Abstract
In the recent years, temperature and pH-sensitive hydrogels were developed as suitable carriers for drug delivery. In this study, four different pH-sensitive nanohydrogels were designed for an oral insulin delivery modeling. NIPAAm–MAA–HEM copolymers were synthesized by radical chain reaction with 80:8:12 ratios respectively. Reactions were carried out in four conditions including 1,4-dioxan and water as two distinct solution under nitrogen gas-flow. The copolymers were characterized with FT-IR, SEM and TEM. Copolymers were loaded with regular insulin by modified double emulsion method with ratio of 1:10. Release study carried out in pH 1.2 and pH 6.8 at 37 °C. For pH 6.8 and pH 1.2, 2 mg of the insulin loaded nanohydrogels was float in a beaker containing 100 mL of PBS with pH 6.8 and 100 mL of HCl solution with pH 1.2, respectively. Sample collection was done in different times and HPLC was used for analysis of samples using water/acetonitrile (65/35) as the mobile phase. Nanohydrogels synthesis reaction yield was 95 %, HPLC results showed that loading in 1,4-dioxan without cross-linker nanohydrogels was more than others, also indicated that the insulin release of 1,4-dioxan without cross-linker nanohydrogels at acidic pH is less, but in pH 6.8 is the most. Results showed that by opting suitable polymerization method and selecting the best nanohydrogels, we could obtain a suitable insulin loaded nanohydrogels for oral administration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is a disorder caused by decreased production of insulin or by decreased ability to use insulin, leading to increase blood glucose levels [1]. Complications in DM causes excess morbidity and mortality, loss of independence, and reduced quality of life. As the disease progresses, severe complications such as retinopathy, nephropathy, neuropathy, cardiovascular disease, and foot ulceration occur [2].
Diabetes mellitus may be categorized into several types but the two major types are type 1 (insulin-dependent diabetes mellitus; IDDM) and type 2 (non-insulin dependent diabetes mellitus; NIDDM) [2].
The World Health Organization (WHO) according to increasing global incidence of diabetes has called diabetes as a hidden epidemic and has invited the world countries to deal with the epidemic since 1993. According to WHO epidemiological studies, 135 million persons in 1995 were diabetic and will reach to 300 million in 2025 [3].
Insulin is used to cure diabetes since 1922 [4]. There are different methods for insulin delivery such as subcutaneous injection, pulmonary, intranasal, and the colon, but up to now there is no consensus on the best method for treatment [4].
Nowadays, the scientists attempt to replace non-invasive methods instead of invasive methods (e.g. injection) for insulin delivery into the body [5]. Oral administration of insulin is one of the interesting methods that enter insulin in the blood through the gastrointestinal tract. According to the rapid enzymatic digestion of insulin in the stomach, insulin is digested and active insulin decreases [5]. So, in order to protect insulin in acidic soap and enzymatic digestion in stomach, different carriers could be used so that they could release and absorbed insulin into the bloodstream through the small intestine [6].
Oral drug delivery offers many advantages in comparison with other prescribed methods, including non-invasive and free from the interference due process for sterile injectable products, easy to use, easy dose adjustment and low cost in comparison with injection method [7]. Oral administration of insulin has great advantages such as direct entry of insulin from the portal vein to the liver and the direct effect of insulin on glucose metabolism in the liver having the same physiological state [8]. Carriers such as micelles, nanoparticles, microcapsules, nanogels, etc. have been used to date for drug delivery [9].
First time, Lowman et al. were loaded insulin on microspheres of poly metacrylic-ethylene glycol and give it orally to normal and diabetic mice [10]. In another study, polymers of acrylic acid–N-isopropyl acrylamide and meta-butyl methacrylic acid with molar ratio of 10:5:85 was used [11, 12]. Alginate microparticles produced by emulsification method was used as a carrier for the drug delivery of insulin by Reis et al. [13]. Used nanoparticles were protect insulin from stomach acid but had little biosafety and showed side effects.
