Abstract
Purpose
Anti-granulocyte-macrophage colony-stimulating factor autoantibodies (anti-GM-CSF Abs) are implicated in the pathogenesis of Cryptococcus gattii (C. gattii) infection and pulmonary alveolar proteinosis (PAP). Their presence has also been noted in nocardiosis cases, particularly those with disseminated disease. This study delineates a case series characterizing clinical features and specificity of anti-GM-CSF Abs in nocardiosis patients.
Methods
In this study, eight patients were recruited to determine the presence or absence of anti-GM-CSF Abs. In addition to the detailed description of the clinical course, we thoroughly investigated the autoantibodies regarding the characteristics, isotypes, subclasses, titers, and neutralizing capacities by utilizing the plasma samples from patients.
Results
Of eight patients, five tested positive for anti-GM-CSF Abs, all with central nervous system (CNS) involvement; patients negative for these antibodies did not develop CNS nocardiosis. Distinct from previously documented cases, none of our patients with anti-GM-CSF Abs exhibited PAP symptoms. The titer and neutralizing activity of anti-GM-CSF Abs in our cohort did not significantly deviate from those found in C. gattii cryptococcosis and PAP patients. Uniquely, one individual (Patient 3) showed a minimal titer and neutralizing action of anti-GM-CSF Abs, with no relation to disease severity. Moreover, IgM autoantibodies were notably present in all CNS nocardiosis cases investigated.
Conclusion
The presence of anti-GM-CSF Abs suggests an intrinsic immunodeficiency predisposing individuals toward CNS nocardiosis. The presence of anti-GM-CSF Abs helps to elucidate vulnerability to CNS nocardiosis, even with low titer of autoantibodies. Consequently, systematic screening for anti-GM-CSF Abs should be considered a crucial diagnostic step for nocardiosis patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The clinical significance of anti-cytokine autoantibodies has been gradually recognized in the past decade; these autoantibodies are considered an adult-onset immunodeficiency disease, and specific phenotypes may correspond to specific types of targeted cytokines [1, 2]. For example, anti-interferon-γ-autoantibody was confirmed as a pathogenic factor for Talaromyces marneffei, salmonellosis, varicella-zoster virus and nontuberculosis mycobacterial infection [3]; anti-interleukin-6 autoantibody is specific for severe staphylococcal infection [4], and neutralizing type-I interferon autoantibodies were reported in elderly individuals with critical COVID-19 or live vaccine-attenuated infection [5]. Current accumulated evidence indicates that anti-cytokine autoantibodies have reshaped the landscape of the pathogenesis of immunodeficiency diseases.
Neutralizing anti-granulocyte-macrophage colony-stimulating factor autoantibodies (anti-GM-CSF Abs) are also among the anti-cytokine autoantibodies identified and are related to several clinical phenotypes. In the 1990s, anti-GM-CSF Abs were first described as pathogenic factors in patients with pulmonary alveolar proteinosis (PAP), a phenomenon of surfactant homeostasis disturbance due to impaired alveolar macrophage function [6]. Under this circumstance, anti-GM-CSF Abs interfered with the maturation of alveolar macrophages, further affecting the clearance of surfactant accumulation. Over a decade after the relationship between PAP and anti-GM-CSF Abs was confirmed, anti-GM-CSF Abs were recognized as a risk factor for cryptococcosis, mostly by Cryptococcus gattii (C. gattii) [7,8,9,10,11,12,13,14,15,16,17], and also occasionally by Cryptococcus neoformans (C. neoformans) [7, 18,19,20,21,22], especially for central nervous system (CNS) manifestations [8]. After the first case series reported by Rosen et al. [7], a few relevant reports have confirmed the clinical significance of anti-GM-CSF Abs in C. gattii cryptococcosis [8, 9, 12, 15, 16, 23, 24].
Recently, there have also been reports regarding the relationship between nocardiosis and anti-GM-CSF Abs [25,26,27,28]. In 2015, Rosen et al. suggested that disseminated nocardiosis should prompt the existence of anti-GM-CSF Abs in previously healthy adults [25]. The primary infection sites of Nocardia include the skin and lungs, and it can disseminate systematically [29]. CNS infection is one of the most severe manifestations of dissemination [30]. Nocardia can evade the human immune system by intracellular survival within macrophages [30]. Nocardiosis is an opportunistic infection affecting immunocompromised subjects, such as cancer patients undergoing chemotherapy or organ transplant recipients [29]. However, one-third of infected patients have no known predisposing risk factors [29], suggesting that the presence of anti-GM-CSF Abs may explain the underlying pathogenesis for a significant portion of nocardiosis patients who do not carry the currently recognized risk.
While it has been established that anti-GM-CSF Abs are associated with C. gattii cryptococcosis, PAP, and disseminated nocardiosis, some unexplained clinical observations remain. First, anti-GM-CSF Abs are strongly linked to cryptococcosis caused by C. gattii, but there is no observed species preference in anti-GM-CSF Abs related nocardiosis [8, 15, 25]. Second, both infections associated with anti-GM-CSF Abs often involve the CNS, suggesting that the CNS may be a susceptible site for anti-GM-CSF Abs related infections [8, 15, 25, 28]. Third, the three clinical phenotypes associated with anti-GM-CSF Abs are rarely observed in the same individual. Despite a few reports of patients with overlapping conditions [7, 28], it appears that these three conditions are largely mutually exclusive [15, 31]. These observations underscore the importance of conducting comprehensive investigations into each anti-GM-CSF Abs phenotype, examining both host factors and antibody properties.
