Abstract
Stable silver nanoparticles have been synthesized using gum karaya acting as both reducing and stabilizing agent without using any synthetic reagent. The reaction is performed using water, which is an environmentally safe solvent. This reaction was carried out in an autoclave at a pressure of 15 psi and 120 °C temperature by varying the time. The influence of different parameters such as time, change of concentration of silver nitrate and concentration of gum karaya on the formation of silver nanoparticles has been studied. The synthesized silver nanoparticles are characterized by UV–Vis spectroscopy, FTIR, XRD and TEM. UV–Vis analysis of the sample confirmed the formation of silver nanoparticles exhibiting a sharp peak at a wavelength of 420 nm. TEM micrographs showed the formation of well-dispersed silver nanoparticles of size 2–4 nm. The antimicrobial activity of silver nanoparticles stabilized in gum karaya is tested against Escherichia coli, Micrococcus luteus and is found to be possessing inhibiting property. The silver nanoparticles stabilized in gum karaya exhibited very good catalytic activity and the kinetics of the reaction was found to be pseudo first order with respect to the 4-nitrophenol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nobel metal nanoparticles have attracted significant attention because of their unusual size-dependent optical and electronic properties. Among these metals, silver nanoparticles show potential applications in various fields such as the biomedicinal, catalysis, optics and electronics [1–4]. Silver has been known for antibacterial activity since the ancient Greece times. There has been a great deal of interest with the discovery that silver nanoparticles are significantly more effective antimicrobial agents in terms of the minimum effective concentration than their Ag+ counterparts [5]. Silver nanoparticles can be used in medicine to reduce infections in burn treatment, to prevent bacteria colonization on dental materials, stainless steel materials, to eliminate microorganisms on textile fabrics, and for water treatment [6–8]. Nanosilver belongs to highly commercialized nanomaterials [9]. Due to the strong antimicrobial activity, silver nanoparticles are also being used in consumer products, household water filters, clothing, cosmetics, antibacterial sprays and detergents, cutting boards, socks, shoes, cell phones, laptop key boards and children’s toys. These are among the retail products that exploit the antimicrobial properties of silver nanomaterials [10, 11].
A number of approaches are available for the preparation of silver nanoparticles. Silver nanoparticles are usually obtained by reducing silver nitrate by sodium borohydride [12, 13], γ radiation [14], UV irradiation [15], photochemical reduction [16], microwave irradiation [17], electrochemical reduction [18] etc. Polymeric materials are usually used as stabilizers to prevent nanoparticles agglomeration, such as polyethylene glycol [19], polyvinyl pyrrolidone [20], polyvinyl alcohol [21], and polyacrylonitrile [22]. Most of these methods are extremely expensive and also involve the use of toxic, hazardous chemicals, which may pose potential environmental and biological risks. To comply with the 12 principles of green chemistry, many researchers tried to avoid the use of hazardous chemicals and solvents [23–25]. Preparation route should address these principles by using environmentally benign solvents and nontoxic chemicals [23]. Earlier reports have dealt with natural polymers like chitosan, heparin [26], and soluble starch [27] as reducing agents and stabilizing agents for preparation of silver nanoparticles.
The manufacturing of many analgesic and antipyretic drugs, such as paracetamol, phenacetin, and so on, needs 4-aminophenol (4-AP) as a potential intermediate. It is also widely used as a photographic developer, corrosion inhibitor, anticorrosion-lubricant, and hair-dyeing agent etc. [28, 29]. Thus, 4-AP, being a common precursor material, a newer and cheaper method for its preparation by catalytic hydrogenation of 4-nitrophenol (4-NP) is always in demand. In particular, 4-NP has been listed as priority pollutant by the US Environmental Protection Agency (EPA) because of its higher solubility and stability in water. 4-NP stays a longer time in water and surface soil without degradation and gets accumulated in deep soil indefinitely [30]. The reduction of nitro compounds to amino compounds is industrially important and catalytic conversion has been observed to be very efficient. NaBH4 is not very effective in this aspect unless some catalyst is provided to remove the kinetic barrier of the reaction to support electron relay for the reduction. In recent years, several research groups have investigated the catalytic reduction of aromatic nitro compounds using a number of noble metal nanoparticles.