Among the carriers, nanohydrogels have attracted much attention. Microspheres with a diameter less than one micrometer is often called nanogels and sensitive microspheres with diameters of micrometer and less is known nanohydrogels [14]. Nanohydrogels are materials that are made from biodegradable natural polymer [15, 16]. Micro nanohydrogels are made from copolymers by photo cross linking and responding to chemical and physical changes such as temperature, pH and composition of the solution and adding special ions and electric fields [14, 17, 18]. Hydrogels can be designed to show significant volume changes in response to small environmental changes such as pH, ionic strength, temperature, electric fields, solution or magnetic fields [19].
Nanohydrogels are made from swellable and water-soluble polymers [20] and convert from powdery substance into the swollen polymer network via absorbing water from the environment and confine the drug to them. This trapped material in suit condition could be released [15, 16].
Nanohydrogels have a special surface that increases its interaction time with various biological components. They could maintain most of the loaded drugs and their small size allow them to pass through the anatomic walls and reach to the capillaries [17].
The main goal of the present study was to provide pH sensitive nanohydrogels containing human insulin and to assess the amount and rate of insulin releasing from nanohydrogels in different pH conditions in order to condense in the gastric pH for insulin protection and decondense in intestine pH. The best nanohydrogel preparation and drug loading method was selected in four different conditions to make a pH sensitive nanohydrogel as insulin delivery system with good smart-releasing behavior for oral administration of insulin.
Poly NIPAAm polymers can exhibit pH-sensitive behaviors by the addition of acidic or basic groups. Hydrophilic characteristic in copolymer is produced by PNIPAAm base. Mechanical strength of PNIPAAm hydrogels was improved by adding MAA to the hydrophilic monomer. pH-sensitive behavior in copolymer was produced by a MAA derivative that is HEMA [21].
Materials and instruments
Materials
N-Iso propyl acryl amide (NIPAAm) obtained from Acros Organics (NJ, USA), hydroxy ethyl methacrylate (HEMA) (Merck chemical Co., Germany), methacrylic acid (MAA) (Merck chemical Co., Germany), HCl (Merck chemical Co., Germany), NaOH (Merck chemical Co., Germany), NaCl (Merck chemical Co., Germany), BPO (Merck chemical Co., Germany), 1,4-dioxan (Di gung chemical Co., Korea), n-hexane (Merck chemical Co., Germany), trichloromethane (Merck chemical Co., Germany), dichlromethane (Merck chemical Co., Germany), ammonium per sulfate (Merck chemical Co., Germany), acetonitrile (Merck chemical Co., Germany), pure human insulin was purchased from EXIR, Iran hormone, N,N-methylene bis acryl amid (N,N-MBAAm) (Merck chemical Co., Germany), centrifuge sigma (5,000 rpm), centrifuge HEROLAB (13,000 rpm), vacuum rotary, sonicator, high performance liquid chromatography (HPLC, Waters), FT-IR spectrometer instrument (FT-IR, Tensor 27 Bruker), scanning electron microscopy (SEM, Mira3 tescan 5.0 kV), transmission electron microscopy (TEM, Zeiss EM10C 80 kV).
Preparation of poly (NIPAAm–MAA–HEMA) nanohydrogel
NIPAAm–MAA–HEMA copolymer was synthesized by radical chain reaction with 80:8:12 ratios respectively. Reactions were carried out in four conditions including 1,4-dioxan and water as two distinct solutions under Nitrogen gas-flow. For preparation of poly NIPAAm–MAA–HEMA hydrogel, cross linker was not used and for preparation of cross-linked nanohydrogel, N,N-MBAAm (300 μL of N,N-MBAAm) was used as cross linker. Benzoyl peroxide and ammonium per sulfate (0.3 mol% with respect to the monomers) for 1,4-dioxan and water distinct solution were added as initiator of polymerization, respectively. The mixture was magnetically stirred and degassed with nitrogen gas for 30 min. The polymerization was fulfilled at 70 °C for 24 h and 25 °C for 24 h under nitrogen atmosphere for 1,4-dioxan and water distinct solution, respectively. The synthesized polymers were precipitated using n-hexane in liquid nitrogen bath. The polymerization yield of the experiment was 86.5 %. Figure 1 shows whole scheme protocol of nanohydrogel synthesizing.
