Abstract
Introduction
Autoantibodies to granulocyte-macrophage colony-stimulating factor (GM-CSF) can cause acquired pulmonary alveolar proteinosis (PAP). Cases of acquired PAP susceptible to typical respiratory pathogens and opportunistic infections have been reported. Anti-GM-CSF autoantibodies have been reported in a few patients with cryptococcal meningitis. This study evaluated the presence of neutralizing anti-GM-CSF autoantibodies in patients without known congenital or acquired immunodeficiency with severe pulmonary or extrapulmonary cryptococcal infection but without PAP.
Methods
We took a clinical history and performed an immunologic evaluation and screening of anti-cytokine autoantibodies in patients with cryptococcal meningitis. The impact of autoantibodies to GM-CSF on immune function was assessed by intracellular staining of GM-CSF-induced STAT5 phosphorylation and MIP-1α production in normal peripheral blood mononuclear cells incubated with plasma from patients or normal control subjects.
Results
Neutralizing anti-GM-CSF autoantibodies were identified in four patients with disseminated cryptococcosis, none of whom exhibited PAP. Plasma from patients blocked GM-CSF signaling and inhibited STAT5 phosphorylation and production of MIP-1α. One patient died of disseminated cryptococcosis involving the central nervous system, which was associated with defective GM-CSF activity.
Conclusions
Anti-GM-CSF autoantibodies increase susceptibility to cryptococcal infection in adults without PAP. Cryptococcal central nervous system infection associated with anti-GM-CSF autoantibodies could result in neurological sequelae or be life-threatening. Therefore, timely detection of neutralizing anti-GM-CSF autoantibodies and development of an effective therapy are necessary to prevent deterioration of cryptococcal infection in these patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-cytokine autoantibodies are important in the pathogenesis of various diseases [1]. Such autoantibodies include anti-interferon (IFN)-γ autoantibodies in disseminated nontuberculous mycobacterial infection (NTM) [2–5]; anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) autoantibodies in pulmonary alveolar proteinosis (PAP), which is a chronic lung disease characterized by abnormalities of surfactant metabolism [6]; anti-interleukin-17 autoantibodies in chronic mucocutaneous candidiasis [7, 8]; and anti-interleukin-6 autoantibodies in Staphylococcus infection [9]. However, the impact of these autoantibodies in infectious diseases and their etiology are unclear.
GM-CSF is essential for the terminal differentiation and function of alveolar macrophages, which are responsible for the catabolism of surfactant lipids and proteins in the lung [10]. Dysfunction of GM-CSF, due to anti-GM-CSF autoantibodies or a genetic defect in the GM-CSF/GM-CSF receptor, can cause defects of alveolar macrophage function, which lead to deposition of lipoproteinaceous material in the alveoli and respiratory insufficiency [11]. Moreover, clearance of GM-CSF by autoantibodies impairs the antimicrobial activity of neutrophils, which might increase susceptibility to infection [12]. Several reports of the susceptibility of patients with acquired PAP to typical respiratory pathogens and opportunistic infections including Nocardia [13], nontuberculous mycobacteria [14], Histoplasma [15], and Cryptococcus [16] are described. However, the association between infection susceptibility due to PAP and anti-GM-CSF autoantibodies is unclear.
Cryptococcosis is a fungal disease caused by Cryptococcus neoformans and Cryptococcus gattii and occurs most frequently in immunocompromised individuals. The organism enters the body through inhalation and establishes a pulmonary infection; it can then disseminate to the meninges and brain, causing meningitis or meningoencephalitis. Disseminated cryptococcal infection is diagnosed by the recovery of Cryptococcus from sterile body fluids or tissues other than the lung. Cryptococcal meningitis has also been described in patients without identified immune defects [17–22]. Rosen et al. [16, 23] recently detected anti-GM-CSF autoantibodies in human immunodeficiency virus (HIV)-uninfected immunocompetent patients with cryptococcal meningitis or disseminated nocardiosis and postulated that anti-GM-CSF autoantibodies may predispose patients to cryptococcosis or nocardiosis with or without PAP.
