Abstract
Purpose
Guillain-Barré syndrome (GBS) is an acute post-infectious immune-mediated demyelinating disease of the peripheral nervous system. Th17 cells and osteopontin (OPN) have been implicated in the development of autoimmune diseases, but little is known about their relationship and roles in the pathogenesis of GBS.
Methods
In this study, we used flow cytometry to evaluate peripheral numbers of Th17, real-time polymerase chain reaction to assay mRNA expression of RORγt, and enzyme-linked immunosorbent assay to determined OPN and IL-17 concentrations.
Results
The frequency of Th17 cells was significantly higher in the peripheral blood of acute-stage GBS patients comparison with other non-inflammatory neurological diseases (OND). In line with these observations, the levels of mRNA expression of RORγt in peripheral blood mononuclear cells and the concentrations IL-17 in both plasma and cerebrospinal fluid (CSF) were significantly higher in the acute-stage GBS than stable-stage GBS. OPN concentrations were significantly increased in the CSF of acute-stage GBS patients compared to OND. Circulating Th17 cell populations and CSF OPN levels, respectively, are correlated with GBS disability scale scores (GDSs), and there was a positive correlation between them.
Conclusion
In summary, our preliminary findings suggest that both Th17 and OPN may be associated with the pathogenesis of GBS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Guillain-Barré syndrome (GBS) is a group of acute, monophasic, and pathophysiologically heterogenous neuropathic disorders triggered frequently by common infections of upper respiratory and gastrointestinal tracts in a susceptible host [1]. According to the electrodiagnostic examination, GBS is currently classified into two primary subtypes: acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor axonal neuropathy (AMAN) [2]. Although the immunological mechanisms underlying the disease have not yet been completely understood, there is strong evidence proving that both humoral and cellular immune responses are involved in the pathogenesis of GBS. Some studies suggests that the AIDP subtype is predominantly caused by T cells directed against peptides from the myelin proteins P0, P2, and PMP22 [3], and the AMAN subtype is caused by antibodies to gangliosides on the axolemma that target macrophages to invade the axon at the node of Ranvier [4]. Elevated serum and/or cerebrospinal fluid levels of pro-inflammatory cytokines, such as TNF-α, were detected in patients with GBS during active disease and were correlated with disease activity [5]. In addition, we found that the number and proportion of CD4+CD25+ Treg cells, which are critical in maintaining immunologic homeostasis and preventing autoimmunity, were significantly reduced in acute-stage GBS patients [6]. Knowledge of the etiology and the pathogenetic steps in immune-mediated peripheral neuropathy that lead to demyelination and axonal damage is rapidly growing but still incomplete.
More recently, a novel CD4+ T cell subset termed Th17 cells has been identified, which associated with retinoic acid receptor-related orphan receptor (ROR) and signal transducer and activator of transcription 3 (STAT3) transcriptional factors in addition to an extensive network of pro-inflammatory cytokines (including interleukin IL-17, IL-6, IL-1, and IL-23) [7, 8]. Various studies have emerged suggesting that Th17 cells may be a potent inducers in autoimmune diseases through enhancing the recruitment and facilitating the activation of neutrophils, stimulating the production of chemokines and other inflammatory cytokines, and promoting the synthesis of tissue-damaging proteases [9]. Differentiation of naive T cells towards a Th17 phenotype is supported by several cytokines including transforming growth factor-β (TGF-β), IL-1β, IL-6, IL-21, and IL-23 in mice and humans [10–12]. It was recently demonstrated that Th17 cells are dominantly associated with human and mouse autoimmune diseases such as rheumatoid arthritis (RA), multiple sclerosis (MS), inflammatory bowel disease (IBD), and systemic lupus erythematosus (SLE) [13–16]. To date, the characteristics and roles of Th17 cells in patients with GBS have been poorly defined. Li and colleagues reported an increase in the concentration of IL-17 in the CSF and plasma of untreated GBS patients compared with healthy controls [17]. Another study reported unstimulated GBS CD4+ T cells and GBS CD4+ T cells stimulated with anti-CD3 and CD28 mAbs had higher relative RORγt mRNA expression compared to controls [18]. All of these findings implied the possibility that Th17 cells play a role in the pathogenesis of GBS.