Preparation of insulin loaded copolymers
Modified double emulsion method was used for loading insulin in the polymers. Poly vinyl alcohol (PVA) was used as stabilizer with 0.1 % concentration. First 100 mg of the synthesized and freeze dried copolymer was dissolved in 5 mL of chloroform. Then 50 IU (international unit) of insulin as aqueous solution was added in solution and sonicated for 60 s. Then 50 mL of PVA was added to the mixture and sonicated 50 s. The mixture was located in vacuum rotary for 30 min for further evaporation of chloroform and loading of insulin and then centrifuged in 13,000 rpm and 25 °C. Loaded polymers lyophilized and stored in 2–8 °C for further use. Figure 1 shows whole scheme protocol of insulin loading to nanohydrogel.
In vitro release study
After loading insulin in nanohydrogels, solutions at 4° C for 30 min (three consecutive times each for 10 min) were centrifuged and the supernatant was removed and the precipitate was collected. The in vitro release of insulin from nanohydrogel was carried out at 37 °C and in two different pH (pH 1.2 and 6.8). For pH 6.8 and pH 1.2, 2 mg of the insulin loaded nanohydrogel was float in a beaker containing 100 mL of PBS with pH 6.8 and 100 mL of HCl solution with pH 1.2, respectively. The beakers were placed in a shaker incubator maintained at 37 °C. 200 μL samples were removed from the external solution and were replaced with fresh solution. Sample collection was done in different times and HPLC was used for analysis of samples using water/acetonitrile (65/35) as the mobile phase. The UV detection was at 214 nm. The run time for the assay was 15 min and the retention time for insulin was 2.56 min. HPLC was determined to opt better loaded nanohydrogel.
Statistical analysis
Analyze of Statistical data was performed by student t test on SPSS version16 and charting was performed by Microsoft Excel software 2010 version.
Results
Synthesis of NIPAm-MAA-HEMA copolymers
The products of synthesized nanohydrogels in four methods are crystal or powdery form. These products for subsequent applications could be kept in refrigerator or in freezer or at room temperature.
Preparation of insulin loaded copolymers and loading percentage
Fifty international units of human insulin were loaded in every four nanohydrogels. In this step nanohydrogels would be in solution state, so we got them in a solution of dichloromethane, but it was low solubility in dichloromethane or was so difficult to solute in it. So nanohydrogels were soluted in trichloromethane (chloroform) solution, and then to prevent solution from evaporation, mouth of the container of solvent and nanohydrogels was closed with aluminum foil and parafilm.
After insulin loading and removing the solvent by vacuum rotary, emulsion should be centrifuged by refrigerated centrifuge at 13,000 rpm. Special falcons were used to separate the sediment from supernatant. HPLC was done for supernatant and sediments were saved to examine the rate of release (at temperature of −20 °C). The under curve area from the corresponding HPLC chromatograms from the supernatant after centrifugation of the emulsion obtained from loading stage, are shown in Table 1.
After performing a modified double emulsion method, drug loading percentage was determined by HPLC and loaded drug is calculated according to the following equation.
FT-IR spectroscopy
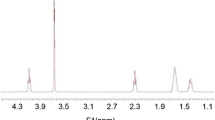
The structure of Insulin loaded on poly(NIPAAm–MAA–HEMA) nanohydrogels was showed using FT-IR spectroscopy (Fig. 2). In IR spectrum, absorption peaks presented at 673, 1282, 1411, 1642, and 3300 cm−1 (OH, which is related to the structure of the polymer that have been synthesized nanoparticles form) belonged to Insulin loaded on (NIPAAm–MAA–HEMA). Bending vibration ofCH2 made peak at 1,040 cm−1 and absorption peaks at 761, 964 and 1,135 cm−1were because of the stretching vibration of the alkyl groups from NIPAAm. However, the peak identification to the stretching vibrations of C–N (normally at about 1,100 cm−1) was problematic due to overlapping of other peaks.