This study evaluated the presence of neutralizing anti-GM-CSF autoantibodies in 11 patients without a known congenital or acquired immunodeficiency who had severe pulmonary or extrapulmonary cryptococcal infection but did not have PAP, together with control subjects.
Methods
Subjects
Eleven patients with cryptococcal infection were screened for anti-GM-CSF autoantibodies. The diagnosis of cryptococcosis was based on culture of blood, cerebrospinal fluid, or tissue. Laboratory tests to differentiate C. neoformans and C. gattii were not performed. This study was performed with the approval of the Ethics Committees of Chang Gung University and China Medical University. Plasma was obtained from heparinized venous whole blood by centrifugation and stored in aliquots at −80 °C. All subjects provided written informed consent under the Institutional Review Board-approved protocols DMR99-IRB-075, 103-7395B, and 104-1357A3.
Identification of Anti-GM-CSF Autoantibodies and Analysis of IgG Subclass in Plasma
A clear polystyrene 96-well, flat-bottomed plates (Nunc) was coated with 100 μl of human GM-CSF recombinant protein (2 μg/ml) in bicarbonate buffer (pH 9.6) per well and incubated at 4 °C overnight. The plate was washed five times with phosphate-buffered saline (PBS)-Tween 0.05% and then blocked with PBS - 5% human normal serum albumin (Aventis) for 2 hr. The plate was washed again, serially diluted plasma (10−2 to 10−4) from patients and healthy donors was added, and the plate was incubated at 4 °C overnight. The plate was thoroughly washed, and Fc-specific peroxidase-conjugated AffiniPure Goat anti-human IgG (Cappel) was added at a ratio of 1:2500. The plate was incubated for 90 min at 37 °C and then washed five times with PBS-Tween 0.05%. p-Nitrophenyl phosphate (pNPP) solution (100 μl/well) was added, and the plate was incubated for 20 min at 37 °C. It was determined absorbance at OD 405 nm with a VICTOR X3 Multilabel Plate Reader (PerkinElmer). Data from a representative experiment are shown. Similar results were obtained in independent experiments. Subsequently, for IgG subclass analysis, the plate was thoroughly washed and peroxidase-conjugated mouse anti-human IgG1, 2, 3, and 4 (Abcam) antibodies were added at a ratio of 1:2000 after the diluted plasma incubation (1:100). The plate was incubated for 60 min at room temperature and then washed five times with PBS-Tween 0.05%. 3,3′,5,5′-Tetramethylbenzidine (TMB) solution (100 μl/well) was then added, and the plate was incubated for 20 min at room temperature. The absorbance was detected at OD 450 nm with a VICTOR X3 Multilabel Plate Reader (PerkinElmer).
Intracellular Staining of Phospho-STAT5
To demonstrate that plasma containing anti-GM-CSF autoantibodies blocked GM-CSF signaling, 2 × 105 PBMCs from a healthy donor were cultured in Roswell Park Memprial Institute (RPMI)-1640 with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin containing control or patient plasma (10 or 50%) and then incubated with or without GM-CSF (10 ng/ml) and IL-3 (10 ng/ml) for 30 min at 37 °C. Monocytes were identified by anti-human CD14-FITC (BD Pharmingen) surface staining, followed by fixation and permeabilization for intracellular staining with an anti-pSTAT5 (Y694)-PE antibody (BD Phosflow™), as described previously [16]. Data were collected using FACSVerse (BD Bioscience) and analyzed using FlowJo software (TreeStar).