OPN, also called ‘early T cell–activation gene 1’ [19], is a negatively charged acidic hydrophilic protein that is produced by various cell types and participates in diverse physiological and pathological processes, such as bone mineralization, oxidative stress, remyelination, wound healing, inflammation and immunity [20–22]. It has been well studied that interactions between OPN and its receptors (αvβ3, α5β1 and CD44) mediated survival, migration and adhesion in many types of cells [23, 24]. As a pro-inflammatory mediator, OPN plays a role in the progression of autoimmune diseases through various mechanisms, including involving in generation of Th1 and Th17 cells that are pathogenic T cells for autoimmune diseases [25–27], inhibiting apoptosis of autoreactive immune cells and recruitment of leukocytes to sites of inflammation [28]. MS and its animal models, experimental autoimmune encephalomyelitis (EAE), are certainly the CNS disease in which the role of OPN has been investigated to a greater extent. The expression of OPN were elevated in the brains of rats with EAE but not in brains of rats protected from EAE [29], and severity of EAE was significantly reduced in OPN deficient mice [30]. In concordance with those findings in animal models, OPN transcripts were frequently detected and were exclusive to the MS mRNA population, but not found in control brain mRNA [30]. However, the role and expression of OPN in patients with GBS, an acquired neuropathy caused by immune-mediated damage to peripheral nerves, remain unclear.
To address the reciprocal relationship of Th17 and OPN and their possible role in the pathogenesis of GBS, we investigated the frequency of ex vivo IL-17-producing CD4+ T cells in peripheral blood and the concentrations of OPN in plasma and CSF from patients with GBS. The results showed that both the increased frequency of Th17 and elevated OPN CSF concentrations were closely associated with the grades on the functional scale which reflects the severity of inflammation in the spinal roots.
Materials and Methods
Study Population
We recruited 51 patients fulfilling international criteria for GBS or its variants [31] from the Medical University of Harbin between 2009 and 2012. The mean age of GBS patients (22 males and 29 females) was 35.3 ± 10.9 years (age range 18–61 years). Patients were classified neurophysiologically as AIDP (n = 24) and AMAN (n = 27), using motor nerve conduction criteria [32]. Severity of GBS was scored as the patients worst deficits at the peak of their illness using a functional disability scale [33]. At the time of sampling, none of the patients had received any immunomodulatory drugs within 3 months. CSF and blood samples were collected on the same day. Patients with chronic-immune-mediated disorders were excluded. The characteristics of all subjects are shown in Table I. Twenty patients with other non-inflammatory neurologic diseases (OND; 11 females and 9 males, age 21–60 years, mean age 34.4 ± 10.1 years) were also enrolled in the study. These patients with the following conditions: 14 chronic intractable headache and 6 normal pressure hydrocephalus. Plasma samples were also obtained from 20 healthy control subjects (HC), age- and sex matched with the patient (10 females and 10 males, age 20–59 years, mean age 34.7 ± 12.5 years). Ethical approval was obtained and informed consent was obtained from all patients and healthy controls. The first peripheral blood and CSF sample was obtained prior to the start of intravenous immunoglobulin treatment, within 2 weeks after the onset of neuropathic symptoms. After standard dose intravenous immunoglobulin (IVIG), 0.4 g/kg per day for 5 days, was given to all patients, most patients showed satisfactory improvement of neuropathic symptoms. The second blood samples were available from 31 patients (16 AMAN and 15 AIDP) more than 4 weeks after the first sample.
Sample Preparations
PBMCs were obtained by standard Ficoll–Hypaque density centrifugation of heparinized peripheral blood obtained from the subjects. They were then harvested by pipetting cells from the Ficoll/serum interface and washed twice. Plasma was obtained after centrifugation and stored at −80 °C for measurement of cytokine levels.
After lumbar puncture, CSF samples (10–12 ml) were obtained and collected in polypropylene tubes. The samples were centrifuged at 2,000 g at 4 °C for 10 min to eliminate cells and other insoluble material, and were then immediately frozen and stored at −80 °C pending biochemical analyses, without being thawed or refrozen. Cell count was performed on the CSF samples and no sample contained more that 500 erythrocytes/μl.
