Abstract
Background
The difference in the clinical course, response to therapy, and distribution of CNS inflammation in primary-progressive (PPMS) and relapsing-remitting multiple sclerosis (RRMS) suggests differences in the underlying immunological characteristics of the disease. We aimed to investigate differences in immunological profiles in relation to intrathecal inflammation in different MS forms.
Methods
The peripheral blood (PB) proportions of CD4 + and CD8 + T-cells and CD19 + B-cells were retrospectively compared with the markers of intrathecal immunoglobulin G (IgG) synthesis at diagnosis: IgG index, percentage of intrathecal IgG synthesis (IF IgG), the number of oligoclonal bands (OCB), depending on the blood-brain barrier (BBB) function, and antibody specific index to neurotrophic viruses (MRZH reaction).
Results
Thirty-six controls, 71 RRMS and 25 PPMS were enrolled. PPMS had higher percentage of CD4 + T-cells compared to RRMS (P = 0.043) and controls (P = 0.003). The percentage of CD8 + T-cells and CD19 + B-cells, and respective absolute cell counts did not differ according to the MS phenotype. In RRMS with the dysfunctional BBB, the IgG index (r = 0.642, P = 0.012) correlated significantly with the CD19 + B-cells while the CD4 + T-cells inversely correlated with IF IgG (r=-0.574, P = 0.039). Interestingly, in PPMS the number of OCB was positively associated with CD4+ (r = 0.603, P = 0.015) and negatively associated with CD8 + T-cells (r=-0.554, P = 0.033), while IF IgG negatively correlated with CD8 + T-cells (r=-0.689, P = 0.003), but only in the preserved BBB function.
Conclusions
The PB CD4 + T-cells and B-cells were associated with the intrathecal inflammation in RRMS with BBB dysfunction while CD8 + T-cells were involved in PPMS with CNS-compartmentalized inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a demyelinating autoimmune and neurodegenerative disease of the central nervous system (CNS). Most commonly patients present with a relapsing-remitting form of MS (RRMS) which is characterized by a neurological relapse followed by full or partial recovery of the affected function. Fewer individuals, approximately 10–15% of people with MS (pwMS), progressively accumulate neurological disability without relapses which is defined as a primary progressive form of MS (PPMS) [1]. People with PPMS (pwPPMS) have a later disease onset, the female gender is not predominately affected, lesions are not restricted to the brain [2] and most available disease-modifying therapies (DMT) are not effective [3], as opposed to pwRRMS. With the disease progression, RRMS can develop into a progressive phenotype (SPMS) with continuing neurodegeneration. Therefore, research is needed to define the underlying immunological profile in different MS forms to recognize future disease courses and provide optimal therapy.
The proposed drivers of MS are dysregulated immune cell interactions in the periphery [4]. Presumably, pathogenic lymphocytes escape the immune tolerance in predisposed individuals, i.e. in case of the past Epstein-Barr virus infection, genetic predisposition, and environmental and lifestyle risk factors such as low vitamin D, obesity and smoking [5]. In contact with the CNS antigen, these cells become re-activated which initiates an autoimmune reaction against CNS autoantigens resulting in local inflammation, demyelination, and neurodegeneration.
In RRMS, the new lesions are the consequence of lymphocyte transmigration in the CNS and are more often accompanied by the disrupted blood-brain barrier (BBB), while tissue-resident lymphocytes propagate diffuse inflammation and ongoing neurodegeneration in the progressive stage. The progressive stage is characterized by the compartmentalization of inflammation in the CNS which is often reflected in the presence of ectopic lymphoid follicles which resemble the structure of the secondary lymphoid organs.
The difference in the clinical course, response to therapy, and distribution of CNS inflammation in PPMS and RRMS, suggests that the underlying immunological characteristics of the disease are different. So far differences in immunological profiles in relation to intrathecal inflammation in different MS forms were not investigated. Therefore, we analyzed lymphocyte subpopulations in peripheral blood (PB) of pwRRMS and pwPPMS, and evaluated the association of PB lymphocytes with the markers of intrathecal inflammation and evaluated their association with disability in pwMS.