Herein, we report eight patients without human immunodeficiency virus (HIV) infection hospitalized due to a primary diagnosis of nocardiosis. Five of these eight patients were positive for anti-GM-CSF Abs, and all presented with CNS nocardiosis. In the present work, we describe the clinical presentation of these patients and the properties of anti-GM-CSF Abs derived from these patients.
Materials and Methods
Eight patients were enrolled in the present study. These eight patients were confirmed not to have HIV infection and were hospitalized due to clinical symptoms or signs of nocardiosis. The diagnosis of nocardiosis was established by microbiologic evidence, including blood culture and tissue culture taken via surgery or biopsy. The present study was approved by the Internal Review Board of Chang Gung Medical Foundation with reference numbers 202100672A3C502 and 202100789B0. Clinical symptoms and signs, laboratory data, previous medical history, personal history, microbiological test results, medical images, and treatment course were all retrieved from medical records. The clinical courses of the patients with positive anti-GM-CSF Abs are briefly presented in the following paragraphs; property analyses of autoantibodies were performed for all eight patients.
Detection of Anti-GM-CSF Abs
The diagnosis of anti-GM-CSF Abs was confirmed by indirect enzyme-linked immunosorbent assay (ELISA), as previously described [15, 23]. Plasma was diluted 1:100 to identify immunoglobulin isotypes: IgG, IgA, IgM, and IgE; Ig subclass, IgG1, IgG2, IgG3, and IgG4. Both isotype and subclass identification were performed by ELISA. We used a fully human anti-GM-CSF monoclonal antibody generated in-house as a standard for anti-GM-CSF Ab titer measurement. Further comparison between titers from patients with nocardiosis (n = 5), selected cryptococcosis (n = 6), and PAP (n = 6) was performed. The Mann–Whitney U test or ANOVA was applied, as appropriate.
Neutralizing Ability of Anti-GM-CSF Abs
The neutralizing ability of anti-GM-CSF Abs was assessed by the signal transducer and activator of transcription 5 (STAT5) reporter assay in U937 cells we applied in previous studies [15, 23]. The U937-STAT5 reporter system was established by lentivirus transfection with the STAT5RE reporter plasmid (Promega, Madison, WI). U937-STAT5 reporter cells (30,000 events) were preincubated with serially diluted plasma from patients and 100 pg/mL recombinant human GM-CSF (rhGM-CSF, BioLegend, San Diego, CA) for 20 h. GM-CSF-induced luciferase activity was measured with a One-Glo™ Luciferase Assay System (Promega, Madison, WI). The IC50 was then calculated using GraphPad Prism 7 software (GraphPad Software Inc. CA). The Mann–Whitney U test or ANOVA was employed, as appropriate.
Results
Case Presentation
Eight nocardiosis patients were enrolled in the present study (Table 1and Supplmentary Table 1). These eight patients were confirmed to be without HIV infection or other obvious immune dysregulation conditions and were hospitalized due to clinical symptoms or signs of nocardiosis. Five patients were positive for anti-GM-CSF Abs, and the neutralizing activity was confirmed by a reporter assay. The clinical features of the anti-GM-CSF-Ab-positive patients are presented below.
Patient 1
Patient 1 was a woman diagnosed with CNS nocardiosis when she was 63 years old. She initially presented with fever and dyspnea. She had multiple comorbidities, including right lung adenocarcinoma with pathological stage I after curative resection eight years before this episode of CNS nocardiosis, congestive heart failure with the New York Heart Association classification III, myelodysplastic syndrome, and chronic viral hepatitis C. No previous history of clinical presentation, such as C. gattii cryptococcosis or PAP, regarding anti-GM-CSF Abs was proven. After hospitalization, left-side pneumonia was impressed based on clinical signs and radiologic findings. After empiric antibiotic treatment, she improved and insisted on being discharged on day 15 of hospitalization. However, seven days after being discharged from the hospital, she returned to the hospital due to right limb weakness and aphagia.
According to the patient’s family, she had neurologic impairment for one week before the previous hospitalization, and she had visited a neurologist’s clinic. Both right limb weakness and right facial palsy were noted at that time. Further exams were arranged but had not been conducted before the previous hospitalization. Due to the progression of neurologic symptoms and signs, she was hospitalized again for further evaluation and treatment. Brain CT without contrast enhancement was performed on day one of the 2nd hospitalization, and a left frontal mass lesion was noted. Magnetic resonance imaging (MRI) was arranged for differential diagnosis (Fig. 1A). Due to the patient’s previous history of lung cancer, a metastatic lesion was highly suspected initially. Craniotomy was performed later on day seven of the 2nd hospitalization to release the intracranial compression. The intraoperative findings suggested the diagnosis of an abscess (Fig. 1B), and the final pathologic report revealed an abscess with nocardiosis. The patient was then treated medically. The patient then showed substantial improvement and was discharged from the hospital on day 22 of her 2nd hospitalization. Two months after this hospitalization, the patient experienced Mycobacteria abscessus infection. Given the high prevalence of anti-IFN-γ autoantibodies, a risk factor for mycobacterial infection, among the Southeast Asia population [32], we also examined the presence of IFN-γ autoantibodies. We confirmed their absence in this patient (Supplementary Fig. 1C).