Flow Cytometric Analysis
Heparinized peripheral whole blood (400 μl) with an equal volume of complete culture medium (RPMI 1640 supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin, 2 mM glutamine and with 10 % heat-inactivated fetal calf serum, Gibco BRL) were incubated for 4 h at 37 °C, 5 % CO2 in the presence of 50 ng/ml of phorbol myristate acetate (PMA), 1 μM of ionomycin, and 500 ng/ml of monensin (all from Alexis Biochemicals, San Diego, CA). PMA and ionomycin are pharmacological T-cell-activating agents that mimic signals generated by the T-cell receptor (TCR) complex and have the advantage of stimulating T cells of any antigen specificity. Monensin was used to block intracellular transport mechanisms, thereby leading to an accumulation of cytokines in the cells. After 4 h of culture, Cells were first stained extracellularly with combinations of phycoerythrin (PE) anti-human CD4 (eBioscience, San Diego, CA) at 4 °C for 20 min. Following surface staining, the cells were fixed and permeabilized according to the manufacturer’s instructions, and then stained with FITC-conjugated IL-17A (BioLegend, San Diego, CA) for Th17 detection, PerCPCy5.5-conjugated IFN-γ (BioLegend, San Diego, CA) for Th1 detection. Isotype controls were treated to enable correct compensation and confirm antibody specificity. Flow cytometric analysis was performed on a fluorescence activated cell sorter (FACS) Calibur cytometer. Data processing was performed with CellQuest software (Becton Dickinson, San Jose, CA, USA).
Enzyme-Linked Immunosorbent Assays for Cytokines
Plasma and CSF OPN and IL-17 concentrations were determined quantitatively using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) and according to the manufactory’s introduction. To determine the concentrations of OPN, serum and CSF samples were diluted respectively 1:10 and 1:25. Briefly, 96-well microtiter plates were precoated overnight at 4 °C with 2 μg per well of respective mouse capturing monoclonal antibody in phosphate buffered saline (PBS). Wells were then blocked at 37 °C for 2 h with 2 % bovine serum albumin and washed 3 times with cold washing solution. Each sample and its control were added to the indicated wells and incubated for 2 h with a biotinylated detecting antibody. Plates were washed and incubated for 30 min with streptavidin-conjugated horseradish peroxidase prior to color development. Optical densities were measured at 450 nm with reference wavelength set at 590 nm. The detection limits for all cytokines were <15 pg/ml in all assays.
RORγt Expression Determined by Real Time-PCR
RORγt mRNA expression was quantified by real-time PCR using ABI PRISM 7700 Sequence Detector (Applied Biosystems, Foster City, CA, USA). The human housekeeping gene β-actin primers and probe set was used as a reference for sample normalization. Total RNA isolated from PBMCs was reverse-transcribed into cDNA using random hexamer primers. The following primer pairs were used: RORγt, F: 5′-GCAATGGAAGTGGTGCTGGTT-3′, R: 5′-AGGATGCTTTGGCGATGAGTC-3′. β-actin, F: 5′-ATCTGCTGGAAGGTGGACAGCGA-3′, R: 5′-CCCAGCACAATGAAGATCAAGATCAT −3′.
The primers and probes used in the real-time PCR were ordered from Sangon (Shanghai, China) and designed not to amplify genomic DNA. Standard curves were generated from serial dilutions of purified plasmid DNA encoding the respective genes with a linear regression R greater than 0.99 and used to quantify mRNA copy numbers for each sample. The amplification protocol used was described as follows: 1 ml of synthesized cDNA product was subsequently added into PCR mix containing 25 ml of TaqMan 2 × PCR master mix (Applied Biosystems), 30 pmol human RORγt primer with 10 pmol probe, 2.5 ml -actin primer/probe set, and distilled water was added to make a total reaction volume of 50 ml. The PCR was programmed as an initial incubation for 10 min at 95 °C followed by 40 thermal cycles of 15 s at 95 °C and 1 min at 60 °C. The normalized values in each sample were calculated as the relative quantity of RORγt mRNA expression divided by the relative quantity of β-actin mRNA expression. All reactions were confirmed by at least one additional independent run.
Statistical Analysis
Normally distributed data sets were analysed by Student’s t-test, paired t-test, analysis of variance (anova) and linear regression and correlation analysis (using ‘Primer for Biostatistics’). P < 0.05 was considered significant.
Results
Acute-Stage GBS Patients Have an Increased Frequency of Th17 Cells in Peripheral Blood
For flow cytometric analysis, lymphocytes were first gated after stimulating for 4 h with PMA and ionomycin. Then cells were gated on CD4+ lymphocytes and studied for expression of IL-17. Th17 cells were identified as IL-17+IFN-γ− cells (Fig. 1). The population of Th17 cell subset as a percentage of total CD4+ cells was evaluated by flow cytometric analysis.