Titration of Neutralizing Anti-GM-CSF Activity in Plasma
U937 cells were cultured in RPMI-1640 with 10% FBS and 1% penicillin/streptomycin. Recombinant human GM-CSF was serially diluted from 5 × 103 to 10−5 ng/ml at 5% human plasma and pre-incubated at 37 °C for 15 min. Then U937 cells were added in a final concentration of 1 × 106 cells/ml. After the incubation with GM-CSF for 30 min at 37 °C, U937 cells were fixed and permeabilized for intracellular staining with an anti-pSTAT5 (Y694)-PE antibody (BD Phosflow™). Data were collected and analyzed using FACSVerse (BD Bioscience) and graphed with Prim6 (GraphPad).
Plasma Inhibition of GM-CSF-Induced MIP-1α
To evaluate inhibition of GM-CSF activity, PBMCs from a healthy donor were incubated with control or patient plasma (10%) overnight with or without GM-CSF (10 ng/ml). Supernatants were collected and MIP-1α levels were determined using a Human MIP-1α ELISA Kit (eBioscience) according to the manufacturer’s instructions.
Results
The clinical manifestations of the following four patients are summarized in Tables 1 and 2.
Case Reports
Patient 1
An otherwise healthy 37-year-old man presented in October 2013 after experiencing at least 1 month of headache, dizziness, nausea, vomiting, and blurred vision. The patient had experienced a 17-kg weight loss in 3 months and had progressive skin lesions on his face for 2 months (Fig. 1a). His initial laboratory studies revealed a leukocyte count of 17,230/μl with 90% neutrophils, hemoglobin of 10.7 g/dl (normocytic anemia), platelets of 364,000 per μl, and a C-reactive protein (CRP) level of 66 mg/l. His chest X-ray and CT showed an upper mediastinal mass with tracheal compression and a consolidation in the right middle lung (Fig. 1b, c). Brain magnetic resonance imaging (MRI) showed an approximately 1.4-cm mass on the right nasopharyngeal roof (Fig. 1d). Lumbar puncture revealed an opening pressure of >60 cm H2O, 40 WBCs/mm3, 20 RBCs/mm3, monocyte predominance (61%), glucose of <10 mg/dl, and protein of 144.7 mg/dl. India ink preparation of cerebrospinal fluid revealed characteristic round budding yeasts with distinct halos. Cerebrospinal fluid and serum cryptococcal antigens were positive with titers of >1:1024, and cerebrospinal fluid culture grew cryptococci. Skin biopsy revealed numerous encapsulated yeasts by hematoxylin and eosin staining (Fig. 1e) and Periodic acid-Schiff staining (Fig. 1f). Chest CT-guided aspiration and bronchoscopic biopsy also demonstrated numerous mucoid encapsulated yeasts consistent with Cryptococcus (Fig. 1g). The immune status of the patient was investigated. He was nonreactive to HIV, and his serum immunoglobulins, complement levels, and lymphocyte subsets were within the normal range except for a low CD4+ T cell percentage. Therefore, the diagnosis of disseminated cryptococcal infection with meningitis and pulmonary and cutaneous involvement in an otherwise immunocompetent individual was made. However, there was no clinical or microbiological improvement after 3 months of amphotericin B plus flucytosine treatment and therapeutic lumbar punctures. The patient died of sequelae of cryptococcal CNS infection.
