Abstract
Immunoglobulin G (IgG) molecules are glycoproteins and residues in the sugar moiety attached to the IgG constant fragment (Fc) are essential for IgG functionality such as binding to cellular Fc receptors and complement activation. The core of this sugar moiety consists of a bi-antennary heptameric structure of mannose and N-acetylglucosamine (GlcNAc), further decorated with terminal and branching residues including galactose, sialic acid, fucose, and GlcNAc. Presence or absence of distinct residues such as fucose and sialic acid can dramatically alter pro- and anti-inflammatory IgG activities which could be harnessed for immunotherapeutic purposes. Here we review recent advances in understanding the role of the IgG-Fc glycan during immune responses and for immunotherapy with a focus on sialic acid and intravenous immunoglobulin (IVIG) treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Immunoglobulin G Effector Functions
Immunoglobulins belong to the most abundant proteins found in serum [3]. Producing these vast amounts of proteins is a huge investment of the body with good cause: Antibodies are an essential component of the immune system bridging humoral and cellular immunity to fulfill inflammatory (assisting the killing of infected cells or tumor cells), non-inflammatory (obsonization, neutralization) and, as most recently recognized, also anti-inflammatory functions. Immunoglobulin G (IgG) represents 75 % of the immunoglobulins in serum reflecting its role in defending us against bacteria and viruses which have penetrated epithelial and mucosal barriers [1]. During a primary infection and if innate immunity and pre-existing low-affinity antibodies (natural antibodies) fail to neutralize the pathogen, immunoglobulins increase the visibility of the pathogen or the infected cell through opsonization and initiate immune responses through binding to cellular receptors specific for the antibodies’ Fc region (Fcγ receptors; FcγRs) and the activation of the complement system. This activates phagocytes such as macrophages and dendritic cells which engulf, destroy, process and transport the pathogen to secondary lymphoid organs for the initiation of a protective adaptive immune response. Furthermore, infected cells can be lysed by a mechanism called “antibody-dependent cell mediated cytotoxicity” (ADCC) by which FcγR-receptor bearing innate immune cells such as natural killer cells lyse antibody-opsonized cells [53]. Similar to Fc-receptor expressing cells, the complement system uses antibodies as specific detectors to fulfill its dual role in both eradicating pathogens and infected cells by complement-dependent cytotoxicity (CDC) and simultaneously assisting their uptake by phagocytes for antigen presentation [1]. The four different subclasses of IgG molecules differ in their efficacy to initiate immune cell activation through FcγR binding and complement activation.
The IgG-Fc N-Glycan
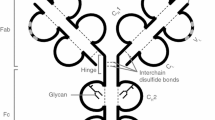
Immunoglobulin G (IgG) molecules are glycoproteins with a sugar moiety attached to each of the asparagin 297 (N297) residues in the CH2-domains of the two Fc-fragments (Fig. 1). Removal of this sugar moiety impairs Fc-dependent effector functions [14, 38]. All human immunoglobulins are Fc-glycosylated and can, depending on the isotype and the sequence of the antigen-binding regions (complementary determining regions, CDR), carry additional glycans in the Fab domains. IgG is unique with respect to a single, highly conserved Asparagine(N)-Glycosylation site (N-X-S/T) in the CH2 domains while the isotypes carry multiple glycosylation sites [7]. Moreover, in contrast to other Ig isotypes, the IgG-associated sugar domain is not exposed on the IgG surface but rather buried within the hydrophobic core between the two Fc-fragments and, therefore, impacts Fc-structure [30, 36]. During protein translation a pre-formed lipid-linked glycan is transfered and covalently attached to asparagine at position 297 in the lumen of the endoplasmatic reticulum (ER). This initial glycan is composed of two N-acetylglucosamines (GlcNAc) followed by branched mannose (Man) residues. Its structure is highly conserved in eukaryotes and serves as an important mechanism for protein folding and quality control of proteins carrying N-glycans [48]. If