Clinical images of nocardiosis patients identified with anti-GM-CSF antibodies. (A) Axial magnetic resonance imaging of two individuals diagnosed with CNS nocardiosis showing affected areas in the left frontal and temporal lobes, respectively. On the left, the T1-weighted image, enhanced with contrast medium, reveals an abscess characterized by a centrally located area of low signal intensity and a distinctly enhanced peripheral ring. On the right, the T2-weighted image displays the same abscess, notable for its high central signal intensity, a rim that appears iso- to hypointense, and a high-intensity surrounding area indicative of vasogenic edema. (B) The presence of an abscess cavity was confirmed in Patient 1 during surgery
Patient 2
This 67-year-old man was incidentally diagnosed with a pulmonary nodule without clinical symptoms (Supplementary Fig. 2). Regarding his medical history, he had two episodes of cerebral vascular accidents, which were diagnosed seven and nine years before his diagnosis of CNS nocardiosis. Similar to Patient 1, he had no clinical presentation related to anti-GM-CSF Abs. Aspiration was arranged to diagnose the pulmonary nodule, and the result was nondiagnostic. His condition was empirically treated as an infectious disease with antibiotics, and regression of the lesion was noted thereafter. Six months later, the patient exhibited bizarre behavior and cognitive impairment. No apparent head trauma history was recorded. No other neurologic symptoms or signs were identified, such as limb weakness, impairment of vision, facial palsy, hearing loss, aphasia, agnosia, amnesia, agraphia, or apraxia. Common presentations regarding infection, such as fever, chillness, or leukocytosis, were also not noted. He was then admitted to our hospital for further survey.
The initial CT scan only demonstrated focal malacia changes in the left temporo-parietal area. Further examination by MRI revealed a rim-enhanced mass with a maximal diameter of 2.5 cm, and perifocal edema around the lesion was also demonstrated (Fig. 1A). The clinical impression included metastatic lesions or brain access. After surgical consultation and a thorough preoperative survey for possible malignancy outside the CNS, an isolated brain mass was the only identified lesion. The neurosurgeon suggested surgical treatment, and the patient then underwent craniotomy on day four of hospitalization.
Intraoperative frozen section pathologic examination showed that the lesion was an abscess rather than a metastatic tumor. Total extirpation of the abscess was performed. The final microbiological diagnosis proved that the pathogen was Nocardia sp. The postoperative course was uneventful. The patient was treated medically after surgery and discharged on day 34 of hospitalization.
Patient 3
This man was diagnosed with nocardiosis when he was 62 years old. He also had squamous cell carcinoma of the lung, AJCC stage IVb. No other underlying chronic medical condition was noted. In addition, no previous history of anti-GM-CSF-Ab-related disease was diagnosed. He suddenly developed limb weakness and dizziness when getting up one night. Later, left-hand clonus was noted. He was then brought to the hospital. On the way to the hospital, he had generalized tonic-clonic seizures. In the emergency department (ED), he regained consciousness. ED physicians arranged a brain CT, and a metastatic brain lesion was suspected. In addition to the brain lesion, bilateral pleural effusion was detected, and a drainage procedure was arranged (Supplementary Fig. 3). The patient was then admitted to the ward for further management. On day 15 of hospitalization, stereotaxic surgery for a brain biopsy was arranged, and an intraoperative frozen section exam revealed a brain abscess. Local debridement was performed instead for brain tumor excision. After surgery, medical treatment with antibiotics was started. On day 21 of hospitalization, or postoperative day six, the microbiological exam demonstrated Nocardia sp. as the pathogen and the regimen of antibiotics was adjusted. The patient was discharged after 43 days of hospitalization.
Patient 4
This patient was a 51-year-old man with a medical history of chronic viral hepatitis B and a surgical history significant for left femoral head avascular necrosis, for which he had undergone total hip replacement years earlier. He had been discharged from another hospital one month prior to this presentation after being hospitalized for one month for a craniectomy to address a left parietal brain abscess. During that hospitalization, no pathogens were isolated from either tissue or blood cultures. However, one month after discharge, he began to experience progressive right-sided weakness and was admitted to the hospital for evaluation. An initial brain CT scan revealed a recurrent left-brain abscess, and craniotomy was performed for abscess drainage. Pus from the craniotomy yielded Nocardia spp. He was started on intravenous imipenem/cilastatin and oral sulfamethoxazole/trimethoprim as consolidation therapy starting on the second postoperative day that continued for four weeks. The treatment was then switched to oral sulfamethoxazole/trimethoprim with doxycycline as maintenance therapy for an additional six months. After clinical improvement, cranioplasty was performed, and he was discharged following a 59-day hospital stay.
During a follow-up brain CT scan arranged in the clinic, no active lesions were found. However, electroencephalography revealed episodic spike waves that were electroactive. Therefore, antiepileptic drugs were prescribed for prophylactic purposes after the antibiotics were discontinued.
Patient 5
A 42-year-old male had a history of a brain abscess for which he underwent craniotomy with drainage and four-month hospitalization. He was subsequently treated with antibiotics for the following months. However, no pathogen was isolated during the previous episode, and he recovered. He had alcoholic liver disease but no chronic viral hepatitis or other systemic diseases. He visited our hospital, presenting with a right-hand tremor and progressive weakness lasting two weeks. An initial brain CT showed a contrast-enhanced 1 cm ring-like lesion with perifocal white matter edema in the left frontal-parietal lobes. Although CT-guided biopsy was performed, it failed to identify any pathogens. The brain abscess was empirically treated with vancomycin, cefepime, and metronidazole. However, brain CT one month later revealed abscess enlargement. Consequently, he underwent a left parietal craniotomy with brain abscess evacuation. A microbiologic examination of the abscess content identified Nocardia wallacei, as confirmed by 16 S DNA analysis. The antibiotic regimen was then adjusted to imipenem/cilastatin, trimethoprim/sulfamethoxazole, and metronidazole. However, the response was limited, and brain CT showed a relapse of the abscess. The antibiotics were changed to intravenous linezolid (600 mg twice daily), meropenem, and metronidazole. After three weeks on this regimen, CT-guided aspiration of the brain lesion was repeated, and the abscess culture showed no growth of Nocardia. The patient’s condition gradually improved, and he was discharged after eight weeks of intravenous linezolid. He continued oral linezolid and ciprofloxacin for another 2.5 months. Throughout the treatment, the patient’s weakness improved, with no hand tremor or other focal neurologic signs. However, in the eighth week of intravenous linezolid treatment, he developed progressive and persistent, symmetrical neuropathy in both lower limbs. The linezolid dose was reduced to 600 mg daily during the eleventh week of treatment to prevent abscess relapse. Neuropathy persisted in his feet even after discontinuing linezolid. During the antibiotic-free period, he did not experience any relapse of right-sided weakness. Brain CT two years later showed residual brain tissue damage but no recurrence of the brain abscess.