Skin lesions, radiographic and cytopathic manifestations of patient 1. a Umbilicated papules of various sizes located mainly on the face. b Chest X-ray showing consolidation of the right middle lobe and mediastinal mass with airway compression (red dashed line). c Chest CT demonstrating a pulmonary cryptococcal lesion (red dashed line). d MRI of the brain showing the right nasopharyngeal roof mass (red dashed line). e Hematoxylin and eosin stain of the cutaneous lesion showing numerous encapsulated yeasts. f Periodic acid-Schiff stain of the cutaneous lesion showing numerous yeast cells. g Sample obtained by CT-guided biopsy revealed numerous encapsulated yeast forms
Patient 2
A 40-year-old man with a history of gout presented in May 2014 after experiencing 1 day of headache and weakness in the left limbs. His initial laboratory studies revealed a leukocyte count of 10,700/μl with 70% neutrophils, hemoglobin of 14.6 g/dl, platelets of 343,000/μl, and CRP level of 67.5 mg/l. His chest X-ray showed a consolidation in the left lower lung (Fig. 2a). Brain MRI showed a large mass on the right frontal lobe and a small nodule in the right caudate head (Fig. 2b, c). Under the impression of glioblastoma multiforme, the patient underwent craniotomy to remove the tumor. However, the pathologic examination revealed cryptococcal spores. Serum cryptococcal antigen was positive with a titer of 1:512. Therefore, the diagnosis was disseminated cryptococcal infection with pulmonary involvement and brain abscess. He received amphotericin B and flucytosine treatment for 3 months, followed until June 2016 by fluconazole. He had severe sequelae of hydrocephalus, seizure, and left hemiplegia.
Radiographic manifestations of patient 2. a Chest X-ray showing consolidation of the left lower lobe (red arrow). b Brain CT showing a large mass in the right frontal lobe with perifocal edema (white arrow). c MRI of brain showing an approximately 5-cm mass in the right frontal lobe with central necrosis (white arrow)
Patient 3
A 59-year-old woman with a history of hypertension presented to our ophthalmologic department due to a red left eye and blurred vision for 2 months and 2 weeks, respectively. Fundoscopic examination disclosed a choroidal tumor in the left eye. Brain computed tomography showed a pseudotumor in the left sclera layer. One week later, this patient underwent microincision vitreoretinal surgery, and the extracted tissue was submitted for pathological examination. The pathologist reported cryptococci within the tissue. A blood cryptococcal antigen test was positive with a titer of 1:64. Under the diagnosis of cryptococcosis of the left eye, intraocular injection of amphotericin B was administered, followed by oral voriconazole. The CD4+ T cell count was within the normal range, and a HIV test was negative. This patient received 14 weeks full-dose (200 mg twice daily) voriconazole therapy, followed by secondary prophylaxis (150 mg daily). No recurrence of disease was reported during the follow-up period.
Patient 4
A 37-year-old man with a history of chronic hepatitis C infection presented to our emergency room with cough and fever for >10 days. In the emergency room, physical examination disclosed bilateral rhonchi in the lungs, and the laboratory data showed an elevated CRP (43.8 mg/l) and leukocytosis (14,900/μl). Numerous consolidated lesions in both lung fields were noted on the chest X-ray. The patient was admitted with a presumptive diagnosis of community-acquired pneumonia. During the course of hospitalization, however, persistent fever, new-onset headache, and vomiting were reported by the patient. Lumbar puncture was performed to exclude meningitis. The lumbar puncture opening pressure was 130 mm H2O. The cerebrospinal fluid showed lymphocyte-predominant pleocytosis (534/μl, 86%) with an elevated protein (92 mg/dl) but reduced glucose (34 mg/dl) level. No pathogen was isolated from the cerebrospinal fluid, but cryptococcal antigen was detected in the cerebrospinal fluid and serum with a titer of 1:256. A sputum culture grew Cryptococcus species. The patient was HIV-negative and had a CD4+ T cell count within the normal range. This patient was treated with amphotericin B and flucytocine. The antifungal therapy resulted in improvement in the clinical symptoms and reduced the size of the pulmonary lesions. Two weeks after systemic antifungal therapy, the patient was discharged with maintenance fluconazole therapy.
Detection of Anti-GM-CSF Autoantibodies
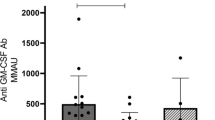
Plasma samples from controls (n = 5) and patients with severe cryptococcal infection (n = 11) at a serial dilution (10−2 to 10−4) were tested for anti-GM-CSF autoantibodies. Plasma samples from five PAP patients served as positive controls. Four patients were positive for anti-GM-CSF autoantibodies (Fig. 3a). The major IgG subclass of anti-GM-CSF autoantibodies in the four patients was IgG1 (Fig. 3b).