Strategy for the analysis of Th17 lymphocytes. Dot plots shown are representative of one healthy volunteer (a) and one patient with Guillain-Barré syndrome (b). Lymphocytes were first gated after heparinized peripheral whole blood from all subjects stimulated for 4 h ex vivo with phorbol myristate acetate (PMA) and ionomycin in the presence of monensin. Then cells were gated on CD4+ lymphocytes and studied for expression of IL-17. Th17 cells were identified as IL-17+IFN-γ− cells and the numbers in each compartment represent the percentage of Th17 cells in CD4+ positive cells
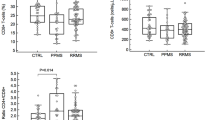
The percentage of Th17 in healthy controls(1.04 ± 0.43 %, n = 20) and ONDs (0.99 ± 0.33 %, n = 20) did not differ significantly. The percentage of Th17 from acute-stage AMAN (AMANa; 2.15 ± 0.45 %, n = 27, p < 0.001) or acute-stage AIDP (AIDPa; 1.99 ± 0.47 %, n = 24, p < 0.001) was significantly increased comparing with the healthy controls. No statistical difference was observed between patients with AMANa and AIDPa. Interestingly, there was a significantly decrease in the percentage of Th17 cells in patients with stable AMAN (AMANs; 1.12 ± 0.51 %, n = 16, p < 0.001) or stable AIDP(AIDPs; 1.21 ± 0.47 %, n = 15, p < 0.001) after they were treated with intravenous immunoglobulin (Fig. 2a). These results demonstrate that, in GBS patients, the frequency of circulating Th17 cells was significantly increased at acute stage and decreased to normal when patient’s condition was stable after treatment with intravenous immunoglobulin.
Acute-stage GBS patients have increased circulating Th17 cell populations and expression levels of RORγt. a Frequency of Th17 cells in peripheral blood of patients with acute-stage AMAN (AMANa, n = 27), acute-stage AIDP (AIDPa, n = 24), stable-stage AMAN (AMANs, n = 16), stable-stage AIDP (AIDPs, n = 15), OND (n = 20) and HC (n = 20). The horizontal lines represent the mean values of the groups, and asterisks show statistical significance (*P < 0.01). b The levels of RORγt expression were much higher in AMANa (n = 10) or AIDPa (n = 10) than those of AMANs (n = 10), AIDPs(n = 10), OND (n = 10) or HC (n = 10). The columns represent mean values, the bars represent SD, and asterisks show statistical significance (*P < 0.01)
Expression of RORγ t in PBMCs from Patients with CIDP
RORγt is an important transcription factor for the differentiation and development of Th17. To further determine the change in the number of Th17 cells, we set out to investigate the expressions of RORγt in PBMCs from AMANa (n = 10), AIDPa (n = 10), AMANs (n = 10), AIDPs (n = 10), healthy controls (n = 10) and OND (n = 10). As shown in Fig. 2b, the levels of RORγt expression were much higher in the AMANa (34.2 ± 8.5, p < 0.01) and AIDPa (35.3 ± 9.3, p < 0.01) than those in the AMANs (22.1 ± 9.5) and AIDPs (24.2 ± 9.9), while there was no significant difference among AMANs, AIDPs, OND (21.3 ± 8.7) and healthy controls (23.6 ± 10.2) (p > 0.05).
Increased IL-17 Concentrations in Plasma and CSF from Patients with GBS
Plasma IL-17 concentrations in healthy controls (53.4 ± 7.7 ng/ml, n = 20) and OND (49.8 ± 8.2 ng/ml, n = 20) did not differ significantly and did not correlate with age and sex. Plasma IL-17 concentrations in acute-stage patients with AMAN (70.7 ± 10.7 ng/ml, n = 27, p < 0.001) or AIDP (71.8 ± 10.5 ng/ml, n = 24, p < 0.001) were significantly increased comparing with the healthy controls and OND patients, whereas plasma concentrations of IL-17 in the stable-stage patients with AMAN (51.4 ± 7.1 ng/ml, n = 16) or AIDP (50.1 ± 6.6 ng/ml, n = 15) did not differ significantly from that in healthy controls or OND (Fig. 3a).