Size and size distribution by scanning electron microscopy (SEM)
The SEM micrographs of pure PNIPAAm–MAA–HEMA nanohydrogels (Fig. 3a) and Insulin loaded on poly(NIPAAm–MAA–HEMA nanohydrogels (Fig. 3b) are shown. Observing the photograph (a), nanohydrogels were aggregated seriously, which was due to the nano size of the polymer and they were about 50 nm. At (Fig. 3b) after insulin loading, the size of particles was changed to be 80–200 nm.
Transient electron microscopy (TEM)
Figure 4 shows delegate TEM images of synthesized poly NIPAAm–MAA–HEMA nanohydrogels and insulin loaded nanohydrogels. The TEM image indicates that nanohydrogels are nano-crystalline, though their shape is primarily spherical with some hexagonal shaped nanohydrogels. The size of the spherical nanohydrogels varies from 70 to 150 nm with average particle size of 80. Moreover, poly NIPAAm–MAA–HEMA nanohydrogels are agglomerates due to high surface area particles.
To evaluate content of insulin release from the nanohydrogels in invitro conditions, 2 mg of the insulin loaded nanohydrogels was float in a beaker containing 100 mL of PBS with pH 6.8 and 100 mL of HCl solution with pH 1.2, respectively. The beakers were placed in a shaker incubator maintained at 37 °C. 200 μL samples were removed from the external solution and were replaced with fresh solution. Sample collection was done in different times Cumulatively (harvested at 15, 30, 45, 60 min and 2 h for acidic environment and harvested at 15, 30, 45, 60 min, 2, 6, 12 and 24 h for the basic environment). HPLC was used for analysis of samples, under curve area was calculated and column charts are plotted for all nanohydrogels. (Fig. 5).
Discussion
To compare the insulin loading and insulin releasing in different pH conditions, four types of nanohydrogels were synthesized in this study. In all of four cases, the amount of synthesized nanohydrogels was approximately equal and had not significant difference between them. Color of synthesized nanohydrogels in aqueous solution was milky and it was almost powdery, but the color of the synthesized nanohydrogels in 1,4-dioxan solution was yellow and they were almost transparent crystals.
PNIPAAm side chains in synthesized NIPAAm–MAA–HEMA copolymer generally have a hydrophilic/hydrophobic balance. At room temperature the good solubility of PNIPAAm is produced by hydrogen bonding interactions between hydrophilic amide groups in the polymers and water molecules. Hydrophilic monomers, MAA and HEMA, increase the hydrophility of the gel [17].
For every four nanoparticles, insulin was loaded and then HPLC was done to choose the nanoparticle that have best loading and protects loaded drug in acidic pH and depletion drug in the intestinal pH. SEM and TEM microscopy was done for selected nanoparticle. Size of nanohydrogels that measured by SEM microscopy is in nano range (less than 100 nm) [14, 22].
In our study, the insulin loading extent on polymers that confirmed by HPLC and FT-IR is 60 % and polymer do not show unwanted side effects on human and its safety is demonstrated, pNIPAAm polymers have been used in eye drop supplies, where no in vitro cytotoxicity was found [23, 24], and as a new embolic material in neurosurgery [25, 26], where no acute toxicity in mice was noted, but in the Reis and colleagues study of the alginate macroparticle that produced with the emulsification method as a carrier for drugs, by using the anc polasion method to uptake the insulin in the polymer, the insulin loading extent was 80 %, in vitro studies showed that the alginate macroparticle inhibited of insulin releases in stomach but it can keep second structure of insulin, also average sizes of particles was less than the size of the carriers required to attract insulin orally and can be used as appropriate for insulin delivery [13]. Used nanoparticles protect the insulin in front of the stomach acid but have little biological safety and shows side effects.