Properties of Anti-GM-CSF Abs
Anti-GM-CSF-Abs are the common etiology of PAP, C. gattii infection and nocardiosis; however, no PAP or cryptococcosis was reported in our patients. Therefore, we analyzed the properties of autoantibodies in our cohort to determine whether the properties of autoantibodies could explain the different clinical manifestations. In our previous work on C. gattii cryptococcosis and other published work on PAP, IgM can be detected coexisting with the dominant IgG isotype [15, 33]. To elucidate the characteristics of anti-GM-CSF Abs in our nocardiosis cohort, we assessed immunoglobulin isotypes (Fig. 2A) and the Ig subclass compositions (Fig. 2B) in the Nocardia-infected patients positive for anti-GM-CSF Abs. The predominant immunoglobulin isotype observed was IgG, whereas neither IgA nor IgE isotypes of anti-GM-CSF Abs were detected in any of the cases (Fig. 2A). Intriguingly, a high prevalence of IgM isotype autoantibodies was noted in all nocardiosis patients, even in P3, who showed limited presence of IgG anti-GM-CSF Abs (Fig. 2A). The primary IgG subclass across most cases was IgG1 (Fig. 2B). Three patients (P1, P2, and P5) displayed diminished presence of IgG2 subclass anti-GM-CSF Abs, and none showed IgG3 subclass autoantibodies. A notable concentration of IgG4 subclass anti-GM-CSF Abs was detected in P2. The specific IgG subclass was undetectable in the case of P3, which had a minimal titer of IgG autoantibodies. Collectively, the composition of anti-GM-CSF Abs related to Nocardia infection resembled that observed in patients with cryptococcosis or PAP. However, the pronounced presence of IgM isotype anti-GM-CSF Abs marked a distinct difference.
Characterization of immunoglobulin isotype and IgG subclass of anti-GM-CSF antibodies in nocardiosis patients. Individual immunoglobulin isotypes and IgG subclasses of anti-GM-CSF antibodies were detected using ELISA on plasma diluted 1:100. (A) In nocardiosis patients P1, P2, P4, and P5, anti-GM-CSF antibodies were predominantly of the IgM and IgG isotypes. In contrast to the low levels of IgG anti-GM-CSF antibodies, IgM anti-GM-CSF antibodies were prominently identified in patient P3. (B) The majority of IgG subclasses revealed the presence of IgG1 anti-GM-CSF antibodies in patients P1, P4, and P5, while patient P2 also exhibited a significant presence of IgG4 anti-GM-CSF antibodies. Patient P3 exhibited rare detection of IgG subclasses, consistent with the low levels of IgG-type anti-GM-CSF antibodies
Comparison of Anti-GM-CSF Abs Among PAP, Cryptococcosis, and Nocardiosis
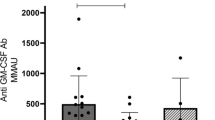
Anti-GM-CSF Abs have been described as having a titer and neutralizing thresholds that induce PAP clinical phenotypes [34]. We sought to analyze the autoantibody titer and neutralizing ability in our nocardiosis cohort compared to the CNS-involved cryptococcosis cohort, which consisted of samples collected during active status (n = 6) and PAP (n = 6) patients described in our previous study [15]. The titer of anti-GM-CSF Abs showed a range of 2.3 ~ 309.2 units (mean = 117.3 ± 124.6) in nocardiosis, 15.2 ~ 105.3 units (mean = 54.6 ± 35.0) in cryptococcosis, and 8.8 ~ 189.4 (mean = 54.6 ± 67.0) in PAP (Fig. 3A). The inhibition abilities of anti-GM-CSF Abs from patients with nocardiosis in our cohort showed a range of 27 ~ 33277 dilution folds (mean = 11414 ± 12795); those from patients with cryptococcosis showed a range of 1871 ~ 12719 dilution folds (mean = 6584 ± 4594), and those from patients with PAP showed a range of 477 ~ 6993 dilution folds (mean = 3318 ± 2574) (Fig. 3B). Correlation analysis exhibited a consistent correlation (R2 = 0.7308) between autoantibody titer and half-maximal inhibitory concentration (IC50) in all cohorts (Fig. 3C). In summary, the titer and neutralizing activities did not significantly differ among these three groups of patients.