Increased IL-17 concentrations in plasma and CSF from patients with GBS. a Plasma IL-17 concentrations in acute-stage patients with AMAN (AMANa, n = 27) or AIDP (AIDPa, n = 24) were significantly increased comparing with OND patients (n = 20) or HC (n = 20), whereas plasma concentrations of IL-17 in the stable-stage AMAN (AMANs, n = 16) or AIDP (AIDPs, n = 15) did not differ significantly from that in HC or OND. b AMANa (n = 25) or AIDPa (n = 24) had significantly higher IL-17 concentrations in the CSF than the neurological controls (n = 20). The horizontal lines represent the mean values of the groups, and asterisks show statistical significance (*P < 0.01)
The CSF represents the fluid compartment that is closest to reflect the immunopathogenic situation in GBS, so we then sought to compare the concentrations of IL-17 in CSF from patients with GBS and OND. Acute-stage patients with AMAN (91.4 ± 8.3 ng/ml, n = 24, p < 0.001) or AIDP (93.3 ± 8.2 ng/ml, n = 25, p < 0.001) had significantly higher IL-17 concentrations in the CSF than the neurological controls (58.8 ± 8.8 ng/ml, n = 20) (Fig. 3b).
In the same GBS patients, the plasma IL-17 concentrations were significantly decreased after they received a standard dose intravenous immunoglobulin (Fig. 3c, d).
Increased OPN Concentrations in CSF from Patients with GBS
Plasma OPN concentrations in acute-stage patients with AMAN (54.9 ± 14.6 ng/ml, n = 27) or AIDP (53.3 ± 9.8 ng/ml, n = 24) did not differ significantly from plasma OPN concentrations in healthy controls (51.4 ± 9.9 ng/ml, n = 20) or OND (53.3 ± 10.3 ng/ml, n = 20) (Fig. 4a). In addition, there was no significant difference in plasma OPN concentrations between the stable-stage patients with AMAN (50.3 ± 9.5 ng/ml, n = 16) or AIDP(52.8 ± 9.4 ng/ml, n = 15) and healthy controls or OND. But the CSF OPN concentrations were significantly increased in acute-stage patients with AMAN (223.1 ± 19.8 ng/ml, n = 25, p < 0.001) or AIDP (218.1 ± 19.6 ng/ml, n = 24, p < 0.001) comparing with the OND patients (134.5 ± 19.3 ng/ml, n = 20) (Fig. 4b).
Increased OPN concentrations in CSF from patients with GBS. a Plasma OPN concentrations in acute-stage patients with AMAN (AMANa, n = 27) or AIDP (AIDPa, n = 24) did not differ significantly from that in stable-stage patients with AMAN (AMANs, n = 16) or AIDP (AIDPs, n = 15), HC (n = 20) and OND (n = 20). b The CSF OPN concentrations were significantly increased in AMANa (n = 25) or AIDPa (n = 24) comparing with the OND patients (n = 20). The horizontal lines represent the mean values of the groups, and asterisks show statistical significance (*P < 0.01)
Increased Circulating Th17 Cell Populations and Elevated CSF OPN and IL-17 Concentrations in Patients with GBS are Correlated with Disease Severity
To determine whether the increase of circulating Th17 cells, elevated CSF OPN and IL-17 concentrations are correlated with peripheral nervous injury, we analyzed the correlation between Th17 frequency, CSF OPN and IL-17 concentrations and GBS disability scale scores (GDSs) which indicate severity of peripheral nervous injury. A positive correlation between the frequency of Th17 cells and GDSs was observed in peripheral blood of acute-stage patients with AIDP (r = 0.541; p = 0.006) or AMAN (r = 0.523; p = 0.005). Similar results were found between the level of IL-17 and OPN in the CSF and GDSs (Fig. 5). Although a trend was noted toward the positive correlation between GDSs and the level of IL-17 in plasma, it was not statistically significant (data not shown). We also found that there was a positive correlation between circulating Th17 cell populations and CSF OPN levels (Fig. 5).
The correlations of Th17 frequency, CSF OPN and IL-17 concentrations and GBS disability scale scores (GDSs). A positive correlation between the frequency of Th17 cells and GDSs was observed in peripheral blood of acute-stage patients with AIDP (r = 0.501; p = 0.024) or AMAN (r = 0.482; p = 0.023). Similar results were found for the level of IL-17 and OPN in the CSF. There was a positive correlation between circulating Th17 cell populations and CSF OPN levels
In a multivariate regression analysis where the CSF concentrations of OPN, IL-17, circulating Th17 cell populations and the plasma concentrations of IL-17 were introduced as independent variables, the CSF concentration of OPN was the only significant, independent predictor of the area of GBS disability score (p = 0.003, OR = 1.668, n = 49).