Karaya tree is one of the commercial and industrial important trees of India. Gum karaya, also called Sterculia gum, is the dried exudation of the Sterculia urens tree, which belong to the family Sterculiaceae. Gum karaya is a negative colloid and a high-molecular-weight complex acidic polysaccharide. The primary structure is shown to be composed of d-glucuronic acid, d-galacturonic acid, d-galactose, l-rhamnose and acetyl groups in different proportions according to the quality, type, and origin of the polysaccharide [31]. It has been used in the treatment of diarrhea [32], irritable bowel syndrome [33], chronic colonic diseases [34] reducing cholesterol and improving glucose metabolism without adversely affecting most mineral balances [35]. In the present work, we report the preparation of silver nanoparticles using gum karaya as both reducing and stabilizing agent and water as solvent. This reaction was carried out in autoclave at 15 psi pressure and 120 °C temperature. During autoclaving under the influence of temperature and pressure, this gum expands and becomes more accessible for the silver ions to interact with the available functional groups on the gum, as observed earlier for starch [36]. Antimicrobial activity of silver nanoparticles stabilized in gum karaya was tested against Escherichia coli (E. coli), Micrococcus luteus (M. luteus) bacteria for finding out the potentiality of the generated nanoparticles for their biomedical applications. We report the catalytic activity of green synthesized silver nanoparticles stabilized in gum karaya towards 4-NP reduction. The silver nanoparticles stabilized in gum karaya catalyst exhibited very good catalytic activity and the kinetics of the reaction was found to be pseudo first order with respect to 4-NP.
Materials and Methods
Materials
Gum karaya grade 1, was purchased from Girijan Co-Operative Corporation, Andhra Pradesh. 4-NP, sodium chloride, silver nitrate and sodium borohydride were obtained from S D Fine-chem Limited, Mumbai, India. The test strains, E. coli MTCC 1303 and M. luteus MTCC 2987 were purchased from IMTECH, Chandigarh, India. Yeast extract, tryptophan and bacterial-grade agar–agar were purchased from Himedia Laboratories, Mumbai, India.
Preparation of Silver Nanoparticles Stabilized in Gum Karaya
Uniform silver nanoparticles can be obtained through the reduction of silver nitrate using gum karaya by autoclaving (Osworld, Model no. 37, India) at 15 psi pressure and 120 °C temperature. In this synthesis process, 5 ml of aqueous solution containing silver nitrate (0.5 g of AgNO3), 5 ml of aqueous solution containing gum karaya (0.5 g of karaya gum), are added in a boiling tube. The boiling tube is sealed with aluminum foil and kept in autoclave. Silver nanoparticles are prepared by varying the time of autoclaving at 15 psi pressure and 120 °C temperature. Uniform silver nanoparticles are obtained by autoclaving for 50 min at 15 psi pressure and 120 °C temperature varying the concentrations of silver nitrate and gum karaya. The colorless reaction mixture was converted to the characteristic clear yellow color after autoclaving. The appearance of color was indicated the formation of silver nanoparticles.
Characterizations
The silver nanoparticles, stabilized in gum karaya solution, were analyzed by UV–Vis absorbance spectroscopy. UV–Vis spectroscopic measurements were made at room temperature using a Shimadzu dual beam UV–Vis spectrophotometer, Japan. Fourier transform infrared (FTIR) spectra of silver nanoparticles stabilized in gum karaya and gum karaya alone were recorded in KBr pellets using an FTIR spectrophotometer (Bruker Optics, Germany, Model no. Tensor 27). The scan was performed in the range 400–4,000 cm−1. X-ray diffraction (XRD) measurement of silver nanoparticles stabilized in gum karaya was carried out on X’pert Pro X-ray diffractometer (Panalytical B.V., The Netherlands) operating at 40 kV and a current of 30 mA at a scan rate of 0.388 min−1. The size distribution and crystallinity of the silver nanoparticles stabilized in gum karaya were obtained by transmission electron microscopy (TEM) measurement, casting nanoparticle dispersion on carbon-coated copper grids and allowing drying at room temperature. Measurements were done on Tecnai G2 FEI F12 operated at an accelerating voltage of 200 kV.
