEGylation of surface amines [128].
An ultrasound-targeted microbubble destruction-promoted delivery system based on DE-AuNPs to co-delivery of miR-21 inhibitor and gemcitabine into pancreatic cancerous cells developed by Lin and coworkers. The co-delivery the miR-21 inhibitor and gemcitabine can be enhanced through using the DE-AuNPs [129].
Peptide-drug-conjugates are designed as a proper technique for delivery of chemotherapeutic drugs to cancer cells specifically. However, their low stability and short half-lives may restrict their applications. Conjugates of peptide-drug-conjugates with AuNPs has the ability to overcome this problem [130].
Recently, the theranostic NPs developed for combination of therapeutic and diagnostic agents for effective therapy of cancer. Elbialy et al. prepared multifunctional magnetic AuNPs that have ability to deliver the drug to tumor sites, induce photothermal therapy by absorption of NIR laser, and act as contrast agents for imaging-guided therapy. The developed AuNPs then coated and conjugated with PEG and Dox to form MAuNP-Dox conjugates. In general, it can be concluded that a promising direction in the application of multifunctionalized AuNPs in biomedicine is the targeted delivery of antitumor drugs and antibiotics for combined photothermal and chemical therapy. To overcome the problems mentioned above, further work should aim to improve smart DDSs based on various nanoparticles, including GNPs. Despite the numerous publications, the number of clinically approved drug delivery nanosystems is still quite limited. Therefore, there is a critical need to further translate laboratory studies with animal models into clinical practice.
Future prospective
Recently, MNPs, in particular, AuNPs have drawn specific attention as an outcome of their intrinsic properties. Obviously, there has been a great promotion in the synthesis, functionalization of AuNPs, which has resulted in advanced diagnostic and therapeutic procedure. Functionalized AuNPs based on functional moieties and their abilities have achieved great advancement in the biomedical field. AuNPs have been applied as contrast agents in bioimaging because of their SPR effect. Also, this effect makes AuNPs applicable as radiotherapy and photothermal therapy agents in primary detection and therapy of cancer. Targeted DDS is a significant field in biomedicine and AuNPs can be utilized as excellent carriers in this system due to their high stability, biocompatibility, low cytotoxicity, and large surface area. However, further investigations are needed to focus on evaluation of in vivo and in vitro targeting efficiency so as to maximize AuNP therapeutic properties in biomedicine.
References
Balakrishnan, S., F.A. Bhat, and A. Jagadeesan, Applications of Gold Nanoparticles in Cancer, in Biomedical Engineering: Concepts, Methodologies, Tools, and Applications. 2018, IGI Global. p. 780–808.
S. Lu, et al. (2018). Self-Assembly of Au Nanoparticles and Quantum Dots by Functional Sol-Gel Silica Layers. Journal of nanoscience and nanotechnology 18 (1), 288–295.
L. Nadav, O.-R. Tsion, and Z. Offer (2020). Improving the properties of a gold nanoparticle barium sensor through mixed-ligand shells. Talanta 208, 120370.
Jiye, X.G.C., Optical Biosensors Based on Localized Surface Plasmon Resonance Effect [J]. Progress in Chemistry, 2010. 1.
A. Khan, et al. (2014). Gold nanoparticles: synthesis and applications in drug delivery. Tropical journal of pharmaceutical research 13 (7), 1169–1177.
P. M. Tiwari, et al. (2011). Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 1 (1), 31–63.
H. Peng, H. Tang, and J. Jiang (2016). Recent progress in gold nanoparticle-based biosensing and cellular imaging. Science China Chemistry 59 (7), 783–793.
H. Daraee, et al. (2016). Application of gold nanoparticles in biomedical and drug delivery. Artificial cells, nanomedicine, and biotechnology 44 (1), 410–422.
P. Baptista, et al. (2008). Gold nanoparticles for the development of clinical diagnosis methods. Analytical and bioanalytical chemistry 391 (3), 943–950.
J. Turkevich, P. C. Stevenson, and J. Hillier (1951). A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussions of the Faraday Society 11, 55–75.
M. Shah, et al. (2014). Gold nanoparticles: various methods of synthesis and antibacterial applications. Front Biosci 19 (8), 1320–1344.