Discussion
GBS is an autoimmune acute peripheral neuropathy. Abnormality of cellular immunity is likely involved in the pathogenesis of the GBS and some forms of experimental autoimmune neuritis (EAN). In addition, various proinflammatory mediators are thought to significantly contribute to this disease development by recruiting effecter cells to the peripheral nervous system or by enabling in situ release of other products toxic for Schwann cells. Accumulating data have suggested that IL-17-producing Th17 cells appear to be important element in the pathogenesis of inflammatory and autoimmune diseases. OPN has been recently recognized as a key pro-inflammatory mediator involved in the development of various inflammatory conditions. It has been demonstrated also that OPN could regulate Th17 cells development in certain ways. However, the exact role of Th17 cells and OPN in the pathogenesis of GBS is not understood.
In this study we have shown for the first time that Th17 cells are present in higher proportions in the peripheral blood of GBS patients compared with OND or healthy control subjects, and paralleled with ascendant expression of critical transcript factors RORγt in PBMCs and special cytokines IL-17 in both plasma and CSF. Furthermore, the increased circulating Th17 cells and CSF IL-17 concentrations were correlated with disease severity in GBS patients. Several studies on animal model and human have given a clue that Th17 cells might be in a key position in the immunolesion of GBS. IL-17 was found in sciatic nerves of EAN, and the accumulation of IL-17 was temporally correlated with severity of neurological signs [17]. Recently, Zhang and colleague found that at the peak of EAN, the proportion of interleukin (IL)-17A expressing cells in cauda equina infiltrating cells, and the levels of IL-17A in sera were elevated in IFN-γ knockout mice when compared with their WT counterparts [34]. The findings of these two studies on animal model suggest a pathological contribution of IL-17+ cells to the development of EAN. In human, study showed that GBS patients had higher relative RORγt mRNA expression in CD4+ T cells, and higher serum concentrations of IL-17 compared to controls [18]. Our findings are in agreement with another study, which show that CSF and plasma levels of IL-17 are elevated in GBS patients compared with HC, and IL-17 levels in CSF are correlated with GBS disability scale scores [19]. In addition, our group has recently demonstrated that the frequency of Th17 cells in CSF and the level of IL-17 in plasma were significantly higher in active chronic inflammatory demyelinating polyradiculoneuropathy which can be considered the chronic equivalent of acute inflammatory demyelinating polyradiculoneuropathy in many ways [27]. These results strongly supported the possible role of Th17 cells and special cytokines IL-17 in damage of the peripheral nerves.
Our studies were not designed to explore the functional role of Th17 cells in GBS. Breakdown of the blood-nerve barrier (BNB) by activated T cells and its cytokines is an early and central event in the pathogenesis of nervous system autoimmune disorders [35]. Anatomically, the BNB is deficient in the distal nerve terminals and nerve roots, and these regions are preferentially affected by an immune attack. It has been demonstrated that Th17 cells could impair blood–brain barrier integrity by disrupting tight junctions by the mutual action of IL-17A and IL-22 [36]. Further observations indicate that IL-17A-induced blood-brain barrier disruption involves the formation of reactive oxygen species by NAD(P)H oxidase and xanthine oxidase, which subsequently are responsible for the disruption and down-regulation of tight junction molecules and the activation of the endothelial contractile machinery [37]. Therefore, we speculate that Th17 cells and IL-17A may assist in the disruption of the BNB in GBS; as a result, immune cells can infiltrate across the barrier and obtain direct access to myelin and Schwann cells, thus causing demyelination and axon degeneration. However, further studies are needed to provide direct insights into the putative immunopathogenesis of the disease associated with Th17 and cytokines IL-17.
