Abstract
B-cell linker (BLNK) protein is a non-redundant adaptor molecule in the signaling pathway activated by (pre) B-cell antigen receptor signals. We present two siblings with a homozygous deleterious frameshift mutation in BLNK, resulting in a block of B cell development in the bone marrow at the preB1 to preB2 stage, absence of circulating B cells and agammaglobulinemia. This is the first description of an enteroviral infection associated arthritis and dermatitis in a patient with BLNK deficiency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Congenital agammaglobulinemia is caused by inborn errors in early B cell development abrogating differentiation, maturation and antibody production. X-linked agammaglobulinemia (XLA), which results from mutations in Bruton tyrosine kinase (BTK), constitutes 85 % of the congenital agammaglobulinemia. Other defects include mutations in μ heavy chain, λ5, Igα, Igβ, BLNK, PIK3R1 and others [1].
B-cell linker (BLNK) adaptor protein is a key molecule in the signaling pathways of B cells activated by pre- and B-cell antigen receptor (BCR) signals [2]. Activation of pre-BCR assembles through phosphorylation induced protein-protein interactions BTK, phospholipase Cγ2 (PLCγ2) and others to the BLNK adaptor protein and thereby facilitates the phosphorylation of PLCγ2 by BTK. This enables downstream signals setting up for recombination-activating gene (RAG) protein expression, light chain recombination and further differentiation of pre-BII cells [3].
Here, we report a child with a homozygous mutation in the BLNK gene with arthritis and dermatitis associated with persistent enteroviral infection.
Case Reports
Patient 1 (P1) is a boy born in 2004 to consanguineous Arab parents and was diagnosed at the age of 6 month with congenital agammaglobulinemia based on his history of recurrent otitis media, diarrhea and a positive family history. Primary care was provided in Queen Rania Children’s Hospital at Amman, Jordan. He has been maintained on a regular intravenous immunoglobulins (IVIG) therapy. Despite trough IgG serum levels between 300 and 400 mg/dl he did not have serious infections till the age of 8 years when he developed diarrhea. Endoscopy and histopathology were unremarkable. At the age of 9 years a polyarthritis of the wrists, ankles, and knees was diagnosed. Seven months later, he had an exacerbation of his diarrhea (with negative cultures) and hypoalbuminemia. The dose of IVIG was increased to 600 mg/kg every 3 weeks. Colonoscopy was performed and demonstrated signs of enteritis with intraepithelial lymphocytes and was suggestive of virally induced colitis or celiac disease. A trial of gluten free diet was unsuccessful. At the age of 10 years he developed generalized edema and dermatitis of the abdomen, upper and lower extremities (Fig. 1a). Skin biopsy demonstrated skin hyperkeratosis and infiltration of lymphocytes into the epidermis compatible with chronic dermatitis. IVIG dose was increased to IgG trough level of above 700 mg/dl and a treatment with antibiotics, prednisone and methotrexate was started. His skin disease and edema however did not improve and he presented with an induration of the skin of the calf affecting knee joint flexion and normal activity. At that time, microbiological workup revealed a positive polymerase chain reaction (PCR) for enterovirus in his peripheral blood. Enterovirus or ureaplasma were not detected on skin biopsy or from synovial fluid. Despite increased IVIG dose and frequency his enteroviral PCR continues to be positive.
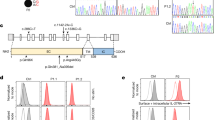
a Dermatitis of patient P1; note edema and induration of the skin. b c.438_439delinsT in the DNA sequence of exon 6 of the human BLNK gene in patients (upper row), obligate heterozygote (middle row), and a healthy control (lower row). The mutation is indicated by the arrow. c B cell precursor populations in a normal bone marrow (control) and bone marrow of patient P1 and P2. Immature and pre B cell stainings are depicted after gating on CD10pos B cell precursors and pro B cells are gated on CD79apos/TdTpos/CD3neg/CD16neg/ CD33neg cells
His older sister (P2) was born as the 4th sibling in 2000. She was diagnosed as congenital agammaglobulinemia at the age of 12 months due to the history of recurrent otitis media, sino-pulmonary infections and absent B lymphocytes. IVIG replacement was started. She was treated with rotating antibiotics, steroids and inhaled steroids for obstructive lung disease. Several episodes of culture negative diarrhea were observed. She suffered from chronic cough and arthralgia. Due to bronchiectatic changes on computed tomography, she was maintained on antibiotics and IVIG. Of note, enterovirus PCR of the blood was negative.
Autosomal recessive agammaglobulinemia was suspected and parental consent was obtained for genetic investigations.