A. J. Mieszawska, et al. (2013). Multifunctional gold nanoparticles for diagnosis and therapy of disease. Molecular pharmaceutics 10 (3), 831–847.
M. Brust, et al. (1994). Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. Journal of the Chemical Society, Chemical Communications 7, 801–802.
R. Herizchi, et al. (2016). Current methods for synthesis of gold nanoparticles. Artificial cells, nanomedicine, and biotechnology 44 (2), 596–602.
E. L. L. Yeo, et al. (2017). Exploiting the protein corona around gold nanorods for low-dose combined photothermal and photodynamic therapy. Journal of Materials Chemistry B 5 (2), 254–268.
J. Piella, N. G. Bastús, and V. Puntes (2016). Size-Controlled Synthesis of Sub-10-nanometer Citrate-Stabilized Gold Nanoparticles and Related Optical Properties. Chemistry of Materials 28 (4), 1066–1075.
J. Niu, T. Zhu, and Z. Liu (2007). One-step seed-mediated growth of 30–150 nm quasispherical gold nanoparticles with 2-mercaptosuccinic acid as a new reducing agent. Nanotechnology 18 (32), 325607.
S. Wang, et al. (2016). Biologically inspired polydopamine capped gold nanorods for drug delivery and light-mediated cancer therapy. ACS applied materials & interfaces 8 (37), 24368–24384.
L. Wang, et al. (2011). Selective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapy. Nano letters 11 (2), 772–780.
X. Wang, et al. (2010). Gold nanorod-based localized surface plasmon resonance biosensor for sensitive detection of hepatitis B virus in buffer, blood serum and plasma. Biosensors and Bioelectronics 26 (2), 404–410.
J. Yu, et al. (2016). Facile one-step green synthesis of gold nanoparticles using Citrus maxima aqueous extracts and its catalytic activity. Materials Letters 166, 110–112.
R. K. DeLong, et al. (2010). Functionalized gold nanoparticles for the binding, stabilization, and delivery of therapeutic DNA, RNA, and other biological macromolecules. Nanotechnology, science and applications 3, 53.
J. S. Suk, et al. (2016). PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Advanced drug delivery reviews 99, 28–51.
N. S. Aminabad, M. Farshbaf, and A. Akbarzadeh (2019). Recent advances of gold nanoparticles in biomedical applications: State of the art. Cell biochemistry and biophysics 77 (2), 123–137.
Pellegrino, T., et al., On the development of colloidal nanoparticles towards multifunctional structures and their possible use for biological applications. small, 2005. 1(1): p. 48–63.
B. Liu, X. Sun, and F. He (2008). Preparation and characterization of a Cu2+ chemosensor based on fluorescent self-assembled sandwich bilayers. Thin solid films 516 (8), 2213–2217.
R. H. Adnan, et al. (2015). Factors influencing the catalytic oxidation of benzyl alcohol using supported phosphine-capped gold nanoparticles. Catalysis Science & Technology 5 (2), 1323–1333.
A. I. Abdelrahman, et al. (2006). Fabrication and electrochemical application of three-dimensional gold nanoparticles: self-assembly. The Journal of Physical Chemistry B 110 (6), 2798–2803.
G. Ajnai, et al. (2014). Trends of gold nanoparticle-based drug delivery system in cancer therapy. Journal of Experimental & Clinical Medicine 6 (6), 172–178.
Locatelli, E., Synthesis and surface modification of silver and gold nanoparticles. Nanomedicine applications against Glioblastoma Multiforme. 2014, alma.
S. Alex and A. Tiwari (2015). Functionalized gold nanoparticles: synthesis, properties and applications—a review. Journal of nanoscience and nanotechnology 15 (3), 1869–1894.
A. G. Kanaras, et al. (2002). Thioalkylated tetraethylene glycol: a new ligand for water soluble monolayer protected gold clusters. Chemical Communications 20, 2294–2295.
J. Ruff, et al. (2018). CLPFFD–PEG functionalized NIR-absorbing hollow gold nanospheres and gold nanorods inhibit β-amyloid aggregation. Journal of Materials Chemistry B 6 (16), 2432–2443.
P. S. Ghosh, et al. (2008). Efficient gene delivery vectors by tuning the surface charge density of amino acid-functionalized gold nanoparticles. ACS nano 2 (11), 2213–2218.