This is also the first study to examine the expression of OPN in the CSF and plasma of GBS patients. Recently, Moon et al. found that, in EAN, OPN was abundantly expressed by infiltrating macrophages in the subarachnoid space and in some astrocytes in the parenchyma, and the expression of OPN paralleled the clinical course of EAN [38]. Another study showed that OPN was upregulated in Schwann cells of the sciatic nerves of rats with EAN, and it might participate in the pro-inflammatory process in the peripheral nervous system during the early stage of EAN [39]. Although the previous reports imply that OPN expression changes during the course of autoimmune injury of the PNS, little is known concerning the changes in OPN expression that may occur in GBS patients. Our results showed that OPN concentrations are significantly increased in the CSF of acute-stage GBS patients compared to OND, whereas there is no significant difference in the plasma. The CSF represents the fluid compartment that is more approximate to target organs of GBS than the peripheral blood, so it more accurately reflect the ongoing inflammatory process in the PNS. We did find that the OPN concentrations in CSF were significantly and positively correlated with GDSs at the acute phase of GBS. Thus, OPN appear to play a role in the pathogenesis of GBS and may be a biomarker for indicating disease severity, which, however, should be confirmed in future studies with a larger sample size. In the current study, we could not explain why the OPN concentrations in CSF were significantly increased in GBS patients. We presume that Schwann cells in the spinal roots and peripheral nerves and infiltrating inflammatory cells, such as macrophages and T cells, may contribute to the origin of OPN in the CSF of the acute phase of GBS.
In the current study, we found that circulating Th17 cell populations and CSF OPN levels, respectively, are correlated with GDSs at the acute phase of GBS, and there was a positive correlation between them. Most recently, OPN was confirmed to play an important role in Th17 differentiation. In rheumatoid arthritis patients, the levels of OPN correlated significantly with IL-17 production and the frequency of Th17 cells in synovial fluid, and the effect of OPN in Th17 differentiation specifically involved OPN receptor CD44 and CD29 and transcription factor ROR [40]. Shinohara and colleagues reported that intracellular OPN in dendritic cells was required for regulation of mouse Th17 cells differentiation through type I interferon receptor by inhibiting IL-27 in dendritic cells [41]. We conjecture that in the inflammation sites of peripheral nerves, elevated OPN might promotes migration and differentiation of Th17 cells from the blood.
Conclusion
In conclusion, we firstly present here evidence that the frequency of Th17 cells in the peripheral blood and the OPN concentrations in the CSF were significantly increased in patients with AMAN and AIDP comparison with other non-inflammatory neurological diseases. Circulating Th17 cell populations, CSF IL-17 and OPN levels, respectively, are correlated with GBS disability scale scores (GDSs). In summary, our preliminary findings suggest that both Th17 and OPN may be associated with the pathogenesis of GBS.
References
Hadden RDM, Karch H, Hartung HP, Zielasek J, Weissbrich B, Schubert J, et al. Preceding infections, immune factors, and outcome in Guillain-Barre syndrome. Neurology. 2001;56:758–65.
Ho TW, Mishu B, Li CY, Gao CY, Cornblath DR, Griffin JW, et al. Guillain-Barré syndrome in northern China. Relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118:597–605.
Csurhes PA, Sullivan AA, Green K, Pender MP, McCombe PA. T cell reactivity to P0, P2, PMP-22, and myelin basic protein in patients with Guillain-Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry. 2005;76:1431–9.
Yuki N, Susuki K, Hirata K. Ataxic form of Guillain-Barré syndrome associated with anti-GD1b IgG antibody. J Neurol Neurosurg Psychiatry. 2000;69:136–7.
Creange A, Belec L, Clair B, Raphael JC, Gherardi RK. Circulating tumor necrosis factor (TNF)-alpha and soluble TNF-alpha receptors in patients with Guillain Barré syndrome. J Neuroimmunol. 1996;68:95–9.
Chi LJ, Wang HB, Zhang Y, Wang WZ. Abnormality of circulating CD4(+)CD25(+) regulatory T cell in patients with Guillain-Barré syndrome. J Neuroimmunol. 2007;192:206–14.
Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, et al. Interleukin17-producing CD4+ effector T develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32.
Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46.
Schwarzenberger P, Huang W, Ye P, Oliver P, Manuel M, Zhang Z, et al. Requirement of endogenous stem cell factor and granulocyte-colony-stimulating factor for IL-17-mediated granulopoiesis. J Immunol. 2000;164:4783–9.
Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, et al. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–2.
Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1β and 6 but not transforming growth factor-β are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9.
Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–7.
Gol-Ara M, Jadidi-Niaragh F, Sadria R, Azizi G, Mirshafiey A. The role of different subsets of regulatory T cells in immunopathogenesis of rheumatoid arthritis. Arthritis. 2012;2012:805–75.
Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–55.
Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, et al. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis. 2008;14:437–45.
Wong CK, Lit LC, Tam LS, Li EK, Wong PT, Lam CW. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–93.