Materials and methods
Participants
This retrospective observational study was conducted at the University Hospital Centre Zagreb, at the Department of Laboratory Diagnostics, on data collected from April 2020 to July 2023. Consecutive adult pwMS who were evaluated before the introduction of anti-CD20 therapy in the treatment of MS were enrolled. The inclusion criteria were: (1) the RRMS or PPMS diagnosis was based on the 2017 McDonald’s criteria, and (2) the decision for the introduction of anti-CD20 therapy was based on the current treatment guidelines for RRMS and PPMS [6, 7]. The exclusion criteria were: (1) the time between the lumbar puncture and PB flow cytometry was longer than three months, (2) the patient was previously on DMT, and (3) the patient received corticosteroid therapy less than four weeks prior to the evaluation for introduction of anti-CD20 therapy. The following clinical data were extracted: age, sex, MS phenotype (RRMS or PPMS), disease duration and Expanded Disability Status scale (EDSS) score.
Controls for PB cell subsets were patients with performed flow cytometry analysis in whom neuroinflammatory, neurodegenerative, autoimmune, or haematological diagnoses were excluded. Therefore, the participants were grouped as PPMS, RRMS and controls.
The study was approved by the institution’s Ethical Committee (approval No. 8.1–23/69 − 2).
Methods
At diagnosis, albumin and immunoglobulin G (IgG) concentration in paired cerebrospinal fluid (CSF) and serum samples were measured by immunonephelometry (Atellica 360 NEPH, Siemens Healthineers, Marburg, Germany). Based on these measurements, the quotients of albumin (Alb) and IgG in cerebrospinal fluid and serum were calculated as follows:
and an IgG index was calculated using the formula:
The percentage of intrathecal synthesis of IgG (IF IgG) was calculated according to the model by Reiber HO [8] based on the following formulae:
The blood-brain barrier (BBB) integrity was assessed using the computational model based on the age dependent QAlb limit [8]:
The BBB integrity was classified as dysfunctional if QAlb was greater than the age appropriate QAlb limit.
Oligoclonal bands (OCBs) testing was performed by isoelectric focusing followed by immunofixation and band visualisation on the Hydrasis 2 Scan Focusing device (Sebia, France). The results of OCBs testing were reported according to the consensus [9]. Types 2 and 3 are considered positive for OCBs if two or more distinct bands were visible in CSF. For this study, all CSF-distinct bands were counted by three experienced specialists in laboratory medicine. Finally, the arithmetic mean of three counts was used in the analysis.
In patients with positive OCB, the antibody specific index (ASI) to measles, rubella, varicella zoster and herpes simplex 1/2 viruses were determined by measuring the titre of virus specific antibodies in CSF and serum with respective ELISA reagent kits (Euroimmun AG, Lübeck, Germany).
The calculation of ASI depended on the presence of IF-IgG, and was calculated as follows:
a) if IF IgG = 0%
b) if IF IgG ≥ 1%
An ASI > 1.5 was considered positive, and participants with at least two positive ASI to either measles, rubella, varicella zoster or herpes simplex 1/2 viruses were considered as having a positive MRZH reaction.
Peripheral blood flow cytometry
Prior to the introduction of anti-CD20 therapy PB imunophenotyping was performed by flow cytometry. Blood samples were collected in K2EDTA tubes and the whole blood was used for staining with adequate fluorochrome-conjugated monoclonal antibodies in one 6-color tube. The antibodies used for B and T lymphocytes characterization were anti-CD45-PerCPCy5.5 (2D1), anti-CD19-APC (SJ25C1), anti-CD-20-FITC (L27), anti-CD3-APC (SK7), anti-CD4-PE (SK3D) and anti-CD8-APCH7 (SK1), all purchased from BD Biosciences. Red blood cells were lysed with BD FACS Lysing solution and washed in phosphate-buffered saline. Stained and washed leukocytes were processed on BD FASCLyric and analysed using BD FACSSuite software. The percentages of B-lymphocytes, as well as CD4 + and CD8 + T-lymphocytes were analyzed with flow cytometer (BD FACSLyric, BD Biosciences). The absolute cell counts were calculated according to white blood cell count measured on a haematological analyser Sysmex XN3000 (Sysmex Europe, Norderstedt, Germany).