G. Dhanya, et al. (2018). Histidine and arginine conjugated starch-PEI and its corresponding gold nanoparticles for gene delivery. International journal of biological macromolecules 120, 999–1008.
C. Thiruppathiraja, et al. (2011). An enhanced immuno-dot blot assay for the detection of white spot syndrome virus in shrimp using antibody conjugated gold nanoparticles probe. Aquaculture 318 (3–4), 262–267.
A. L. Ginzburg, et al. (2018). Synergistic toxicity produced by mixtures of biocompatible gold nanoparticles and widely used surfactants. ACS nano 12 (6), 5312–5322.
Sasidharan, A. and N. Monteiro-Riviere, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol, 2015. 7: p. 779–796.
X. Li, et al. (2018). The systematic evaluation of size-dependent toxicity and multi-time biodistribution of gold nanoparticles. Colloids and Surfaces B: Biointerfaces 167, 260–266.
M. Tsoli, et al. (2005). Cellular uptake and toxicity of Au55 clusters. Small 1 (8–9), 841–844.
Dykman, L. and N. Khlebtsov, Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Naturae (aнглoязычнaя вepcия), 2011. 3(2 (9)).
Li, X., et al., Biocompatibility and toxicity of nanoparticles and nanotubes. Journal of Nanomaterials, 2012. 2012.
Rani, K., Biomedical applications of silver and gold nanoparticles: effective and safe non-viral delivery vehicles. Journal of Applied Biotechnology and Bioengineering, 2017. 3(2).
J.-G. Piao, et al. (2018). pH-sensitive zwitterionic coating of gold nanocages improves tumor targeting and photothermal treatment efficacy. Nano Research 11 (6), 3193–3204.
N. Pantidos and L. E. Horsfall (2014). Biological synthesis of metallic nanoparticles by bacteria, fungi and plants. Journal of Nanomedicine & Nanotechnology 5 (5), 1.
K. Nejati-Koshki, A. Akbarzadeh, and M. Pourhassan-Moghaddam (2014). Curcumin inhibits leptin gene expression and secretion in breast cancer cells by estrogen receptors. Cancer cell international 14 (1), 1–7.
S. Rasouli, et al. (2020). Synergistic anticancer effects of electrospun nanofiber-mediated codelivery of Curcumin and Chrysin: Possible application in prevention of breast cancer local recurrence. Journal of Drug Delivery Science and Technology 55, 101402.
E. Priyadarshini and N. Pradhan (2017). Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: a review. Sensors and Actuators B: Chemical 238, 888–902.
R. A. Reynolds, C. A. Mirkin, and R. L. Letsinger (2000). Homogeneous, nanoparticle-based quantitative colorimetric detection of oligonucleotides. Journal of the American Chemical Society 122 (15), 3795–3796.
V. Raj, A. N. Vijayan, and K. Joseph (2014). Naked eye detection of infertility using fructose blue–A novel gold nanoparticle based fructose sensor. Biosensors and Bioelectronics 54, 171–174.
N. N. Vinita and R. Prakash (2018). One step synthesis of AuNPs@ MoS2-QDs composite as a robust peroxidase-mimetic for instant unaided eye detection of glucose in serum, saliva and tear. Sensors Actuators B Chem 263, 109–119.
N. R. Nirala, P. S. Saxena, and A. Srivastava (2018). Colorimetric detection of cholesterol based on enzyme modified gold nanoparticles. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 190, 506–512.
M. António, et al. (2018). Functionalized Gold Nanoparticles for the Detection of C-Reactive Protein. Nanomaterials 8 (4), 200.
V. Raj and K. Sreenivasan (2010). Selective detection and estimation of C-reactive protein in serum using surface-functionalized gold nano-particles. Analytica chimica acta 662 (2), 186–192.
K. E. Sapsford, L. Berti, and I. L. Medintz (2006). Materials for fluorescence resonance energy transfer analysis: beyond traditional donor–acceptor combinations. Angewandte Chemie International Edition 45 (28), 4562–4589.
J. Shi, et al. (2015). A fluorescence resonance energy transfer (FRET) biosensor based on graphene quantum dots (GQDs) and gold nanoparticles (AuNPs) for the detection of mecA gene sequence of Staphylococcus aureus. Biosensors and Bioelectronics 67, 595–600.