Comparison of anti-GM-CSF antibody characteristics among nocardiosis, cryptococcosis, and PAP patients. The titer of anti-GM-CSF antibodies was measured using an in-house ELISA, and the STAT5 inhibition levels were detected using a reporter system established in a previous study [23]. Patients with CNS-involved cryptococcosis (n = 6, black circle and line) and PAP (n = 6, blue square and line) were collected to compare with our nocardiosis patients (n = 5, red symbol). Healthy controls (n = 3) were shown in gray line. (A) Antibody titers and (B) STAT5 inhibition levels showed no significant differences among the three patient cohorts. (C) Correlation analysis revealed a strong correlation but no difference between antibody titer and IC50 across all cohorts
Discussion
We enrolled eight Nocardia-infected non-HIV patients and identified five anti-GM-CSF-Ab-positive patients. The anti-GM-CSF-Ab-positive patients were all accompanied by CNS-involved Nocardia infection but without a specific Nocardia sp. Regarding serological analyses, the anti-GM-CSF Ab-positive nocardiosis patients were found to have IgM-type autoantibodies. We compared the properties of the autoantibodies among anti-GM-CSF Ab-positive cryptococcosis, PAP, and nocardiosis, revealing no significant differences in either concentrations or neutralizing activities. Our findings suggest that adult-onset immunodeficiency caused by anti-GM-CSF Abs is the major etiology underlying CNS nocardiosis, even without the sign of pulmonary proteinosis.
The CNS is the second most common site affected by nocardiosis, with the percentage of cases ranging from 3 to 26%, depending on the specific population studied [35]. Anti-GM-CSF Abs involved in nocardiosis in our cohort primarily involved the CNS. Moreover, combining previous research and our cohort, 14/16 (87.5%) cases with anti-GM-CSF Abs were identified in CNS nocardiosis [14, 25, 27, 28, 36]. Among cryptococcosis patients reported in the literature, 43/50 (86.0%) cases with anti-GM-CSF Abs were reported to have CNS involvement [7,8,9,10,11,12,13,14,15,16, 18,19,20,21, 23], and the anti-GM-CSF Abs could be identified in their cerebrospinal fluid [7]. Current clinical observations suggest that anti-GM-CSF antibodies may impair the central nervous system’s ability to defend against infections caused by Cryptococcus gattii and Nocardia, without species specificity. GM-CSF is known to play an essential role in supporting the CNS environment, such as promoting monocytes to cross the blood-brain barrier and microglial proliferation [37,38,39,40,41]. Gavino et al. reported a cohort of spontaneous CNS candidiasis patients with CARD9 mutation causing GM-CSF production deficiency [42]. However, the exact role of GM-CSF deficiency driven by autoantibodies in CNS susceptibility to infection is unclear. Furthermore, no studies have examined the presence of anti-GM-CSF antibodies in the cerebrospinal fluid of patients with nocardiosis. Therefore, further investigation into the underlying pathogenesis of clinical presentations and localization associated with anti-GM-CSF antibodies is warranted and may provide a better understanding of the influence of GM-CSF on central nervous system immunity.
Patient 3 in the present work was identified with equivocally detectable IgG-type anti-GM-CSF Abs, even under 1:100 plasma dilution (Supplementary Fig. 1A). However, the autoantibody from Patient 3 did have 50% inhibition of GM-CSF activity after 100-fold dilution (Fig. 3B). In a recent study, Bastard et al. applied a high-sensitivity luciferase assay to detect the presence of anti-type-I-interferon-autoantibody. They reported that critical COVID-19 patients produced anti-type-I-interferon-autoantibodies; although they had weak neutralizing activity, the autoantibodies sufficiently impaired the type-I interferon biological protection from viral infection [43]. In our work, we also used U937-STAT5 reporter system to confirm the plasma from Patient 6, Patient 7, and Patient 8. No neutralizing activity was identified in those samples (Supplementary Fig. 1B). This observation indicates that low titer but potentially neutralizing autoantibodies may have existed without detection by methods in previous studies, and a novel approach for identifying pathogenic autoantibodies should be developed and applied. Concerning clinical observation in our series, the severity of the disease did not necessarily correlate with the titer of anti-GM-CSF Abs. Therefore, increasing the sensitivity for detecting low-titer autoantibody doses might be worth further investigation.
The immunoglobulin isotype analysis in the present work demonstrated that IgM-type anti-GM-CSF Abs were present in all (5/5) nocardiosis patients. IgM-type anti-GM-CSF Abs have rarely been addressed in previous studies. Nei et al. described that as an etiologic bystander, IgM-type anti-GM-CSF-Abs were present in more than 80% of PAP patients and approximately 20% of healthy subject serum samples, with extremely weak neutralizing and binding avidity [33]. However, isotype analysis of anti-GM-CSF Abs were detected in none (0/6) of the PAP and healthy controls and 26.7% (4/15) of the cryptococcosis patients with IgM-type anti-GM-CSF Abs in our previous studies [15]. Soluble IgM-type antibodies are defined via their constant region Fcμ, showing pentamer formation, which is the first line of host defense [44]. In addition to direct blockade, IgM-type antibodies have a high affinity for complement and Fc receptor (Fcα/µR, Poly-IgR, and FcµR) interactions, regulating B-cell development [45]. Recently, several studies have discussed the importance of Fc-mediated antibody function, even in anti-cytokine autoantibodies [46, 47]. Overall, the dynamics of IgM-type and IgG-type anti-GM-CSF Ab levels in our nocardiosis cohort and the manifestations of IgM-type anti-GM-CSF Abs are worth investigating and follow-up.