successfully produced and folded, the IgG polypeptide is transfered from the ER to the Golgi where glycosyl-hydrolases and -transferases can modify the core glycan leading to such diverse and highly complex glycans as seen in the IgG-Fc. The monosaccharide composition of the glycan is impacted by the amino-acid sequence [29] as well as glycan modifying enzyme- and substrate availability. The presence of glycosylhydrolases and glycosyltransferases in the golgi can be regulated at the level of transcription and location within the secretory pathway [43]. Peptide-glycan interactions and the three-dimensional structure of the Fc are thought to limit the glycan variability and the extent of galactosylation and sialylation by limiting the accessability of the glycan to glycosyltransferases [29, 57]. A total of around 30 different glycan structures is found in the IgG-Fc pool [41]. Compared to the Fab glycans, reduced bisecting GlcNAc, less galactose and less sialylation is found on the Fc glycans [57]. The restricted diversity may be essential for the use of specific IgG-Fc glycan modification for the regulation of antibody effector functions. How and which immunological factors such as cytokines influence antibody glycosylation is yet to be defined but much progress has been made in understanding the structural and functional consequences of the presence or absence of different sugar moieties on the IgG-Fc carbohydrate. In addition to the oligosaccharide core, more than 95 % of the biantennary complex type structure of the final IgG glycan carries a N-acetylglucosamine on both arms [40, 41] and 85 % are fucosylated [34] (Fig. 1). In contrast, the presence of galactose is less homogenous with 40 % of glycans carrying one galactose (G1 glycan) and the frequency of non-galactosylated (G0) or bi-galactosylated glycans (G2) ranging between 20 and 40 % depending on age and gender [40, 51]. Additional heterogeneity is conferred by the addition of a bisecting N-acetylglucosamine present on approximately 10 to 15 % of total IgG-Fc glycans [58].
IgG-Fc glycan structure. The Asparagine 297 linked complex-type glycan found on the Fc domain of immunoglobulin G antibodies consists of a set of constant (depicted bold) and variable (depicted regular) sugars. Although theoretically possible, this fully processed glycan will be found only in trace amounts as the majority of antibodies will carry either no, one or two galactose residues and a fraction of those carrying galactose will additionally possess sialic acid. Further complexity is added by the addition of the bisecting N-acetylglucosamine. Man Mannose, Neu5Ac Neuraminic acid (Sialic acid), Gal Galactose, GlcNAc N-acetylglucosamine, Fuc Fucose, N Asparagine
Based on the structure of the glycan, the conformation of the IgG-Fc homodimer in the binding interface to Fc-receptors and the complement protein C1q (CH2 domain) changes from an open (maximal C2-C2 domain distance) to a closed conformation (minimal C2-C2 domain distance) [16, 33]. Analysis of crystal structures revealed that removal of terminal galactose and N-acetylglucosamine residues favours a closed conformation which in turn disfavours Fc-receptor and C1q binding and impacts on the thermal stability [28, 32, 33]. The most distal sugar on the glycan is sialic acid (neuraminic acid, Neu5Ac). Around 10 % of glycans carry sialic acid on one arm and bi-sialylated glycans are found only in trace amounts. The linkage of sialic acid to the penultimate galactose is found in either α2-3 or α2-6 confirmation with a strong preference for the latter [7]. Addition of galactose has a strong preference for the 1–6 arm, whereas sialic acid is almost exclusively found on the 1–3 arm [57]. While the 1–6 arm bias of galactose may be largely explained by accessability [57] the 1–3 arm specific addition of sialic acid by human sialyltransferase is retained even in vitro using the released Fc glycan, substrate and recombinant human sialyltransferase arguing for an enzyme-intrinsic bias [8]. The opposing glycan arm-bias of galactose and sialic acid provides a rationale for the inefficient addition of sialic acid. The functional consequence of sialylation may be reduced FcγR binding [45] and enhancing the anti-inflammatory properties of IgG molecules [6] as discussed below.