A. N. Shipway, E. Katz, and I. Willner (2000). Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem 1 (1), 18–52.
ZHAO, J., et al., Gold nanoparticles-based biosensors for biomedical application. Nano Life, 2012. 2(04): p. 1230008.
I. Bhatnagar, et al. (2018). Chitosan stabilized gold nanoparticle mediated self-assembled glip nanobiosensor for diagnosis of invasive aspergillosis. International journal of biological macromolecules 110, 449–456.
Mohammadi, H., G. Yammouri, and A. Amine, Current advances in electrochemical genosensors for detecting microRNA cancer markers. Current Opinion in Electrochemistry, 2019.
L. Tian, et al. (2018). Gold nanoparticles superlattices assembly for electrochemical biosensor detection of microRNA-21. Biosensors and Bioelectronics 99, 564–570.
V. Buk, M. E. Pemble, and K. Twomey (2019). Fabrication and evaluation of a carbon quantum dot/gold nanoparticle nanohybrid material integrated onto planar micro gold electrodes for potential bioelectrochemical sensing applications. Electrochimica Acta 293, 307–317.
L. Cui, et al. (2018). An ultrasensitive electrochemical biosensor for polynucleotide kinase assay based on gold nanoparticle-mediated lambda exonuclease cleavage-induced signal amplification. Biosensors and Bioelectronics 99, 1–7.
M. H. Ghalehno, M. Mirzaei, and M. Torkzadeh-Mahani (2019). Electrochemical aptasensor for activated protein C using a gold nanoparticle–Chitosan/graphene paste modified carbon paste electrode. Bioelectrochemistry 130, 107322.
B. A. Du, Z. P. Li, and C. H. Liu (2006). One-step homogeneous detection of DNA hybridization with gold nanoparticle probes by using a linear light-scattering technique. Angewandte Chemie International Edition 45 (47), 8022–8025.
T. Špringer, et al., Surface plasmon resonance biosensor for the detection of tau-amyloid β complex. (Chemical, Sensors and Actuators B, 2020), p. 128146.
Y. Wang, et al. (2007). SERS opens a new way in aptasensor for protein recognition with high sensitivity and selectivity. Chemical Communications 48, 5220–5222.
E. A. Vitol, et al. (2009). In situ intracellular spectroscopy with surface enhanced Raman spectroscopy (SERS)-enabled nanopipettes. Acs Nano 3 (11), 3529–3536.
J. H. Choi, W. A. El-Said, and J.-W. Choi (2020). Highly sensitive surface-enhanced Raman spectroscopy (SERS) platform using core/double shell (Ag/polymer/Ag) nanohorn for proteolytic biosensor. Applied Surface Science 506, 144669.
G. Maiorano, et al. (2010). Effects of cell culture media on the dynamic formation of protein− nanoparticle complexes and influence on the cellular response. ACS nano 4 (12), 7481–7491.
Y. Zhang, H. Hong, and W. Cai (2010). Imaging with Raman spectroscopy. Current pharmaceutical biotechnology 11 (6), 654–661.
Nie, S. and S.R. Emory, Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. science, 1997. 275(5303): p. 1102–1106.
J. He, et al. (2019). Design of Raman tag-bridged core–shell Au@ Cu 3 (BTC) 2 nanoparticles for Raman imaging and synergistic chemo-photothermal therapy. Nanoscale 11 (13), 6089–6100.
Soni, J., Use of nanostructures based on noble metals in nanobiomedicine, in Nanostructures for Novel Therapy. 2017, Elsevier. p. 685–712.
Aioub, M., L.A. Austin, and M.A. El-Sayed, Gold nanoparticles for cancer diagnostics, spectroscopic imaging, drug delivery, and plasmonic photothermal therapy, in Inorganic Frameworks as Smart Nanomedicines. 2018, Elsevier. p. 41–91.
T. Gong, et al. (2013). Engineering bioconjugated gold nanospheres and gold nanorods as label-free plasmon scattering probes for ultrasensitive multiplex dark-field imaging of cancer cells. Journal of biomedical nanotechnology 9 (6), 985–991.
W. Li and X. Chen (2015). Gold nanoparticles for photoacoustic imaging. Nanomedicine 10 (2), 299–320.
Conde, J., et al., Multifunctional Gold Nanocarriers for Cancer Theranostics: From Bench to Bedside and Back Again?, in Nano-Oncologicals. 2014, Springer. p. 295–328.