Characterization of anti-GM-CSF autoantibodies in patients with Cryptococcus infection. a Detection of anti-GM-CSF autoantibodies in controls (n = 5), patients with cryptococcal infection (patients 1–11), and patient with PAP (PAP 1–5). b Identification of IgG subclass in plasma containing anti-GM-CSF autoantibodies from patient with cryptococcal infection
Inhibition by Anti-GM-CSF Autoantibodies of Phospho-STAT5 Signaling
Non-neutralizing anti-GM-CSF autoantibodies and a low titer thereof have been described in healthy individuals and those with various diseases [24]. To determine the inhibitory capacity of plasma containing anti-GM-CSF autoantibodies, control PBMCs were incubated with or without GM-CSF or IL-3 in the presence of normal or patient plasma (10%) to detect the phosphorylation level of STAT5. The plasma of six patients containing anti-GM-CSF autoantibodies blocked STAT5 phosphorylation to a greater degree than plasma from healthy controls, whereas IL-3 which inducing STAT5 phosphorylation through sharing the β unit of the GM-CSF receptor served as control (Fig. 4a). Moreover, the inhibitory level of anti-GM-CSF autoantibodies in patient plasma was evaluated through stimulating U937 cells in patient or control plasma (5%) with increasing amounts of GM-CSF and calculated the phosphorylation level of STAT5 protein. The amount of GM-CSF required to achieve 50% of maximum STAT5 phosphorylation (half-maximal effective concentration = EC50) was determined from each dose-response curve (Fig. 4b). Plasma samples from health donors had the similar response to GM-CSF. However, patient plasma containing anti-GM-CSF autoantibodies required a 2–5 log higher concentration of GM-CSF to obtain 50% of maximum pSTAT5 level compared to control plasma.
The inhibitory capacity of anti-GM-CSF autoantibodies in patient plasma. a Inhibition by anti-GM-CSF autoantibodies of phospho-STAT5 signaling. Control PBMCs were incubated with control or patient plasma and stimulated with or without GM-CSF. Intracellular staining for phospho-STAT5 was analyzed by flow cytometry. Only patient 4 was under 50% plasma, the remaining subjects were under 10% plasma. b Representative dose-response curves for phospho-STAT5 level in U937 cells incubated with plasma from healthy donors, patient with cryptococcal infection or PAP and stimulated with increasing amounts of GM-CSF. The concentration required for 50% pSTAT5 level (half-maximal effective concentration = EC50) was shown and presented as nanogram per milliliter in the column, respectively. c Anti-GM-CSF autoantibodies in plasma inhibited GM-CSF-induced MIP-1α protein expression. Control PBMCs were incubated with control or patient plasma and stimulated with or without GM-CSF (10 ng/ml). The MIP-1α protein level was measured by ELISA and is presented as picogram per milliliter
Plasma Containing Anti-GM-CSF Autoantibodies Inhibits MIP-1α Production
The effect of plasma containing anti-GM-CSF autoantibodies on GM-CSF-induced MIP-1α production from PBMCs of controls was determined. Control PBMCs were incubated with or without GM-CSF in the presence of control or patient plasma (10%) (Fig. 4c). Plasma containing anti-GM-CSF autoantibodies decreased GM-CSF-induced MIP-1α production in comparison with control plasma (p < 0.05).
Discussion
We have herein reported an association between biologically inhibitory anti-GM-CSF autoantibodies and four cases of severe cryptococcal infection without concurrent PAP. Fourteen cases of cryptococcal meningitis [16, 24] and three cases of CNS nocardiosis [23] associated with anti-GM-CSF autoantibodies have been reported in previous studies; none had concurrent PAP, although it developed later in two patients. Therefore, our data support the hypothesis that anti-GM-CSF autoantibodies increase susceptibility to fungal infection in the absence of PAP. The presence of anti-GM-CSF autoantibodies should be considered in patients with CNS cryptococcal infection, and earlier diagnosis and antifungal treatment, including depletion of autoantibodies, might be critical to prevent mortality.