Function of Fc N-glycan Fucose-Residues
The majority of circulating IgG is fucosylated [34], which has been shown to reduce the binding to the activating FcγRIII (CD16) and consequently antibody-dependent cell mediated cytotoxicity [50]. Direct carbohydrate-carbohydrate interactions between the FcγRIII Asn162 linked glycan and the Fc glycan are responsible for this effect [15]. The in vivo relevance of increased antibody binding to CD16 was first indicated in patients carrying a high affinity receptor polymorphism which increased the efficacy of the B cell depleting antibody Rituximab [10]. Similarly, clinical trials using non-fucosylated antibodies for cell depletion therapy in cancer have shown promising results [24, 37]. Largely reduced levels of fucosylated IgG have been reported on alloantigen-specific (anti-HPA1a) antibodies in neonatal alloimmune thrombocytopenia (FNAIT) in contrast to normal levels in the total antibody pool suggesting specific regulation of fucosylation and a potential role in autoimmunity [26].
Function of Fc N-glycan Galactose-Residues
The absence of galactose in the core glycan (IgG-G0) has long been known to be associated with rheumatoid arthritis (RA) and primary osteoarthritis [41]. Similar observations were made in other autoimmune diseases, infectious diseases and cancer [20, 49]. The first suggested mechanism for a functional consequence of galactosylation was based on the in vitro finding that galactosylation prevents binding of the initiator of the lectin pathway of the complement system, mannose binding lectin (MBL) [31]. However, in vivo studies in mouse models could not confirm a role of MBL for the function of agalactosylated antibodies [35] and analysis of patients carrying MBL promotor polymorphisms leading to differential MBL expression level could not find a role for MBL in RA [55, 56]. A dual role for MBL was found in juvenile RA where MBL deficiency led to younger age of juvenile polyarthritis onset but more frequent remission of juvenile oligoarthritis [13]. A recent study in mice shows that antibody galactosylation can contribute to limiting anaphylatoxin C5a mediated complement-activation by a mechanism involving Dectin-1 and FcγRIIb dependent inhibition of C5a receptor signaling [27]. The observation that antiviral activity and spontaneous control of HIV infection is associated with increased prevalence of total and in particular antigen-specific IgG-G0 antibodies additionally argues for a functional significance of antibody galactosylation in humans [2]. An important aspect to consider when looking at the functional impact of galactose is that it provides the basis for the addition of sialic acid, the most distal sugar moiety on the IgG-Fc glycan.
Function of Fc N-glycan Sialic Acid-Residues
In mice, T cell independent B cell activation or tolerogenic immunization (in the absence of pro-inflammatory stimuli) with protein antigens induces antigen-specific IgG with high levels of terminal galactose and sialic acid capable of blocking antigen-specific B and T cell responses [19, 39]. Similarly, successful treatment of birch pollen allergy was found to induce antigen-specific IgG partially carrying sialic acid [39]. The mechanisms that mediate such anti-inflammatory properties are not yet clear but may involve reduced binding to Fc receptors [25] or sialic acid binding lectins such as DC-SIGN [4].