J. A. Copland, et al. (2004). Bioconjugated gold nanoparticles as a molecular based contrast agent: implications for imaging of deep tumors using optoacoustic tomography. Molecular Imaging & Biology 6 (5), 341–349.
X. Xu, et al. (2019). Multifunctional nanotheranostic gold nanocages for photoacoustic imaging guided radio/photodynamic/photothermal synergistic therapy. Acta biomaterialia 84, 328–338.
I.-C. Sun, et al. (2019). Photoacoustic imaging of cancer cells with glycol-chitosan-coated gold nanoparticles as contrast agents. Journal of biomedical optics 24 (12), 121903.
H. Liu, et al. (2013). Targeted dendrimer-stabilized gold nanoparticles for computed tomography imaging of cancer cells. Journal of Controlled Release 1 (172), e37–e38.
J. Beik, et al. (2017). A nanotechnology-based strategy to increase the efficiency of cancer diagnosis and therapy: folate-conjugated gold nanoparticles. Current Medicinal Chemistry 24 (39), 4399–4416.
M. Keshavarz, et al. (2018). Alginate hydrogel co-loaded with cisplatin and gold nanoparticles for computed tomography image-guided chemotherapy. Journal of biomaterials applications 33 (2), 161–169.
S. Khademi, et al. (2019). Targeted gold nanoparticles enable molecular CT imaging of head and neck cancer: an in vivo study. The international journal of biochemistry & cell biology 114, 105554.
L. Zou, et al. (2016). Current approaches of photothermal therapy in treating cancer metastasis with nanotherapeutics. Theranostics 6 (6), 762.
J.-L. Li and M. Gu (2009). Gold-nanoparticle-enhanced cancer photothermal therapy. IEEE Journal of selected topics in quantum electronics 16 (4), 989–996.
Y. Panahi, et al. (2017). Preparation, surface properties, and therapeutic applications of gold nanoparticles in biomedicine. Drug research 11 (02), 77–87.
M. A. Khiavi, et al. (2019). Enzyme-conjugated gold nanoparticles for combined enzyme and photothermal therapy of colon cancer cells. Colloids and Surfaces A: Physicochemical and Engineering Aspects 572, 333–344.
N. S. Abadeer and C. J. Murphy (2016). Recent progress in cancer thermal therapy using gold nanoparticles. The Journal of Physical Chemistry C 120 (9), 4691–4716.
E. C. Dreaden, et al. (2012). The golden age: gold nanoparticles for biomedicine. Chemical Society Reviews 41 (7), 2740–2779.
I. Grabowska-Jadach, et al. (2019). Synthesis, characterization and application of plasmonic hollow gold nanoshells in a photothermal therapy—New particles for theranostics. Biomedicine & Pharmacotherapy 111, 1147–1155.
H. S. Kim and D. Y. Lee (2018). Near-infrared-responsive cancer photothermal and photodynamic therapy using gold nanoparticles. Polymers 10 (9), 961.
S. Pan, et al. (2018). The effect of photothermal therapy on osteosarcoma with polyacrylic acid–coated gold nanorods. Dose-Response 16 (3), 1559325818789841.
S. E. Skrabalak, et al. (2008). Gold nanocages: synthesis, properties, and applications. Accounts of chemical research 41 (12), 1587–1595.
E. J. Hong, et al. (2018). Cancer-targeted photothermal therapy using aptamer-conjugated gold nanoparticles. Journal of Industrial and Engineering Chemistry 67, 429–436.
J. Narang, et al. (2015). Electrochemical impediometric detection of anti-HIV drug taking gold nanorods as a sensing interface. Biosensors and Bioelectronics 66, 332–337.
N. Kutsevol, et al. (2019). New hybrid composites for photodynamic therapy: synthesis, characterization and biological study. Applied Nanoscience 9 (5), 881–888.
K. Haume, et al. (2016). Gold nanoparticles for cancer radiotherapy: a review. Cancer nanotechnology 7 (1), 8.
D. R. Cooper, D. Bekah, and J. L. Nadeau (2014). Gold nanoparticles and their alternatives for radiation therapy enhancement. Frontiers in chemistry 2, 86.