Our in vitro study showed that nanohydrogel-bonded insulin has lowest release in the acidic environment similar to the stomach, Our result was consistent with the findings of Peppas and Ramkisson-Ganorkar that studied from polymers of acrylic acid-N-isopropyl acrylamide and butyl metacrylic acid was used in molar ratio of 10:5:85, the results showed that insulin in the acidic environment of the stomach had minimal liberalization while in pH 7.4 of intestinal insulin release done well [11, 12],and also our result was consistent with the Lowman and colleagues that loaded insulin on metacrylic-ethylene glycol microspheres and administered it orally to normal and diabetic rats. They observed that in the acidic environment of the stomach due to formation of intramolecular polymeric complexes, insulin trapped within the polymer network and remained safe from the photolytic analysis, and the complex was decomposed in neutral and alkaline intestinal environment and the drug released [10].
The results of HPLC show that in nanoparticle that synthesis in the 1,4-dioxan solvent without cross-linker loading is the most and the liberation in pH 6.8 after 2 h has been more than other three nanoparticles, and this is probably due to the existence of larger cavities in nanoparticles for insertion of insulin and the insulin releasing in acidic pH in the lowest value is because nanoparticles in the pH 1.2 has been dense but it is open in pH 6.8, probably this depletion increases over time with the effect of pH 6.8 after 2 h and insulin released and subsequently it reached to the maximum amount. So it was the best option for our purpose. Polymer synthesis in hydrogel form in water soluble is not complete and the drug has not been loaded well to release to be done. In the case of the cross-linked nanoparticles the drug is confined within it and its liberation at different pH is similar. The n value is number of repetitions for each sample in this case we did 3 times (n = 3).
Conclusion
Our goal was merging of two methods together (nano encapsulation and pH sensitive property of smart hydrogels), to obtain a nanohydrogel to protect insulin from acidic condition in stomach and release it in intestine pH for oral delivery of insulin. From four synthesized nanoparticles, copolymer that was synthesized in 1,4 dioxan solvent without cross linker was capable for the most loaded insulin and had the lowest insulin release in the environment similar to stomach, but in environment similar to the gut had the largest release of insulin. This release was after 2 h. So by improving conditions, and altering the monomers ratio, and selecting different solvents and cross-linkers, we can achieve better results to have oral insulin delivery.
References
Morçöl T, Nagappan P, Nerenbaum L, Mitchell A (2004) Calcium phosphate–PEG–insulin-casein (CAPIC) particles as oral delivery systems for insulin. Int J Pharm 277(1–2):91–97
Sweetman S (ed) (2007) Martindale, the complete drug reference. Pharmaceutical Press, London Electronic version
King H, Aubert RE, Herman WH (1998) Global burden of diabets 1995–2005: prevalence, numerical, estimates and projection. Diabets Care 21:1414–1431
Akhter DT, Nijhu RS (2012) Diabetes mellitus: a journey of insulin. Int Curr Pharm J 1(2):32–42
Morishita M, Goto T, Nakamura K, Lowman AM, Takayama K, Peppas NA (2006) Novel oral insulin delivery systems based on complexation polymer hydrogels: single and multiple administration studies in type 1 and 2 diabetic rats. J Control Release 110:587–594
Kinesh VP, Neelam DP, Punit BP, Bhavesh SB, Pragna KS (2010) Novel approaches for oral delivery of insulin and current status of oral insulin products. Int J Pharm Sci Nanotechnol 3(3):1057–1064
Davaran S, Jafari B, Rafie F (2011) Pharmaceutical sciences, oral insulin: a review on its current condition and future aspects. Pharm Sci 17(3):151–162
Carino GP, Mathiowitz E (1999) Oral insulin delivery. J Adv Drug Deliv Rev 35:249–257