Statistical analysis
The type of distribution was tested with Shapiro-Wilks test on each group of data used. Normally distributed data were presented as mean and standard deviation (SD). Non-normally distributed quantitative data, or in case of less than 30 cases per group, were presented as median and interquartile range (IQR). Patients’ age was presented as median, with minimum and maximum range. Categorical data were presented with ratios or percentages. Chi-square test was used for testing the differences of proportions. Differences in quantitative measurements between patient subgroups were tested with Kruskal Wallis test. The Dunn’s post-hoc test was used for pairwise comparison of subgroups. The Spearman rank correlation test was used for correlation testing. The P < 0.05 was considered significant. The Bonferroni correction was applied in case of multiple comparisons. Logistic regression was used for moderate disability prediction (EDSS ≥ 3) and the model accuracy was assessed by comparing the area under the curve (AUC) of the Receiver operating curve (ROC) analysis. The statistical analysis was performed using MedCalc® Statistical Software version 20.216 (MedCalc Software Ltd, Ostend, Belgium).
Results
In total, 96 pwMS and 36 controls were included. Of pwMS, 25 (26%) had PPMS, and the rest had RRMS. PwMS did not differ from controls in age and sex. The clinical and laboratory data of subjects included in the study are presented in Table 1. The median time difference between LP and PB flow cytometry analysis was 0 (IQR: 0–26) days in pwMS. Median time from the first symptom until the evaluation for anti-CD20 therapy was 15 (4–42) months in pwRRMS and 115 (60–161) months in pwPPMS (P < 0.001). PwPPMS were older, median age 49 (30–67 years), compared to pwRRMS, median age 36 (18 to 70 years) (P < 0.001). No differences were found in the sex distribution, the biomarkers of intrathecal inflammation or BBB function between MS phenotypes (Table 2).
Fisher’s exact test was for testing the differences in sex distribution. Mann-Whitney test was used for testing the differences in quantitative data. Multiple comparisons for lymphocyte subsets were corrected by applying the Bonferroni correction and reported in the table. Abbreviations: EDSS, Expanded Disability Status Scale; IQR, interquartile range; na, not applicable; PPMS, primary-progressive multiple sclerosis; pwMS, people with multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis.
Fisher’s exact test was used for testing the differences in the distribution EDSS ≥ 3, OCB types, frequency of BBB dysfunction and MRZH positivity. Mann-Whitney test was used for testing the differences in quantitative data. Abbreviations: ASI, antibody specific index; BBB, blood-brain barrier; IgG, immunoglobulin G; na, not applicable; EDSS, Expanded Disability Status Scale; MRZH reaction, at least two positive antibody specific indices to either measles, rubella, varicella zoster virus or herpes simplex 1 and 2 viruses; OCB, oligoclonal bands; PPMS, primary-progressive multiple sclerosis; QAlb, albumin cerebrospinal fluid to serum quotient; QIgG, IgG cerebrospinal fluid to serum quotient; RRMS, relapsing-remitting multiple sclerosis; VZV, varicella zoster virus; HSV1/2, herpes simplex virus 1 and 2.
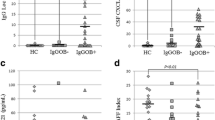
The PB proportions of CD4 + and CD8 + T-cells and CD19 + B-cells were not different in pwMS compared to controls. However, the CD4 + T-cell proportion was related to the MS phenotype, as CD4% were higher in PPMS compared to RRMS (P = 0.043) and controls (P = 0.005). The CD4+/CD8 + ratio was significantly higher (P = 0.014) in PPMS compared to controls. Interestingly, CD8 + T-cells and CD19 + B-cells did not differ according to the MS phenotype and compared to the controls (Fig. 1).