The Role of IgG Fc-glycosylation for IVIG Treatment of Autoimmunity
The anti-inflammatory effect of pooled high-dose gammaglobulins from healthy donors infused in patients (so-called intravenous immunoglobulin, IVIG) suffering from autoimmune diseases was first recognized during immunoglobulin replacement therapy of a patient suffering hypogammaglobulinemia and immune thrombocytopenia (ITP) [21–23]. Subsequently, IVIG has been approved for the treatment of the autoimmune diseases ITP, Kawasaki’s disease, chronic inflammatory demyelinating polyneuropathy (CIDP) and multifocal motor neuropathy but is used for many other neuromuscular, hematologic or dermatologic disorders [17]. Numerous mechanisms for the anti-inflammatory action of IVIG have been suggested. Examples therefore are ranging from increased clearance of pathologic antibodies due to the saturation of the neonatal Fc-receptor [18], anti-idiotypic antibodies [52] and suppression of dendritic cell maturation [9] to the activation of regulatory T cells via Fc-derived peptides [11]. Studies in mouse models [5, 44] and humans [12] clearly point towards a major role for the IgG-Fc for the anti-inflammatory properties of IVIG. Because an extremely high dose of IVIG is required for its efficacy (1–2 g per kg), it was speculated that the beneficial effect may be mediated by a small sub-fraction of IVIG. Ravetch and colleagues identified sialylated Fc fragments as the anti-inflammatory mediator of IVIG in the K/N experimental arthritis mouse model [5, 25]. Subsequent studies implicated a protective mechanism involving the inhibitory Fc receptor (FcγRIIb) and the C-type lectin SIGNR1 or its human ortholog DC-SIGN [4, 46]. Signaling downstream of DC-SIGN was found to induce IL-33 which in turn induces IL-4 in basophils leading to the upregulation of FcγRIIb on macrophages [4]. The requirement of sialic acid and FcγRIIb has been recently confirmed in four mouse models of antibody-mediated autoimmune diseases whereas SIGNR1 was not always required [47]. Although the relevance of DC-SIGN (or SIGNR1) for the initiation of a sialic-acid specific response is supported by several studies [4, 5, 46], it remains unclear whether direct binding of Fc-linked sialic acid to these receptors [6] is mandatory as no binding of IgG-Fc (independent of its glycosylation) to DC-SIGN was detected by others [59].
Studies in humans support a role for FcγRIIb expression as being associated with the clinical efficacy of IVIG therapy. Chronic inflammatory demyelinating polyneuropathy (CIDP) is the most common treatable acquired chronic polyneuropathy, and IVIG is widely used as a first-line initial and maintenance treatment. Untreated patients with CIDP, compared with demographically matched healthy controls, showed consistently lower FcγRIIb expression levels. FcγRIIb expression was, however, up-regulated on circulating monocytes and B cells after clinically effective IVIG therapy [54]. Preliminary data from our laboratory suggest that the frequency of sialic acid-containing IgG Fc-glycoforms is associated with disease remission (unpublished observation).
Thus, there is accumulating evidence that both FcγRIIb and sialic acid play a major role for the anti-inflammatory effect of IVIG but depending on the experimental conditions, other yet to be defined mechanisms might have additional Fc-glycan and FcγRIIb-independent effects. It is important to consider that all available data on the requirement of sialic acid for IVIG efficacy have been generated in mouse models infused with human IVIG (or modified derivatives thereof). Furthermore, all animal models that show a dependency on sialic acid involve the transfer of serum or pathogenic autoantibodies [4, 25, 46, 47], whereas expansion of protective regulatory T cells as well as augmentation of their effector function during HSV-induced encephalitis was found to be independent of sialylation [42]. Studies in humans and complementary humanized animal models are, therefore, mandatory to better define the role of Fc-glycosylation and the requirements for anti-inflammatory IgG activity.
References
Abbas AK, Lichtman AH, Pillai S. Cellular and molecular immunology. 6th ed. Philadelphia: Saunders (Elsevier); 2007.
Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest. 2013;123:2183–92.
Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–67.
Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–3.
Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–6.
Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A. 2008;105:19571–8.
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50.
Barb AW, Brady EK, Prestegard JH. Branch-specific sialylation of IgG-Fc glycans by ST6Gal-I. Biochemistry. 2009;48:9705–7.
Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. 2003;101:758–65.
Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8.
De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. 2008;112:3303–11.
Debre M, Bonnet MC, Fridman WH, Carosella E, Philippe N, Reinert P, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. 1993;342:945–9.
Dolman KM, Brouwer N, Frakking FN, Flato B, Tak PP, Kuijpers TW, et al. Mannose-binding lectin deficiency is associated with early onset of polyarticular juvenile rheumatoid arthritis: a cohort study. Arthritis Res Ther. 2008;10:R32.
Duncan AR, Winter G. The binding site for C1q on IgG. Nature. 1988;332:738–40.
Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–74.
Gaboriaud C, Juanhuix J, Gruez A, Lacroix M, Darnault C, Pignol D, et al. The crystal structure of the globular head of complement protein C1q provides a basis for its versatile recognition properties. J Biol Chem. 2003;278:46974–82.
Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 2012;367:2015–25.
Hansen RJ, Balthasar JP. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thromb Haemost. 2002;88:898–9.
Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J Clin Invest. 2013;123:3788–96.
Huhn C, Selman MH, Ruhaak LR, Deelder AM, Wuhrer M. IgG glycosylation analysis. Proteomics. 2009;9:882–913.
Imbach P. Immune thrombocytopenic purpura and intravenous immunoglobulin. Cancer. 1991;68:1422–5.
Imbach P, Barandun S, Baumgartner C, Hirt A, Hofer F, Wagner HP. High-dose intravenous gammaglobulin therapy of refractory, in particular idiopathic thrombocytopenia in childhood. Helv Paediatr Acta. 1981;36:81–6.
Imbach P, Barandun S, d’Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981;1:1228–31.
Junttila TT, Parsons K, Olsson C, Lu Y, Xin Y, Theriault J, et al. Superior in vivo efficacy of afucosylated trastuzumab in the treatment of HER2-amplified breast cancer. Cancer Res. 2010;70:4481–9.
Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–3.
Kapur R, Kustiawan I, Vestrheim A, Koeleman CA, Visser R, Einarsdottir HK, et al. A prominent lack of IgG1-Fc fucosylation of platelet alloantibodies in pregnancy. Blood. 2014;123:471–80.
Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nat Med. 2012;18:1401–6.
Krapp S, Mimura Y, Jefferis R, Huber R, Sondermann P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J Mol Biol. 2003;325:979–89.
Lund J, Takahashi N, Pound JD, Goodall M, Jefferis R. Multiple interactions of IgG with its core oligosaccharide can modulate recognition by complement and human Fc gamma receptor I and influence the synthesis of its oligosaccharide chains. J Immunol. 1996;157:4963–9.
Lux A, Nimmerjahn F. Impact of differential glycosylation on IgG activity. Adv Exp Med Biol. 2011;780:113–24.
Malhotra R, Wormald MR, Rudd PM, Fischer PB, Dwek RA, Sim RB. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;1:237–43.
Mimura Y, Church S, Ghirlando R, Ashton PR, Dong S, Goodall M, et al. The influence of glycosylation on the thermal stability and effector function expression of human IgG1-Fc: properties of a series of truncated glycoforms. Mol Immunol. 2000;37:697–706.
Mimura Y, Sondermann P, Ghirlando R, Lund J, Young SP, Goodall M, et al. Role of oligosaccharide residues of IgG1-Fc in Fc gamma RIIb binding. J Biol Chem. 2001;276:45539–47.
Mizuochi T, Taniguchi T, Shimizu A, Kobata A. Structural and numerical variations of the carbohydrate moiety of immunoglobulin G. J Immunol. 1982;129:2016–20.
Nimmerjahn F, Anthony RM, Ravetch JV. Agalactosylated IgG antibodies depend on cellular Fc receptors for in vivo activity. Proc Natl Acad Sci U S A. 2007;104:8433–7.
Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:513–33.
Niwa R, Shoji-Hosaka E, Sakurada M, Shinkawa T, Uchida K, Nakamura K, et al. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004;64:2127–33.
Nose M, Wigzell H. Biological significance of carbohydrate chains on monoclonal antibodies. Proc Natl Acad Sci U S A. 1983;80:6632–6.
Oefner CM, Winkler A, Hess C, Lorenz AK, Holecska V, Huxdorf M, et al. Tolerance induction with T cell-dependent protein antigens induces regulatory sialylated IgGs. J Allergy Clin Immunol. 2012;129:1647–55. e1613.