S. Her, D. A. Jaffray, and C. Allen (2017). Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements. Advanced drug delivery reviews 109, 84–101.
Liu, M., et al., Radiotherapy enhancement with gold nanoparticles. Nuclear Techniques, 2015. 38(9).
L. Sancey, et al. (2014). The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. The British journal of radiology 87 (1041), 20140134.
X.-D. Zhang, et al. (2012). Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials 33 (27), 6408–6419.
N. Ma, et al. (2017). Shape-dependent radiosensitization effect of gold nanostructures in cancer radiotherapy: comparison of gold nanoparticles, nanospikes, and nanorods. ACS applied materials & interfaces 9 (15), 13037–13048.
A. Kefayat, et al. (2019). Investigation of different targeting decorations effect on the radiosensitizing efficacy of albumin-stabilized gold nanoparticles for breast cancer radiation therapy. European Journal of Pharmaceutical Sciences 130, 225–233.
X. Yang, et al. (2015). Gold nanomaterials at work in biomedicine. Chemical reviews 115 (19), 10410–10488.
P. Ghosh, et al. (2008). Gold nanoparticles in delivery applications. Advanced drug delivery reviews 60 (11), 1307–1315.
A. Madhusudhan, et al. (2014). Efficient pH dependent drug delivery to target cancer cells by gold nanoparticles capped with carboxymethyl chitosan. International journal of molecular sciences 15 (5), 8216–8234.
C. M. Cobley, et al. (2011). Gold nanostructures: a class of multifunctional materials for biomedical applications. Chemical Society Reviews 40 (1), 44–56.
S. Guo, et al. (2010). Enhanced gene delivery and siRNA silencing by gold nanoparticles coated with charge-reversal polyelectrolyte. ACS nano 4 (9), 5505–5511.
S. Fekri Aval, et al. (2016). Gene silencing effect of SiRNA-magnetic modified with biodegradable copolymer nanoparticles on hTERT gene expression in lung cancer cell line. Artificial cells, nanomedicine, and biotechnology 44 (1), 188–193.
A. Graczyk, et al. (2020). Gold nanoparticles in conjunction with nucleic acids as a modern molecular system for cellular delivery. Molecules 25 (1), 204.
J. Karnoosh-Yamchi, et al. (2014). Preparation of pH sensitive insulin-loaded Nano hydrogels and evaluation of insulin releasing in different pH conditions. Molecular biology reports 41 (10), 6705–6712.
K. Nejati, et al. (2020). GDNF gene-engineered adipose-derived stem cells seeded Emu oil-loaded electrospun nanofibers for axonal regeneration following spinal cord injury. Journal of Drug Delivery Science and Technology 60, 102095.
H. Mellatyar, et al. (2018). 17-DMAG-loaded nanofibrous scaffold for effective growth inhibition of lung cancer cells through targeting HSP90 gene expression. Biomedicine & Pharmacotherapy 105, 1026–1032.
M. Dadashpour, et al. (2017). Emerging importance of phytochemicals in regulation of stem cells fate via signaling pathways. Phytotherapy Research 31 (11), 1651–1668.
P. Luo, et al. (2014). Aptamer biosensor for sensitive detection of toxin A of Clostridium difficile using gold nanoparticles synthesized by Bacillus stearothermophilus. Biosensors and Bioelectronics 54, 217–221.
D. Pissuwan, T. Niidome, and M. B. Cortie (2011). The forthcoming applications of gold nanoparticles in drug and gene delivery systems. Journal of controlled release 149 (1), 65–71.
B. Saha, et al. (2007). In vitro structural and functional evaluation of gold nanoparticles conjugated antibiotics. Nanoscale Research Letters 2 (12), 614.
H. Gu, et al. (2003). Presenting vancomycin on nanoparticles to enhance antimicrobial activities. Nano letters 3 (9), 1261–1263.
M. Pourhassan-Moghaddam, et al. (2014). Watercress-based gold nanoparticles: biosynthesis, mechanism of formation and study of their biocompatibility in vitro. Micro & Nano Letters 9 (5), 345–350.
Y.-H. Chen, et al. (2007). Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model. Molecular pharmaceutics 4 (5), 713–722.
A. E. Kel, et al. (2016). Multi-omics “upstream analysis” of regulatory genomic regions helps identifying targets against methotrexate resistance of colon cancer. EuPA open proteomics 13, 1–13.