Antibacterial Property of Samples
Luria–Bertani (LB) agar medium was prepared by adding yeast extract (0.5 g), tryptophan (1 g), sodium chloride (1 g) and bacterial grade agar (2.5 g) in distilled water (100 ml). Then the agar medium was sterilized by autoclaving at a pressure of 15 psi and 120 °C temperature for 30 min. This medium was transferred into sterilized Petri dishes in a laminar air flow. After solidification of media, overnight culture of E. coli (100 μl) and M. luteus (100 μl) was spread separately on the solid surface of the media. Sterile discs were kept on these inoculated plates with the help of sterile forceps. Sample (10 μl) solutions were placed on these discs and were incubated at 37 °C for 24 h in a bacterial incubator. The inhibition zone that appeared around the disc was measured and recorded as the antibacterial effect of gum karaya and the various concentrations of nanosilver stabilized in gum karaya.
Catalytic Activity
The catalytic reduction of 4-NP was carried out in a well stoppered quartz cuvette in the presence of aqueous borohydride and silver nanoparticles stabilized in gum karaya. To a 1 ml solution of 0.2 M NaBH4, 1.9 ml of 0.2 mM 4-NP solution was added. At this stage, the change of color from light yellow to yellow–green was observed. After that 10 μl of 0.0006 M silver nanoparticles stabilized in gum karaya solution was added. The reaction was spectrophotometrically monitored in the wavelength range of 200–600 nm at different time intervals. The rate constant of the redox reaction was dependent on the change in absorbance at 400 nm as a function of time.
Results and Discussions
UV Analysis
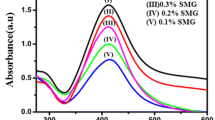
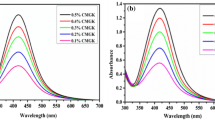
UV–Vis spectroscopy is one of the most widely used techniques for structural characterization of silver nanoparticles. The electronic transitions involving the Ag+ ion give rise to absorption bands located between 200 and 230 nm, whereas the electronic transitions of metallic Ag (0) appear in the 250–330 nm spectral range [37]. The obtained silver nanoparticles displayed the characteristic surface plasmon resonance (SPR) band for silver, centered from 418 to 428 nm [14]. There are no peaks located around 335 and 560 nm, indicating the absence of nanoparticle aggregation. The UV–Vis spectra of silver nanoparticles prepared by varying the time of autoclaving at 15 psi pressure and 120 °C temperature shown in Fig. 1. The formation of silver nanoparticles increases with increasing autoclaving time. Various concentrations of silver nitrate were reduced with gum karaya for 50 min of autoclaving at 15 psi pressure and 120 °C temperature. The typical UV–Vis absorption spectra of the resulting solutions are shown in Fig. 2. It is evident from Fig. 2 that, as the concentration of silver nitrate increases, there is a progressive enhancement in the intensity of the SPR band. This indicates the increased formation of silver nanoparticles in gum karaya solution. Various concentrations of gum karaya were oxidized with silver nitrate by autoclaving at 15 psi pressure and 120 °C temperature, and the typical UV–Vis absorption spectra of the resulting solutions are shown in Fig. 3.
FTIR Analysis
The evidence for the interaction of hydroxyl groups of gum karaya in the stabilization of the silver nanoparticles was obtained from the FTIR spectra. The FTIR spectra of silver nanoparticles stabilized in gum karaya is shown in Fig. 4. FTIR spectrum of gum karaya absorption bands at 3447 and 2943 cm−1, represent the –OH and –CH2, –CH3 aliphatic groups. The band at 1620 cm−1 is due to asymmetric and symmetric stretching of COO− group. The band at 1427 cm−1 is due to –OH group bending of acid group. The variations in the shape and peak positions of –OH, –COO− groups at 3447 and 1620 cm−1 respectively, are observed because of the contribution of gum karaya towards the reduction and stabilization process.