For whole exome analysis, exonic sequences were enriched in the DNA samples of P1 using SureSelect Human All Exon 50 Mb Kit (Agilent Technologies, Santa Clara, California, USA). Sequences were determined by HiSeq2000 (Illumina, San Diego, California, USA) and 100-bp were read paired-end. Reads alignment and variant calling were performed with DNAnexus software (Palo Alto, California, USA) using the default parameters with the human genome assembly hg19 (GRCh37) as a reference. This analysis yielded 47.05 million confidently mapped reads with a mean coverage of X55. We removed variants which were called less than X8, were off-target, heterozygous, synonymous, MAF>0.1 % at dbSNP138 and MAF>1 % in the Hadassah in-house dbSNP. 15 variants survived this filtering but only one affected a gene with immune relevance. This was a homozygous frameshift mutation in the BLNK gene, Hg19:Chr10:97983671delTC/InsA, NM_001114094 c.435_436delTCInsA, p.E145fs25* (Fig. 1b). Genotyping the family members by Sanger sequencing confirmed that the sister (P2) is homozygous for the same mutation, whereas the parents and four healthy siblings are heterozygous.
Further immunological examinations were performed as previously described [4] and demonstrated no peripheral B cells in both siblings. A severe block at the pre B I to pre B II cell stage was observed by flow cytometric analysis of bone marrow aspirates of P1 with nearly absent immature B cells (Fig. 1c).
The T cell phenotype did not demonstrate a common deviation between both patients. In P1 especially absolute numbers of terminally differentiated and effector memory CD8 T cells were oligoclonaly expanded, probably in reaction to the enteroviral infection. Both patients had a low percentage of circulating CXCR5+ CD4 memory T cells as previously reported for BTK deficiency (P1: 13.1 %, P2: 17.4 %, norm: 18.2–31.3 % CXCR5+ of memory CD4 T cells), [5] which was also confirmed for bona-fide circulating T follicular helper (TFH) like CXCR5+CXCR3−PD1+ CD4 memory CD4 T cells (data not shown).
Discussion
We present two siblings with a homozygous deleterious frameshift mutation in BLNK, resulting in a developmental block of B cells at the preB1 to preB2 stage, absence of circulating B cells and agammaglobulinemia. BLNK −/− mice also have a block in pre-B cell development in the bone marrow and reduced numbers of splenic mature B cells [6]. But compensatory mechanisms may allow for a leaky block at the pre B cell stage in BLNK −/− mice, while the function of BLNK in human B cells seems non redundant [3]. This finding resembles the presentation of BTK and λ5 deficiencies, which are also more severe in humans than in the murine model [1].
Only five patients with BLNK deficiency have been reported (Table 1) [1–3, 7]. All reported patients presented with bacterial respiratory tract infections, nearly absent circulating B cells and agammaglobulinemia. Systemic enteroviral infections (especially CNS) were previously reported in patients with XLA and common variable immune deficiencies [8–10]. However, to the best of our knowledge this infection has not been reported in the context of BLNK deficiency. Interestingly, the patient presented with skin and joint manifestations, as had been described in BTK-deficient patients. Thus, corroborating the special role of antibody-mediated mechanisms in the control of systemic enteroviral disease [8, 11]. However, as enteroviral infection was not isolated from skin biopsy or synovial fluid, its role in the etiology of P1 dermatitis and arthritis remains unclear [12–14].
With the improved diagnosis and the prompt wide use of IVIG fewer patients develop enteroviral meningoencephalitis [14, 15]. However, once infection occurs, IVIG treatment often fails to achieve clinical remission [16–18]. High doses IVIG and Pleconaril were suggested for the treatment of enteroviral meningoencephalitis, although more reports need to be awaited [18, 19]. The two cases described here provide support for the notion that in BLNK patients agammaglobulinemia, absent B cells, low TFH cells and the infection profile closely resemble findings in BTK deficiency and so far no abnormalities due to the absent expression of the protein in cells other than B cells have been identified.
References
Conley ME, Dobbs AK, Farmer DM, Kilic S, Paris K, Grigoriadou S, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi:10.1146/annurev.immunol.021908.132649.
Minegishi Y, Rohrer J, Coustan-Smith E, Lederman HM, Pappu R, Campana D, et al. An essential role for BLNK in human B cell development. Science. 1999;286(5446):1954–7.
Lagresle-Peyrou C, Millili M, Luce S, Boned A, Sadek H, Rouiller J, et al. The BLNK adaptor protein has a nonredundant role in human B-cell differentiation. J Allergy Clin Immunol. 2014;134(1):145–54. doi:10.1016/j.jaci.2013.12.1083S0091-6749(14)00070-0.
Stepensky P, Keller B, Abuzaitoun O, Shaag A, Yaacov B, Unger S, et al. Extending the clinical and immunological phenotype of human Interleukin-21 receptor deficiency. Haematologica. 2014. doi:10.3324/haematol.2014.112508.
Martini H, Enright V, Perro M, Workman S, Birmelin J, Giorda E, et al. Importance of B cell co-stimulation in CD4(+) T cell differentiation: X-linked agammaglobulinaemia, a human model. Clin Exp Immunol. 2011;164(3):381–7. doi:10.1111/j.1365-2249.2011.04377.x.