No clinical sign of PAP in anti-GM-CSF Ab-positive Nocardia-infected patients suggests that PAP and other anti-GM-CSF Ab-associated infections do not overlap. In a systematic literature review of seventy-five reported cases of PAP with opportunistic infections, thirty-two patients (43%) with opportunistic infections were infected with Nocardia [48]. However, these highly selective case series only enrolled PAP patients with opportunistic infection during a period of six decades (1950 ~ 2010), and only nine more patients were reported in a review from Berthoux et al. in 2021 [28]. A summary of published relevant reports is summarized in Supplementary Table 2. Overall, publication bias should be considered, and the observation cannot be simply recognized that PAP and nocardiosis are present in a single subject due to the existence of anti-GM-CSF Abs. Of note, opportunistic infection might be at least partially explained by the immune compromise related to treatments in which steroids or immunosuppressive treatment are the most commonly applied to manage PAP. In contrast, no PAP patient had nocardiosis in a large well-studied cohort of autoimmune PAP from Japan with 223 anti-GM-CSF Ab-positive patients [31]. In our published works on PAP and C. gattii cryptococcosis [15, 23] and the present work on nocardiosis, all evidence suggests that overlapping presentation is rare. Based on the summary of clinical observation and evidence from the literature, it is conceivable that a determining mechanism may influence the clinical presentation of individuals with anti-GM-CSF. Therefore, studies investigating the pathogenesis of diseases associated with anti-GM-CSF Abs may need to be specifically designed for each of the three clinical conditions.
Recent studies, including our investigations on anti-IFN-γ autoantibodies, showed that antibodies recognizing distinct epitopes can have variable biological implications [46]. The pathogenic mechanisms of anti-GM-CSF Abs in infectious conditions and PAP might be inherently different. A thorough exploration of the pathogenesis associated with anti-GM-CSF Abs is imperative. Given the complex clinical presentations and limited insights into this specific anti-cytokine autoantibody, understanding its properties might clarify its diverse clinical implications.
Conclusions
In summary, we describe that anti-GM-CSF Abs are an underlying immune deficiency that increases the risk of non-HIV CNS nocardiosis. IgM-type autoantibodies were abundant in serum from the patients in our series. The existence of anti-GM-CSF Abs can explain the etiology of CNS nocardiosis, even with low-titer autoantibodies. Routine screening of anti-GM-CSF Abs is imperative for patients with nocardiosis.
Data Availability
No datasets were generated or analysed during the current study.
References
Puel A, Bastard P, Bustamante J, Casanova JL. Human autoantibodies underlying infectious diseases. J Exp Med. 2022;219(4):e20211387. https://doi.org/10.1084/jem.20211387.
Ku CL, Chi CY, von Bernuth H, Doffinger R. Autoantibodies against cytokines: phenocopies of primary immunodeficiencies? Hum Genet. 2020;139:783–94. https://doi.org/10.1007/s00439-020-02180-0.
Shih HP, Ding JY, Yeh CF, Chi CY, Ku CL. Anti-interferon-γ autoantibody-associated immunodeficiency. Curr Opin Immunol. 2021;72:206–14. https://doi.org/10.1016/j.coi.2021.05.007.
Vincent T, Plawecki MM, Goulabchand R, Guilpain P, Eliaou JFF. Emerging clinical phenotypes associated with anti-cytokine autoantibodies. Autoimmun Rev. 2015;14:528–35; https://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=25633324&retmode=ref&cmd=prlinks
Casanova JL, Anderson MS. Unlocking life-threatening COVID-19 through two types of inborn errors of type I IFNs. J Clin Invest. 2023;133(3):e166283. https://doi.org/10.1172/JCI166283.
Ben-Dov I, Segel MJ. Autoimmune pulmonary alveolar proteinosis: clinical course and diagnostic criteria. Autoimmun Rev. 2014;13:513–7. https://linkinghub.elsevier.com/retrieve/pii/S1568997214000585.
Rosen LB, Freeman AF, Yang LM, Jutivorakool K, Olivier KN, Angkasekwinai N, et al. Anti–GM-CSF autoantibodies in patients with cryptococcal meningitis. J Immunol. 2013;190:3959–66. https://doi.org/10.4049/jimmunol.1202526.
Saijo T, Chen J, Chen SC-ACA, Rosen LB, Yi J, Sorrell TC, et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio. 2014;5:e00912–14. https://doi.org/10.1128/mBio.00912-14.
Crum-Cianflone NF, Lam PV, Ross-Walker S, Rosen LB, Holland SM. Autoantibodies to Granulocyte-Macrophage colony-stimulating factor Associated with severe and unusual manifestations of Cryptococcus gattii infections. Open Forum Infect Dis. 2017;4:1–6.
Demir S, Chebib N, Thivolet-Bejui F, Cottin V. Pulmonary alveolar proteinosis following cryptococcal meningitis: a possible cause? BMJ Case Rep. 2018;2018:2017–9.
Huynh J, Saddi V, Cooper P, Cheng AT, Meyer W, Chen S, et al. Unusual presentation of severe endobronchial obstruction caused by Cryptococcus gattii in a child. J Pediatr Infect Dis Soc. 2019;9:67–70.
Applen Clancey S, Ciccone EJ, Coelho MA, Davis J, Ding L, Betancourt R, et al. Cryptococcus deuterogattii vgiia infection associated with travel to the pacific northwest outbreak region in an anti- granulocyte-macrophage colony-stimulating factor autoantibody-positive patient in the United States. mBio. 2019;10:1–14.
Stevenson B, Bundell C, Mulrennan S, McLean-Tooke A, Murray R, Brusch A. The significance of anti-granulocyte-macrophage colony-stimulating factor antibodies in cryptococcal infection: case series and review of antibody testing. Intern Med J. 2019;49:1446–50.