XRD Analysis
The XRD patterns of the prepared sample indicate the formation of the silver nanoparticles. Figure 5 clearly shows well defined diffraction peaks at 37.7623°, 45.8697°, 64.1992° and 76.9467°, corresponding to the (111), (200), (220) and (331) crystallographic planes of face centered cubic (FCC) silver crystals, respectively. The size of the nanoparticles are calculated over the (111) reflection using the classical Scherrer formula with a geometric factor of 0.97 is applied. The crystallite diameters of sample are found to be about 4 nm. This result is in agreement with TEM image.
TEM Analysis
A typical transmission electron micrograph of green synthesized silver nanoparticles stabilized in gum karaya using 0.4 % silver nitrate and 0.5 % gum karaya was shown in Fig. 6a. The TEM image shows the silver nanoparticles are spherical and are well distributed in the gum polymer matrix. To obtain size distributions of silver nanoparticles, approximately 105 particles were counted and then converted into histograms. Figure 6b presents a histogram of the particle size distribution of silver nanoparticles. Histogram shows average particle size of silver nanoparticles 4 ± 2 nm. The selected area electron diffraction pattern of this sample (Fig. 6c) exhibits polycrystalline diffraction rings, which can be indexed to cubic-phase metal silver, indicating that these nanoparticles are crystalline metallic silver.
Microbial Activity
The mechanism of the bactericidal effect of silver and silver nanoparticles is not well understood. The silver nanoparticles which show interaction with the bacteria were all between 1 and 10 nm. The reason for this size dependency is probably a combination of the particles ability to react with and penetrate the cell membrane and the higher surface to volume ratio of smaller particles [38]. In present study antibacterial studies of silver nanoparticles stabilized in gum karaya and gum karaya alone were tested by paper disc method using both E. coli and M. luteus and shown in Fig. 7. The details of the antimicrobial experiment were provided in the experimental section. Sterile discs were kept on these inoculated plates with the help of sterile forceps. Sample (10 μl) solutions of different concentrations of silver nanoparticles stabilized in gum karaya, gum karaya alone and ampicillin were placed on these discs and were incubated at 37 °C for 24 h in a bacterial incubator. Ampicillin was used as positive control. The inhibition zone appeared around the disc was measured and recorded as the antibacterial effect of nanosilver stabilized in gum karaya and gum karaya. The diameter of the zone of inhibition for the 5 mg ml−1 Ag+ per gum karaya was 12 mm, where as that of 1 mg ml−1Ag+ per gum karaya it was 8 mm for both E. coli and M. luteus. The inhibition zone decreases with decrease in concentration of silver nanoparticles. The pure gum karaya sample showed no inhibition ability.
Antibacterial test results of E. coli and M. luteus after 24 h of incubation. 1 5 mg ml−1 AgNO3 per gum karaya, 2 4 mg ml−1 AgNO3 per gum karaya, 3 3 mg ml−1 AgNO3 per gum karaya, 4 2 mg ml−1 AgNO3 per gum karaya, 5 1 mg ml−1 AgNO3 per gum karaya, 6 pure gum karaya, 7 Ampicillin 1 mg ml−1 used as positive control
Catalytic Activity
Catalytic activity of silver nanoparticles was demonstrated by the study of reduction of 4-NP to 4-AP by sodium borohydride. The details of the catalysis experiment were provided in the experimental section. 