The reason for the diverse clinical manifestations of anti-GM-CSF autoantibodies is unclear. Non-neutralizing anti-GM-CSF autoantibodies and a low concentration thereof have been reported in patients with various diseases and in healthy individuals [24]. Immune complex formation in the presence of a high concentration of anti-GM-CSF autoantibodies was recently reported to block the function of GM-CSF in patients with PAP [25]. Consistent with this observation, anti-GM-CSF antibody therapy did not cause PAP in a clinical trial, suggesting that immune complex formation or neutralizing epitopes are linked to PAP.
The brain is protected by the blood–brain barrier, and only 0.1% of antibodies are found in the cerebrospinal fluid. The anti-GM-CSF autoantibody concentration in cerebrospinal fluid was not measured in the present study due to lack of availability. Anti-GM-CSF autoantibodies in cerebrospinal fluid were reported in a previous study; however, their concentration and inhibitory activity were not measured [16]. The antibody concentration in the cerebrospinal fluid and the presence of neutralizing epitopes or immune complex formation may explain the diverse clinical manifestations in patients with anti-GM-CSF autoantibodies. Investigation of the concentration or even the neutralizing titer of anti-GM-CSF autoantibodies in patients’ cerebrospinal fluid, cloning and characterization of anti-GM-CSF autoantibodies from patients with CNS infection are thus necessary to improve our understanding of the two clinical manifestations of anti-GM-CSF autoantibody disease.
Rosen et al. [16] reported that five of seven patients with anti-GM-CSF autoantibodies responded well to standard antifungal treatment. In contrast, two of our patients with anti-GM-CSF autoantibodies showed a poor response to treatment, resulting in mortality and severe sequelae, respectively. The reason for this difference is unclear. However, the increased severity might reflect lower residual GM-CSF activity in the presence of anti-GM-CSF autoantibodies, particularly in the CNS. Patients with complete loss-of-function mutations of IFNGR1 or IFNGR2 can suffer from lethal NTM infections. Despite suffering from NTM infections, patients with hypomorphic mutations have a better clinical outcome, likely due to residual IFN-γ activity. It seems that anti-IFN-γ autoantibody disease is a phenocopy of hypomorphic IFNG receptor deficiency because patients with anti-IFN-γ autoantibodies retain a degree of IFN-γ activity. This might also be the case for anti-GM-CSF autoantibodies and cryptococcal CNS infection: patients with the most severe Cryptococcus infection might have minimal GM-CSF activity in the CNS. In this situation, removing autoantibodies by plasmapheresis and B cell depletion therapy, which have been reported for treating anti-IFN-γ autoantibody disease [26], should be considered in addition to standard antifungal therapy.
C. neoformans and C. gattii are the two major species of the genus Cryptococcus considered to be pathogenic in humans, and both can cause pulmonary and CNS disease. C. gattii is mainly associated with immunocompetent patients, but can also infect immunosuppressed patients, while C. neoformans infection is reported predominantly in immunocompromised patients [27]. C. gattii, but not C. neoformans, has been linked to meningitis associated with anti-GM-CSF autoantibodies [24]. However, molecular techniques that can distinguish these two species are not widely available, including in Taiwan. Therefore, we were unable to specify the Cryptococcus isolates from our patients. Identification of C. gattii should be performed in all patients with CNS cryptococcal infection, and if C. gattii is identified, the presence of anti-GM-CSF autoantibodies should be evaluated due to their influence on clinical management.