Lee E, Miller C, Ataya A, Wang T. Opportunistic Infection Associated With Elevated GM-CSF Autoantibodies: A Case Series and Review of the Literature. Open Forum Infect Dis. United States; 2022. p. ofac146.
Wang SY, Lo YF, Shih H-, Ho MW, Yeh CF, Peng JJ, et al. Cryptococcus gattii infection as the Major Clinical Manifestation in patients with autoantibodies against Granulocyte-Macrophage colony-stimulating factor. J Clin Immunol. 2022;42:1730–41.
Kuo PH, Wu UI, Pan YH, Wang JT, Wang YC, Sun HY, et al. Neutralizing anti-granulocyte-macrophage colony-stimulating factor autoantibodies in patients with Central Nervous System and localized cryptococcosis: Longitudinal Follow-Up and Literature Review. Clin Infect Dis. 2022;75:278–87.
Goupil de Bouillé J, Epelboin L, Henaff F, Migaud M, Abboud P, Blanchet D, et al. Case Report: Invasive Cryptococcosis in French Guiana: Immune and Genetic Investigation in six Non-HIV patients. Front Immunol. 2022;13:881352. https://doi.org/10.3389/fimmu.2022.881352.
Panackal AA, Rosen LB, Uzel G, Davis MJ, Hu G, Adeyemo A, et al. Susceptibility to Cryptococcal Meningoencephalitis Associated with idiopathic CD4(+) Lymphopenia and secondary germline or acquired defects. Open Forum Infect Dis. 2017;4:ofx082.
Perrineau S, Guery R, Monnier D, Puel A, Lanternier F. Anti-GM-CSF autoantibodies and Cryptococcus neoformans var. Grubii CNS vasculitis. J Clin Immunol. 2020;40:767–9.
Viola GM, Malek AE, Rosen LB, DiNardo AR, Nishiguchi T, Okhuysen PC, et al. Disseminated cryptococcosis and anti-granulocyte-macrophage colony-stimulating factor autoantibodies: an underappreciated association. Mycoses. 2021;64:576–82.
Lim WT, Mudalige S, Nilushi T, Shanka J, Parakrama K. Crazy-paving pattern: a rare case of Autoimmune Pulmonary Alveolar Proteinosis (PAP) with positive Anti-GM-CSF antibody following cryptococcal infection in an otherwise Healthy Individual and Review of Literature. Eur J Respiratory Med. 2021;3:200–5.
Arango-Franco CA, Migaud M, Ramírez-Sánchez IC, Arango-Bustamante K, Moncada-Vélez M, Rojas J, et al. Anti-GM-CSF neutralizing autoantibodies in Colombian patients with disseminated cryptococcosis. J Clin Immunol. 2023;43:921–32.
Kuo CY, Wang SY, Shih HP, Tu KH, Huang WC, Ding JY, et al. Disseminated Cryptococcosis due to anti-granulocyte-macrophage colony-stimulating factor autoantibodies in the absence of pulmonary alveolar proteinosis. J Clin Immunol. 2017;37:143–52.
Yang DH, England MR, Salvator H, Anjum S, Park YD, Marr KA, et al. Cryptococcus Gattii Species Complex as an opportunistic Pathogen: Underlying Medical conditions Associated with the infection. mBio. 2021;12:e0270821.
Rosen LB, Rocha Pereira N, Figueiredo CCC, Fiske LC, Ressner RA, Hong JC, et al. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin Infect Dis. 2015;60:1017–25. https://doi.org/10.1093/cid/ciu968.
Salvator H, Cheng A, Rosen LB, Williamson PR, Bennett JE, Kashyap A, et al. Neutralizing GM-CSF autoantibodies in pulmonary alveolar proteinosis, cryptococcal meningitis and severe nocardiosis. Respir Res. 2022;23:1–9. https://doi.org/10.1186/s12931-022-02103-9.
Wu X-KK, Lin Q. Pulmonary alveolar proteinosis complicated with nocardiosis: a case report and review of the literature. World J Clin Cases. 2021;9:2874–83.
Berthoux C, Mailhe M, Vély F, Gauthier C, Mège JL, Lagier JC, et al. Granulocyte Macrophage colony-stimulating factor-specific autoantibodies and cerebral Nocardia with Pulmonary Alveolar Proteinosis. Open Forum Infect Dis. 2021;8:1–5.
Mehta HH, Shamoo Y. Pathogenic nocardia: a diverse genus of emerging pathogens or just poorly recognized? PLoS Pathog. 2020;16:1–7.
Lerner PI, Nocardiosis. Clin Infect Dis. 1996;22:891–5.
Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, et al. Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan. Am J Respir Crit Care Med. 2008;177:752–62.
Chi CY, Chu CC, Liu JP, Lin CH, Ho MW, Lo WJ, et al. Anti-IFN-gamma autoantibodies in adults with disseminated nontuberculous mycobacterial infections are associated with HLA-DRB1*16:02 and HLA-DQB1*05:02 and the reactivation of latent varicella-zoster virus infection. Blood. 2013;121:1357–66. https://doi.org/10.1182/blood-2012-08-452482.
Nei T, Urano S, Motoi N, Takizawa J, Kaneko C, Kanazawa H, et al. IgM-type GM-CSF autoantibody is etiologically a bystander but associated with IgG-type autoantibody production in autoimmune pulmonary alveolar proteinosis. Am J Physiol Lung Cell Mol Physiol. 2012;302:959–64.
Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol [Internet]. 2010;135:223–35. https://doi.org/10.1016/j.clim.2010.02.017.