Jumaa H, Bossaller L, Portugal K, Storch B, Lotz M, Flemming A, et al. Deficiency of the adaptor SLP-65 in pre-B-cell acute lymphoblastic leukaemia. Nature. 2003;423(6938):452–6. doi:10.1038/nature01608.
van Zelm MC, Geertsema C, Nieuwenhuis N, de Ridder D, Conley ME, Schiff C, et al. Gross deletions involving IGHM, BTK, or artemis: a model for genomic lesions mediated by transposable elements. Am J Hum Genet. 2008;82(2):320–32. doi:10.1016/j.ajhg.2007.10.011S0002-9297(08)00090-6.
Wilfert CM, Buckley RH, Mohanakumar T, Griffith JF, Katz SL, Whisnant JK, et al. Persistent and fatal central-nervous-system ECHOvirus infections in patients with agammaglobulinemia. N Engl J Med. 1977;296(26):1485–9. doi:10.1056/NEJM197706302962601.
Wagner DK, Marti GE, Jaffe ES, Straus SE, Nelson DL, Fleisher TA. Lymphocyte analysis in a patient with X-linked agammaglobulinemia and isolated growth hormone deficiency after development of echovirus dermatomyositis and meningoencephalitis. Int Arch Allergy Appl Immunol. 1989;89(2–3):143–8.
Bardelas JA, Winkelstein JA, Seto DS, Tsai T, Rogol AD. Fatal ECHO 24 infection in a patient with hypogammaglobulinemia: relationship to dermatomyositis-like syndrome. J Pediatr. 1977;90(3):396–9.
Rhoades RE, Tabor-Godwin JM, Tsueng G, Feuer R. Enterovirus infections of the central nervous system. Virology. 2011;411(2):288–305. doi:10.1016/j.virol.2010.12.014S0042-6822(10)00768-3.
Thyss A, el Baze P, Lefebvre JC, Schneider M, Ortonne JP. Dermatomyositis-like syndrome in X-linked hypogammaglobulinemia. Case-report and review of the literature. Acta Derm Venereol. 1990;70(4):309–13.
Rudge P, Webster AD, Revesz T, Warner T, Espanol T, Cunningham-Rundles C, et al. Encephalomyelitis in primary hypogammaglobulinaemia. Brain. 1996;119(Pt 1):1–15.
McKinney Jr RE, Katz SL, Wilfert CM. Chronic enteroviral meningoencephalitis in agammaglobulinemic patients. Rev Infect Dis. 1987;9(2):334–56.
Webster AD, Rotbart HA, Warner T, Rudge P, Hyman N. Diagnosis of enterovirus brain disease in hypogammaglobulinemic patients by polymerase chain reaction. Clin Infect Dis. 1993;17(4):657–61.
Misbah SA, Spickett GP, Ryba PC, Hockaday JM, Kroll JS, Sherwood C, et al. Chronic enteroviral meningoencephalitis in agammaglobulinemia: case report and literature review. J Clin Immunol. 1992;12(4):266–70.
Mease PJ, Ochs HD, Wedgwood RJ. Successful treatment of echovirus meningoencephalitis and myositis-fasciitis with intravenous immune globulin therapy in a patient with X-linked agammaglobulinemia. N Engl J Med. 1981;304(21):1278–81. doi:10.1056/NEJM198105213042107.
Quartier P, Foray S, Casanova JL, Hau-Rainsard I, Blanche S, Fischer A. Enteroviral meningoencephalitis in X-linked agammaglobulinemia: intensive immunoglobulin therapy and sequential viral detection in cerebrospinal fluid by polymerase chain reaction. Pediatr Infect Dis J. 2000;19(11):1106–8.
Schmugge M, Lauener R, Bossart W, Seger RA, Güngör T. Chronic enteroviral meningo-encephalitis in X-linked agammaglobulinaemia: favourable response to anti-enteroviral treatment. Eur J Pediatr. 1999;158(12):1010–1.
Acknowledgments
We would like to thank Howard Lederman MD, Ph.D from Johns Hopkins Hospital and Sara Sebnem Kilic, MD from Uludag University, School of Medicine, Bursa, Turkey, for providing clinical follow up regarding their patients.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Naser Eddin, O. Shamriz and B. Keller contributed equally in the making of this manuscript and should all be considered as “first authors” .
K. Warnatz, O. Elpeleg, and P. Stepensky contributed equally in the making of this manuscript and should all be considered as “last authors”
Rights and permissions
About this article
Cite this article
NaserEddin, A., Shamriz, O., Keller, B. et al. Enteroviral Infection in a Patient with BLNK Adaptor Protein Deficiency. J Clin Immunol 35, 356–360 (2015). https://doi.org/10.1007/s10875-015-0164-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-015-0164-2