Anti-IFN-γ autoantibodies are a common etiology of disseminated NTM infection, particularly in patients in Southeast Asia [5, 28–31]. Recently, we demonstrated that production of these autoantibodies was associated with specific genetic factors, the human leukocyte antigen (HLA) class II molecules DRB1*15:02/16:02 and DQB1*05:01/05:02. Moreover, these HLA molecules mediated the geographic/ethnic specificity of this disease [5, 30]. Due to the small number of patients, it is unclear whether or not anti-GM-CSF autoantibodies are linked to a particular geographic location or ethnicity. Also, whether production of anti-GM-CSF autoantibodies is associated with specific HLA molecules should be the subject of future research. Further studies and evaluation of a larger number of patients with fungal infections that have anti-GM-CSF autoantibodies are needed to address this issue.
We did not observe the sign of PAP in our patients; however, it is still possible that the patients might develop the PAP later. Reported by Rosen et al., two patients of cryptococcal meningitis with anti-GM-CSF autoantibodies later developed evidence of PAP [16]. Long-term follow-up in cryptococcal infection patients with anti-GM-CSF autoantibodies is necessary to dissect the relationship between PAP and cryptococcal meningitis. Further prospective work must be conducted to dissect the molecular mechanism of these two clinical manifestations induced by anti-GM-CSF autoantibodies.
Conclusions
Cryptococcus infection of the CNS in otherwise healthy patients suggests acquired immunodeficiency due to anti-GM-CSF autoantibodies, even in the absence of signs of PAP. The manifestations of CNS cryptococcal infections in these patients range from moderate to severe and life-threatening illness. The presence of anti-GM-CSF autoantibodies should be considered in patients with cryptococcal CNS infection because earlier diagnosis and treatment might prevent mortality.
References
Browne SK, Holland SM. Anticytokine autoantibodies in infectious diseases: pathogenesis and mechanisms. Lancet Infect Dis. 2010;10(12):875–85.
Doffinger R, Helbert MR, Barcenas-Morales G, Yang K, Dupuis S, Ceron-Gutierrez L, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38(1):e10–4.
Hoflich C, Sabat R, Rosseau S, Temmesfeld B, Slevogt H, Docke WD, et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood. 2004;103(2):673–5.
Kampmann B, Hemingway C, Stephens A, Davidson R, Goodsall A, Anderson S, et al. Acquired predisposition to mycobacterial disease due to autoantibodies to IFN-gamma. J Clin Invest. 2005;115(9):2480–8.
Chi CY, Chu CC, Liu JP, Lin CH, Ho MW, Lo WJ, et al. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent Varicella-Zoster virus infection. Blood. 2013;121(8):1357–66.
Kitamura T, Tanaka N, Watanabe J, Uchida, Kanegasaki S, Yamada Y, et al. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J Exp Med. 1999;190(6):875–80.
Kisand K, Wolff ASB, Podkrajsek KT, Tserel L, Link M, Kisand KV, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207(2):299–308.
Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207(2):291–7.
Puel A, Picard C, Lorrot M, Pons C, Chrabieh M, Lorenzo L, et al. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol. 2008;180(1):647–54.
Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15(4):557–67.
Trapnell BC, Carey BC, Uchida K, Suzuki T. Pulmonary alveolar proteinosis, a primary immunodeficiency of impaired GM-CSF stimulation of macrophages. Curr Opin Immunol. 2009;21(5):514–21.
Uchida K, Beck DC, Yamamoto T, Berclaz PY, Abe S, Staudt MK, et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med. 2007;356(6):567–79.
Fried J, Hinthorn D, Ralstin J, Gerjarusak P, Liu C. Cure of brain abscess caused by Nocardia asteroides resistant to multiple antibiotics. South Med J. 1988;81(3):412–3.
Witty LA, Tapson VF, Piantadosi CA. Isolation of mycobacteria in patients with pulmonary alveolar proteinosis. Medicine (Baltimore). 1994;73(2):103–9.