Parekh R, Roitt I, Isenberg D, Dwek R, Rademacher T. Age-related galactosylation of the N-linked oligosaccharides of human serum IgG. J Exp Med. 1988;167:1731–6.
Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316:452–7.
Ramakrishna C, Newo AN, Shen YW, Cantin E. Passively administered pooled human immunoglobulins exert IL-10 dependent anti-inflammatory effects that protect against fatal HSV encephalitis. PLoS Pathog. 2011;7:e1002071.
Rich JR, Withers SG. Emerging methods for the production of homogeneous human glycoproteins. Nat Chem Biol. 2009;5:206–15.
Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6.
Scallon BJ, Tam SH, McCarthy SG, Cai AN, Raju TS. Higher levels of sialylated Fc glycans in immunoglobulin G molecules can adversely impact functionality. Mol Immunol. 2007;44:1524–34.
Schwab I, Biburger M, Kronke G, Schett G, Nimmerjahn F. IVIg-mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur J Immunol. 2012;42:826–30.
Schwab I, Mihai S, Seeling M, Kasperkiewicz M, Ludwig RJ, Nimmerjahn F. Broad requirement for terminal sialic acid residues and FcgammaRIIB for the preventive and therapeutic activity of intravenous immunoglobulins in vivo. Eur J Immunol. 2014.
Schwarz F, Aebi M. Mechanisms and principles of N-linked protein glycosylation. Curr Opin Struct Biol. 2011;21:576–82.
Selman MH, Niks EH, Titulaer MJ, Verschuuren JJ, Wuhrer M, Deelder AM. IgG fc N-glycosylation changes in Lambert-Eaton myasthenic syndrome and myasthenia gravis. J Proteome Res. 2011;10:143–52.
Shields RL, Lai J, Keck R, O’Connell LY, Hong K, Meng YG, et al. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human Fcgamma RIII and antibody-dependent cellular toxicity. J Biol Chem. 2002;277:26733–40.
Shikata K, Yasuda T, Takeuchi F, Konishi T, Nakata M, Mizuochi T. Structural changes in the oligosaccharide moiety of human IgG with aging. Glycoconj J. 1998;15:683–9.
Shoenfeld Y, Rauova L, Gilburd B, Kvapil F, Goldberg I, Kopolovic J, et al. Efficacy of IVIG affinity-purified anti-double-stranded DNA anti-idiotypic antibodies in the treatment of an experimental murine model of systemic lupus erythematosus. Int Immunol. 2002;14:1303–11.
Sun PD. Structure and function of natural-killer-cell receptors. Immunol Res. 2003;27:539–48.
Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci U S A. 2009;106:4788–92.
van de Geijn FE, de Man YA, Wuhrer M, Willemsen SP, Deelder AM, Hazes JM, et al. Mannose-binding lectin does not explain the course and outcome of pregnancy in rheumatoid arthritis. Arthritis Res Ther. 2011;13:R10.
van de Geijn FE, Hazes JM, Geleijns K, Emonts M, Jacobs BC, Dufour-van den Goorbergh BC, et al. Mannose-binding lectin polymorphisms are not associated with rheumatoid arthritis–confirmation in two large cohorts. Rheumatology (Oxford). 2008;47:1168–71.
Wormald MR, Rudd PM, Harvey DJ, Chang SC, Scragg IG, Dwek RA. Variations in oligosaccharide-protein interactions in immunoglobulin G determine the site-specific glycosylation profiles and modulate the dynamic motion of the Fc oligosaccharides. Biochemistry. 1997;36:1370–80.
Yamada E, Tsukamoto Y, Sasaki R, Yagyu K, Takahashi N. Structural changes of immunoglobulin G oligosaccharides with age in healthy human serum. Glycoconj J. 1997;14:401–5.
Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. 2013;425:1253–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quast, I., Lünemann, J.D. Fc Glycan-Modulated Immunoglobulin G Effector Functions. J Clin Immunol 34 (Suppl 1), 51–55 (2014). https://doi.org/10.1007/s10875-014-0018-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-014-0018-3