Pan YJ, Chen YY, Wang DR, Wei Ch, Guo J, Lu DR, Chu CC, Wang CC (2012) Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 33(27):6570–6579
Lowman AM (1999) Oral delivery of insulin using pH sensitive complexation gels. J Pharm Sci 88(9):933–937
Peppas NA (2004) Devices based on intelligent biopolymers for oral protein delivery. Int J Pharm 277:11–17
Ramkisson-Ganorkar C (1999) Modulating insulin release profile from pH/thermosensitive polymeric beads through polymer molecular weight. J Control Release 59(3):287–298
Reis CP, Ribeiro AJ, Neufeld RJ, Veiga F (2007) Alginate microparticles as novel carrier for oral insulin delivery. J Biotechnol Bioeng 96(5):97
Gupta S, Kuckling D, Kretschmer K, Choudhary V, Rgen Adler H (2006) Synthesis and characterization of stimuli-sensitive micro- and nanohydrogels based on photocrosslinkable poly(dimethylaminoethyl methacrylate). J Polym Sci Part A Polym Chem. doi:10.1002/pola.21846
Akiyosh K, Kobayashi S, Schichibe S, Mix D, Baudys M, Kim SW, Sunamoto J (1998) Self-assembled hydrogel nanoparticle of cholesterol-bearing pullulan as a carrier of protein drugs: complexation and stabilization of insulin. J. Control Release 54(3):313–320
Na K, Bae YH (2004) Self-assembled hydrogel nanoparticles responsive to tumor extracellular pH from pullulan derivative/sulfonamide conjugate: characterization, aggregation and adriamycin release in vitro. Pharm Res 19(5):681–688
Argentiere S, Blasi L, Ciccarella G, Barbarella G, Cingolani R, Gigli G (2010) Nanogels of poly (acrylic acid): uptake and release behavior with fluorescent oligothiophene-labeled bovine serum albumin. J Appl Polym Sci 116(5):2808–2815
Liu KH, Liu TY, Chen SY, Liu DM (2008) Drug release behavior of chitosan–montmorillonite nanocomposite hydrogels following electrostimulation. Acta Biomater 4:1038–1045
Sahiner N, Godbey WT, McPherson GL, John VT (2006) Microgel, nanogel and hydrogel–hydrogel semi-IPN composites for biomedical applications: synthesis and characterization. Colloid Polym Sci 284:1121–1129. doi:10.1007/s00396-006-1489-4
Üzüm Ö, Karadağ E (2007) Swelling characterization of poly (acrylamide-co-N-vinylimidazole) hydrogels crosslinked by TMPTA and semi-IPN’s with PEG. J Polym Res 14:483–488
Jafari B, Rafie F, Davaran S (2011) Preparation and characterization of a novel smart polymeric hydrogel for drug delivery of insulin. Bio Impacts 1(2):135–143
Geever LM, Devine DM, Nugent MJD, Kennedy JE, Lyons JG, Higginbotham CL (2006) The synthesis, characterisation, phase behaviour and swelling of temperature sensitive physically crosslinked poly (1-vinyl-2-pyrrolidinone)/poly (N-isopropylacrylamide) hydrogels. Eur Polym J 42(1):69–80
Hsiue GH, Hsu SH, Yang CC, Lee SH, Yang IK (2002) Preparation of controlled release ophthalmic drops, for glaucoma therapy using thermosensitive poly-N-isopropylacrylamide. Biomaterials 23:457–462
Hsiue GH, Chang RW, Wang CH, Lee SH (2003) Development of in situ thermosensitive drug vehicles for glaucoma therapy. Biomaterials 24:2423–2430
Matsumaru Y, Hyodo A, Nose T, Ito S, Hirano T, Ohashi S (1996) Application of thermosensitive polymers as a new embolic material for intravascular neurosurgery. J Biomater Sci Polym Ed 7:795–804
Lee BH, Leon C, McLemore R, Macias JV, Vernon BL (2011) Synthesis and characterization of thermo-sensitive radio-opaque poly (N isopropylacrylamide-co-PEG-2-iodobenzoate). J Biomater Sci Polym Ed 22:2357–2367
Acknowledgments
The authors thank Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University for all supports provided. This work was funded by 2013 Drug Applied Research Center Grant.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karnoosh-Yamchi, J., Mobasseri, M., Akbarzadeh, A. et al. Preparation of pH sensitive insulin-loaded nano hydrogels and evaluation of insulin releasing in different pH conditions. Mol Biol Rep 41, 6705–6712 (2014). https://doi.org/10.1007/s11033-014-3553-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-014-3553-3