Meena DS, Kumar D, Bohra GK, Midha N, Garg MK. Clinical characteristics and treatment outcome of Central Nervous System Nocardiosis: a systematic review of reported cases. Med Principles Pract. 2022;31:333–41.
Courbin V, Riller Q, Amegnizin J-LL, Gricourt G, Demontant V, Fihman V, et al. Case Report: cerebral nocardiosis caused by Nocardia cyriacigeorgica detected by Metagenomics in an apparently immunocompetent patient. Front Immunol. 2022;13:719124. https://doi.org/10.3389/fimmu.2022.719124.
Vogel DYSS, Kooij G, Heijnen PDAMAM, Breur M, Peferoen LANN, van der Valk P, et al. GM-CSF promotes migration of human monocytes across the blood brain barrier. Eur J Immunol. 2015;45:1808–19.
Shang DS, Yang YM, Zhang H, Tian L, Jiang JS, Dong YB, et al. Intracerebral GM-CSF contributes to transendothelial monocyte migration in APP/PS1 Alzheimer’s disease mice. J Cereb Blood Flow Metab. 2016;36:1978–91.
Dikmen HO, Hemmerich M, Lewen A, Hollnagel JO, Chausse B, Kann O. GM-CSF induces noninflammatory proliferation of microglia and disturbs electrical neuronal network rhythms in situ. J Neuroinflammation. 2020;17:1–13.
Zhang H, Zhang S, Zhang J, Liu D, Wei J, Fang W, et al. ZO-1 expression is suppressed by GM-CSF via miR-96/ERG in brain microvascular endothelial cells. J Cereb Blood Flow Metab. 2018;38:809–22.
Zhao J, Sun L, Li X, Commanding CNS, Invasion. GM-CSF. Immunity. 2017;46:165–7; https://doi.org/10.1016/j.immuni.2017.02.003
Gavino C, Hamel N, Zeng J, Bin, Legault C, Guiot MC, Chankowsky J, et al. Impaired RASGRF1/ERK-mediated GM-CSF response characterizes CARD9 deficiency in french-canadians. J Allergy Clin Immunol. 2016;137:1178–e11887.
Bastard P, Gervais A, Voyer T, Le, Rosain J, Philippot Q, Manry J, et al. Autoantibodies neutralizing type I IFNs are present in ~ 4% of uninfected individuals over 70 years old and account for ~ 20% of COVID-19 deaths. Sci Immunol. 2021;6. https://doi.org/10.1126/sciimmunol.abl4340.
Jones K, Savulescu AF, Brombacher F, Hadebe S. Immunoglobulin M in Health and diseases: how far have we come and what Next? Front Immunol. 2020;11:595535. https://doi.org/10.3389/fimmu.2020.595535.
Liu J, Wang Y, Xiong E, Hong R, Lu Q, Ohno H, et al. Role of the IgM fc receptor in immunity and tolerance. Front Immunol. 2019;10:529. https://doi.org/10.3389/fimmu.2019.00529.
Shih HP, Ding JY, Sotolongo BJ, Lo YF, Chung PH, Ting HT, et al. Pathogenic autoantibodies to IFN-γ act through the impedance of receptor assembly and Fc-mediated response. J Exp Med. 2022;219:e20212126. https://doi.org/10.1084/jem.20212126.
Van Erp EA, Luytjes W, Ferwerda G, Van Kasteren PB. Fc-mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. 2019:10:548; https://doi.org/10.3389/fimmu.2019.00548
Punatar AD, Kusne S, Blair JE, Seville MT, Vikram HR. Opportunistic infections in patients with pulmonary alveolar proteinosis. J Infect. 2012;65:173–9. https://doi.org/10.1016/j.jinf.2012.03.020.
Acknowledgements
We thank Dr. Huan-Wu Chen (Division of Emergency and Critical Care Radiology, Department of Medical Imaging and Intervention, Chang Gung Memorial Hospital, Taoyuan City, Taiwan) for interpreting the MRI images.
Funding
This work was supported by Chang Gung Memorial Hospital (CMRPG3L0961, CMRPG3L0962 and CMRPG3P0201) and the Ministry of Science and Technology of Taiwan (104-2314-B-182 A-042-, 105-2314-B-182 A-033- and 112-2314-B-182 -017-).
Author information
Authors and Affiliations
Contributions
CL.K, SY.W, and YF.L conceived of and designed the experiments; YF.L conducted the experiments; YC.C collected and summarized patient information; YF.L and SY.W conducted the data analysis; YF.L, SY.W, and CL.K wrote the manuscript; YH.W, MW.H, CF.Y, CS.R, CY.K, KH.T, WT.L, and YC.C provided the patient samples and clinical information; YF.L, YN.L, and JY.D helped in the sample collection and autoantibody screening. WT.L, JJ.P, TY.W, YP.C, CC.L, YN.L, and HP.S provided experimental support and expertise. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The study was conducted with permission from the Institutional Review Board of Chang Gung Memorial Hospital (IRB number: 202100672A3C502 and 202100789B0).
Consent to Participate
All patients provided written informed consent approved by the Institutional Review Board of Chang Gung Memorial Hospital. Blood samples were obtained from patients with written informed consent in accordance with the Declaration of Helsinki.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lo, YF., Wang, SY., Wu, YH. et al. The Pathogenic Role of Anti-Granulocyte-Macrophage Colony-Stimulating Factor Autoantibodies in the Nocardiosis with the Central Nervous System Involvement. J Clin Immunol 44, 176 (2024). https://doi.org/10.1007/s10875-024-01775-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10875-024-01775-w