Li S, Yu M, Li H, Zhang H, Jiang Y. IL-17 and IL-22 in cerebrospinal fluid and plasma are elevated in Guillain-Barré syndrome. Mediators Inflamm. 2012;260473.
Liang SL, Wang WZ, Huang S, Wang XK, Zhang S, Wu Y. Th17 helper cell and T-cell immunoglobulin and mucin domain 3 involvement in Guillain-Barré syndrome. Immunopharmacol Immunotoxicol. 2012;34:1039–46.
Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–5.
Chellaiah MA, Hruska KA. The integrin alpha(v)beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int. 2003;72:197–205.
Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–45.
Cho HJ, Kim HS. Osteopontin: a multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep. 2009;11:206–13.
Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9-beta1 to osteopontin. Matrix Biol. 2005;24:418–27.
Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science. 1996;271:509–12.
Cantor H, Shinohara ML. Regulation of T-helper-cell lineage development by osteopontin: the inside story. Nat Rev Immunol. 2009;9:137–41.
Chi LJ, Lu HT, Li GL, Wang XM, Su Y, Xu WH, et al. Involvement of T helper type 17 and regulatory T cell activity in tumour immunology of bladder carcinoma. Clin Exp Immunol. 2010;161:480–9.
Chi LJ, Xu WH, Zhang ZW, Huang HT, Zhang LM, Zhou J. Distribution of Th17 cells and Th1 cells in peripheral blood and cerebrospinal fluid in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010;15:345–56.
Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat Immunol. 2007;8:74–83.
Chabas D, Baranzini SE, Mitchell D, Bernard CC, Rittling SR, Denhardt DT, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–5.
Jansson M, Panoutsakopoulou V, Baker J, Klein L, Cantor H. Cutting edge: attenuated experimental autoimmune encephalomyelitis in eta-1/osteopontin-deficient mice. J Immunol. 2002;168:2096–9.
Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barre syndrome. Ann Neurol. 1990;27:21–4.
Hadden RDM, Cornblath DR, Hughes RAC, Zielasek J, Hartung HP, Toyka KV, et al. Electrophysiological classification of Guillain-Barre syndrome: clinical associations and outcome. Ann Neurol. 1998;44:780–8.
Van der Meche FG, Schmitz PI, Dutch Guillain-Barre Study Group. A randomized trial comparing intravenous immune globuli and plasma exchange in Guillain-Barre syndrome. N Engl J Med. 1992;326:1123–9.
Zhang HL, Azimullah S, Zheng XY, Wang XK, Amir N, Mensah-Brown EP, et al. IFN-γ deficiency exacerbates experimental autoimmune neuritis in mice despite a mitigated systemic Th1 immune response. J Neuroimmunol. 2012;246:18–26.
Kieseier BC, Kiefer R, Gold R, Hemmer B, Willison HJ, Hartung HP. Advances in understanding and treatment of immune-mediated disorders of the peripheral nervous system. Muscle Nerve. 2004;30:131–56.
Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, et al. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5.
Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010;24:1023–34.
Moon C, Shin T. Increased expression of osteopontin in the spinal cords of Lewis rats with experimental autoimmune neuritis. J Vet Sci. 2004;5:289–93.
Ahn M, Lee Y, Moon C, Jin JK, Matsumoto Y, Koh CS, et al. Upregulation of osteopontin in Schwann cells of the sciatic nerves of Lewis rats with experimental autoimmune neuritis. Neurosci Lett. 2004;372:137–41.
Chen G, Zhang X, Li R, Fang L, Niu X, Zheng Y, et al. Role of osteopontin in synovial Th17 differentiation in rheumatoid arthritis. Arthritis Rheum. 2010;62:2900–8.
Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29:68–78.
Acknowledgments
This study was supported by Heilongjiang Province Science Foundation for Youths (project number: QC2009C05), China Postdoctoral Science Foundation (project number: 70802) and Foundation of the First Affiliated Hospital of Harbin Medical University (project number: 2012BS002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Han Rong kun, Cheng Yue feng, Zhou Shan shan, Guo Hong, He Rui dong, and Zhang Li ming contributed equally to the work.
Rights and permissions
About this article
Cite this article
Han, R.k., Cheng, Y.f., Zhou, S.s. et al. Increased Circulating Th17 Cell Populations and Elevated CSF Osteopontin and IL-17 Concentrations in Patients with Guillain-Barré Syndrome. J Clin Immunol 34, 94–103 (2014). https://doi.org/10.1007/s10875-013-9965-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-013-9965-3