4-NP shows its characteristic absorption maximum at 317 nm, but a red-shift was observed at 400 nm immediately after addition of sodium borohydride as shown in Fig. 8. This was due to the formation of 4-nitrophenolate ion. In the absence of silver nanoparticles catalyst, the absorption peak at 400 nm remained unaltered for a long time, indicating the inability of the strong reducing agent, NaBH4 itself, to reduce 4-nitrophenolate ion. However, the addition of a small amount of silver nanoparticles stabilized in gum karaya solution causes fading and ultimate bleaching of the yellow color of the reaction mixture in quick succession. Time-dependent UV–Vis absorption spectrum of this catalytic reaction mixture shows the disappearance of the 400 nm peak and the gradual development of a new peak at 300 nm as given in Fig. 8. This indicates that the reduction of 4-NP by NaBH4 can be catalyzed by silver nanomaterials. The concentration of sodium borohydride greatly exceeds that of 4-NP and the catalyst nanoparticles. The excess of sodium borohydride used increases the pH of the reacting system, thereby, retarding the degradation of the borohydride ions, and the liberated hydrogen is purged out, thereby, checking the aerial oxidation of the reduced product of 4-NP. It is well known that the metal nanoparticles catalyze this reaction by facilitating electron relay from the donor BH4 − to acceptor 4-nitrophenolate ion to overcome the kinetic barrier. The catalytic reduction proceeds on the surface of the metal nanoparticles. As soon as the electron donor (BH4 −) and electron acceptor (4-nitrophenolate ion) are adsorbed on the surface of the silver nanoparticles, catalytic reaction starts by the transfer of electron from BH4 − to 4-nitrophenolate ion. Thus, silver nanoparticles help in facilitating the reduction of 4-NP by lowering the activation energy of the reaction and play the role of catalyst. The absorbance at time t = 0 (A0) and at t (At) are proportional to the initial concentration C0 and concentration at time t, i.e., Ct, of 4-NP, respectively. The conversion percentage (α) of 4-NP to 4-AP was calculated by the formula:
The conversion percentage of 4-NP to 4-AP was shown in Fig. 9. It has been found that the reduction of 4-NP to 4-AP by sodium borohydride in the presence of silver nanoparticles as catalyst follows pseudo first order rate equation with respect to 4-NP because the concentration of sodium borohydride was too high as compared to 4-NP. So, concentration of sodium borohydride was considered constant throughout the reaction. UV–Vis spectra of successive reduction of 4-NP to 4-AP using silver nanoparticles stabilized in gum karaya with time interval of 10 min were shown in Fig. 10. The rate equation can be written as
Figure 11 shows a good linear correlation of ln(C0/Ct) versus time and the rate constant of the reaction is obtained as 0.03159 min−1 for silver nanoparticles stabilized in gum karaya.
Conclusion
A novel sustainable low cost approach was developed for the synthesis of silver nanoparticles using gum karaya. Stable silver nanoparticles were synthesized using gum karaya acting as both reducing and stabilizing agent without using any synthetic reagent. The reaction was carried out in water as an environmentally safe solvent. The silver nanoparticles stabilized in gum karaya had significant antibacterial action on E. coli and M. luteus bacteria. The pure gum karaya sample had no significant antibacterial action on E. coli and M. luteus bacteria. The silver nanoparticles stabilized in gum karaya exhibited very good catalytic activity and the kinetics of the reaction was found to be pseudo first order with respect to the 4-NP.
References
J. Tain, K. K. Wong, C. M. Ho, C. N. Lok, W. Y. Yu, C. M. Che, J. F. Chiu, and P. K. Tam (2007). Chem. Med. chem. 2, 129.
J. Liu, D. A. Sonshine, S. Shervani, and R. H. Hurt (2010). ACS Nano. 4, 6903.
D. Tain, G. Yong, Y. Dai, X. Yan, and S. Liu (2009). Catal. Lett. 130, 211.
Y. F. Chau and H. H. Yeh (2011). J. Nanopart. Res. 13, 637.
C. N. Lok, C. M. Ho, R. Chen, Q. Y. He, W. Y. Yu, H. Sun, P. K. H. Tam, J. F. Chiu, and C. M. Che (2006). J. Proteome. Res. 5, 916.
A. L. Panacek, L. Kivtek, R. Prucek, K. Milan, R. Vecerova, and N. Pizurova (2006). J. Phys. Chem. B. 110, 16248.
L. Bo, W. Yang, M. Chen, J. Gao, and Q. Xue (2009). Chem. Biodivers. 6, 111.
B. Tomsic, B. Simoncic, B. Orel, L. Cerne, P. Tavcer, M. Zorko, and A. Jerman (2008). Sol–Gel Sci. Technol. 47, 44–57.
X. Chen and H. J. Schluesener (2008). Toxicol. Lett. 176, 1.
R. Kaegi, B. Sinnet, S. Zuleeg, H. Hagendorfer, E. Mueller, R. Vonbank, M. Boller, and M. Burkhardt (2010). Environ. Pollut. 158, 2900.