R. Agabeigi, et al. (2020). Novel Chemo-Photothermal Therapy in Breast Cancer Using Methotrexate-Loaded Folic Acid Conjugated Au@ SiO 2 Nanoparticles. Nanoscale Research Letters 15 (1), 1–14.
J. Akinyelu and M. Singh (2019). Folate-tagged chitosan-functionalized gold nanoparticles for enhanced delivery of 5-fluorouracil to cancer cells. Applied Nanoscience 9 (1), 7–17.
V. Ramalingam, et al. (2018). Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer. Scientific reports 8 (1), 1–12.
Kesharwani, P., et al., Dendrimer-entrapped gold nanoparticles as promising nanocarriers for anticancer therapeutics and imaging. Progress in Materials Science, 2019.
L. Lin, et al. (2018). UTMD-promoted co-delivery of gemcitabine and miR-21 inhibitor by dendrimer-entrapped gold nanoparticles for pancreatic cancer therapy. Theranostics 8 (7), 1923.
K. Kalimuthu, et al. (2018). Gold nanoparticles stabilize peptide-drug-conjugates for sustained targeted drug delivery to cancer cells. Journal of nanobiotechnology 16 (1), 34.
N. Rizk, N. Christoforou, and S. Lee (2016). Optimization of anti-cancer drugs and a targeting molecule on multifunctional gold nanoparticles. Nanotechnology 27 (18), 185704.
B. Cheng, et al. (2016). Gold nanosphere gated mesoporous silica nanoparticle responsive to near-infrared light and redox potential as a theranostic platform for cancer therapy. Journal of biomedical nanotechnology 12 (3), 435–449.
Q. Zhao, Z.-J. Yang, and J. He (2018). Fano resonances in heterogeneous dimers of silicon and gold nanospheres. Frontiers of Physics 13 (3), 137801.
A. R. Rastinehad, et al. (2019). Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proceedings of the National Academy of Sciences 116 (37), 18590–18596.
B. K. Poudel, et al. (2018). In situ fabrication of mesoporous silica-coated silver-gold hollow nanoshell for remotely controllable chemo-photothermal therapy via phase-change molecule as gatekeepers. International journal of pharmaceutics 548 (1), 92–103.
J. Y. Zeng, et al. (2018). Porphyrinic metal–organic frameworks coated gold nanorods as a versatile nanoplatform for combined photodynamic/photothermal/chemotherapy of tumor. Advanced Functional Materials 28 (8), 1705451.
J.-T. Cao, et al. (2018). Graphene oxide@ gold nanorods-based multiple-assisted electrochemiluminescence signal amplification strategy for sensitive detection of prostate specific antigen. Biosensors and Bioelectronics 99, 92–98.
Y.-Y. Cai, et al. (2018). Photoluminescence of gold nanorods: Purcell effect enhanced emission from hot carriers. Acs Nano 12 (2), 976–985.
X. Wang, et al. (2019). Surface-enhanced Raman scattering by composite structure of gold nanocube-PMMA-gold film. Optical Materials Express 9 (4), 1872–1881.
L. Li, et al. (2019). Gap-mode excitation, manipulation, and refractive-index sensing application by gold nanocube arrays. Nanoscale 11 (12), 5467–5473.
R. Liang, et al. (2018). Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@ manganese dioxide to inhibit tumor growth and metastases. Biomaterials 177, 149–160.
C. Wang, et al. (2018). Pretreated Macrophage-Membrane-Coated Gold Nanocages for Precise Drug Delivery for Treatment of Bacterial Infections. Advanced Materials 30 (46), 1804023.
S. Abalde-Cela, et al. (2018). Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Scientific reports 8 (1), 1–6.
Y. Zou, et al. (2019). Synthesis of mesoporous-silica coated multi-branched gold nanoparticles for surface enhanced Raman scattering evaluation of 4-bromomethcathinone. Journal of Saudi Chemical Society 23 (3), 378–383.
Acknowledgements
The authors express their sincere thanks to the pharmaceutical sciences research center of Ardabil University of Medical Sciences for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nejati, K., Dadashpour, M., Gharibi, T. et al. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J Clust Sci 33, 1–16 (2022). https://doi.org/10.1007/s10876-020-01955-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-020-01955-9