Hartung M, Salfelder K. Pulmonary alveolar proteinosis and histoplasmosis: report of three cases. Virchows Arch A Pathol Anat Histol. 1975;368(4):281–7.
Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013;190(8):3959–66.
Mitchell DH, Sorrell TC, Allworth AM, Heath CH, McGregor AR, Papanaoum K, et al. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin Infect Dis. 1995;20(3):611–6.
Bichile LS, Gokhale YA, Sridhar V, Gill NH. Disseminated cryptococcal infection in immune competent patients. J Assoc Physicians India. 2001;49:377–8.
Pappas PG, Perfect JR, Cloud GA, Larsen RA, Pankey GA, Lancaster DJ, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33(5):690–9.
Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci U S A. 2004;101(49):17258–63.
Lui G, Lee N, Ip M, Choi KW, Tso YK, Lam E, et al. Cryptococcosis in apparently immunocompetent patients. QJM: Mon J Assoc Phys. 2006;99(3):143–51.
Chau TT, Mai NH, Phu NH, Nghia HD, Chuong LV, Sinh DX, et al. A prospective descriptive study of cryptococcal meningitis in HIV uninfected patients in Vietnam—high prevalence of Cryptococcus neoformans var grubii in the absence of underlying disease. BMC Infect Dis. 2010;10:199.
Rosen LB, Rocha Pereira N, Figueiredo C, Fiske LC, Ressner RA, Hong JC, et al. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin Infect Dis. 2015;60(7):1017–25.
Saijo T, Chen J, Chen SC, Rosen LB, Yi J, Sorrell TC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. MBio. 2014;5(2):e00912–14.
Piccoli L, Campo I, Fregni CS, Rodriguez BM, Minola A, Sallusto F, et al. Neutralization and clearance of GM-CSF by autoantibodies in pulmonary alveolar proteinosis. Nat Commun. 2015;6:7375.
Browne SK, Zaman R, Sampaio EP, Jutivorakool K, Rosen LB, Ding L, et al. Anti-CD20 (rituximab) therapy for anti-IFN-gamma autoantibody-associated nontuberculous mycobacterial infection. Blood. 2012;119(17):3933–9.
Capoor MR, Nair D, Deb M, Gupta B, Aggarwal P. Clinical and mycological profile of cryptococcosis in a tertiary care hospital. Indian J Med Microbiol. 2007;25(4):401–4.
Patel SY, Ding L, Brown MR, Lantz L, Gay T, Cohen S, et al. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J Immunol. 2005;175(7):4769–76.
Browne SK, Burbelo PD, Chetchotisakd P, Suputtamongkol Y, Kiertiburanakul S, Shaw PA, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367(8):725–34.
Ku CL, Lin CH, Chang SW, Chu CC, Chan JF, Kong XF, et al. Anti-IFN-gamma autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J Allergy Clin Immunol. 2016;137(3):945–8. e8.
Lee WI, Huang JL, Wu TS, Lee MH, Chen IJ, Yu KH, et al. Patients with inhibitory and neutralizing auto-antibodies to interferon-gamma resemble the sporadic adult-onset phenotype of Mendelian Susceptibility to Mycobacterial Disease (MSMD) lacking Bacille Calmette-Guerin (BCG)-induced diseases. Immunobiology. 2013;218(5):762–71.
Acknowledgements
We thank all clinicians and patients who participated in this study. This study was supported by grants from the Ministry of Science and Technology (MOST 104-2314-B-182A-042) and Chang Gung Memorial Hospital (CMRPG3D0281 and BMRPE44).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kuo, CY., Wang, SY., Shih, HP. et al. Disseminated Cryptococcosis Due to Anti-Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies in the Absence of Pulmonary Alveolar Proteinosis. J Clin Immunol 37, 143–152 (2017). https://doi.org/10.1007/s10875-016-0364-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-016-0364-4