C. M. Jones and E. Hoek (2010). J. Nanopart Res. 12, 1531.
K. S. Chou and C. Y. Ren (2000). Mater. Chem. Phys. 64, 241.
F. Douglas, R. Yanez, J. Ros, S. Marın, A. E. Muniz, S. Alegret, and A. Merkoci (2008). J. Nanopart. Res. 10, 97.
R. Yoksan and S. Chirachanchai (2009). Mater. Chem. Phys. 115, 296.
M. Darroudi, M. B. Ahmad, K. shameli, A. H. Abdullah, and N. A. Ibrahim (2009). Solid state sci 11, 1621.
K. Mallick, M. J. Witcomb, and M. S. Scurrel (2004). J. Mater. Sci. 39, 4459.
R. Bhat, S. Ganachari, R. Deshpande, G. Ravindra, and A. Venkataraman (2013). J. Clust. Sci. 24, 107.
M. starowicz, B. B. stypula, and J. Bana (2006). Electrochem. commun. 8, 227.
A. Shkilnyy, M. Souce, P. Dubois, F. Warmont, M. L. Saboungi, and I. Chourpa (2009). Analyst 134, 1868.
S. kheybari, N. Samadi, S. V. Hosseini, A. Fazeli, and M. R. Fazeli (2010). DARU 18, 168.
E. Filippo, D. Manno, and A. Serra (2009). Sens. Actuators. B. 138, 625.
C. Zhang, Q. Yang, N. Zhan, L. Sun, H. Wang, Y. Song, and Y. Li (2010). Coll. Surf. A: Physicochem. Eng. Aspects. 362, 58.
P. T. Anstas and J. C. Warner Green Chemistry: Theory and Practice (Oxford University Press, New York, 1998).
R. A. Cross and B. Kalra (2002). Science 297, 803.
P. Raveendran, J. Fu, and S. L. Wallen (2003). J. Am. Chem. Soc. 125, 13940.
H. Huang and X. Yang (2004). Carbohydr. Res. 339, 2627.
N. Vigneshwaran, R. P. Nachane, R. H. Balasubramanya, and P. V. Varadarajan (2006). Carbohydr. Res. 341, 2012.
J. F. Corbett (1999). Dyes and Pigments 41, 127.
C. V. Rode, M. J. Vaidya, and R. V. Chaudhari (1999). Org. Process. Res. Dev. 3, 465.
E. Pocurull, R. M. Marce, and F. Borrull (1996). J. Chromatogr. A. 738, 1.
D. L. Cerfa, F. Irineib, and G. Mullera (1990). Carbohydr. Polym. 13, 375.
E. Huttel (1983). Med. Welt. 34, 1383.
J. P. Capron, P. Zeitoun, and D. Julien (1981). Gastroenterol. Clin. Biol. 5, 67.
J. Guerre and M. Neuman (1979). Med. Chir. Dig. 8, 679.
K. M. Behall (1990). Adv. Exp. Med. Biol. 270, 7.
N. Vigneshwaran, R. P. Nachane, R. H. Balasubramanya, and P. V. Varadarajan (2006). Carbohydr. Res. 341, 2012.
J. Lu, J. J. Bravo-Suarez, A. Takahashi, M. Haruta, and S. T. Oyama (2005). J. Catal. 232, 85.
J. R. Morones, J. L. Elechiguerra, A. Camacho, K. Holt, J. B. Kouri, J. T. Ramırez, and M. J. Yacaman (2005). Nanotechnology 16, 2346.
Acknowledgments
The authors wish to thank the Coordinator, DBT-OU-ISLARE, Instrumentation Laboratory (Funded by UGC), Osmania University and Center for Nanotechnology, University of Hyderabad for the use of their facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venkatesham, M., Ayodhya, D., Madhusudhan, A. et al. A Novel Green Synthesis of Silver Nanoparticles Using Gum Karaya: Characterization, Antimicrobial and Catalytic Activity Studies. J Clust Sci 25, 409–422 (2014). https://doi.org/10.1007/s10876-013-0620-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-013-0620-1