Differences in percentages and absolute counts of peripheral blood T and B-cells in primary-progressive (PPMS) and relapsing-remitting MS (RRMS) compared to controls (CTRL). The boxes in the boxplot represent interquartile range (IQR) and the middle line the median. The whiskers of the boxplot extend 1.5 times the IQR from both percentiles (Q1–1.5 * IQR or Q3 + 1.5 * IQR). Differences were tested using the Kruskal-Wallis test and Dunn’s post-hoc test. Multiple comparisons were corrected by applying the Bonferroni correction and only the statistically significant differences (P < 0.05) between groups were reported above the horizontal line
In all pwMS, the PB immunological status was not associated with any of the biomarkers of IgG intrathecal synthesis. While the overall analysis across all MS phenotypes did not reveal an association between PB immunological status and IgG intrathecal synthesis, further examination within specific clinical phenotypes suggests distinction between RRMS and PPMS. The PB immunological status was associated with the MS phenotype and the BBB functionality. In PPMS, the CD8 + T-cells percentage correlated with the IF IgG (r=-0.584, P = 0.006). The association was more pronounced in participants with the functional BBB, as the number of OCB bands (r=-0.554, P = 0.033) and IF IgG (r=-0.689, P = 0.003) negatively correlated with the percentage of CD8 + T-cells. The number of OCB bands also positively correlated (r = 0.603, P = 0.015) with the percentage of CD4 + T-cells in this cohort. The association of PB lymphocytes with the dysfunction of the BBB in PPMS could not be tested due to the small number of cases. In RRMS, PB immunological status was strongly associated with the IgG intrathecal synthesis in case of the BBB dysfunction: IF-IgG negatively correlated with the percentage of CD4 + T-cells (r=-0.574, P = 0.039) and IgG index positively correlated with the percentage of CD19 + B-cells (r = 0.642, P = 0.012) (Fig. 2). The same associations of intrathecal IgG synthesis biomarkers and absolute T- and B-cell counts in PB were found (data not shown).
Correlations (Spearman rank test) of the percentage of peripheral blood T and B-cells with the cerebrospinal fluid markers of intrathecal IgG synthesis in relation to the blood-brain barrier (BBB) function. The association of PB lymphocytes with the dysfunction of the BBB in PPMS could not be tested due to the small number of cases. Multiple comparisons were corrected by applying the Bonferroni correction and reported. The statistically significant (P < 0.05) correlations were highlighted with color indicating the strenght and direction of the correlation coefficient. Abbreviations: IgG, immunoglobulin G; IF IgG, the percentage of the intrathecally synthetised IgG; PwMS, people with multiple sclerosis; PPMS, primary progressive MS; RRMS, relapsing-remitting MS
The MRZH reaction status was not associated with the PB immunological status, regardless of the MS phenotype (Table 3).
The Mann-Whitney test was used for testing the differences between groups. The differences in PPMS could not be tested due to the small number of cases. Abbreviations: na, not applicable; MRZH reaction, at least two positive antibody specific indices to either measles, rubella, varicella zoster virus or herpes simplex 1 and 2 viruses; pwMS, people with multiple sclerosis; PPMS, primary progressive MS; RRMS, relapsing-remitting MS.
The associations of PB immunophenotyping, CSF analysis and clinical data parameters with moderate or higher disability (EDSS ≥ 3) in pwMS were tested to evaluate whether the PB immunophenotype has any additional influence on disability compared to other data. The AUC was 0.910 (95% CI 0.827–0.961) for attaining EDSS ≥ 3, the equation had a high sensitivity of 75.6% (95% CI 59.7–87.6%) and an excellent specificity of 95.3% (95% CI 87.7–99.9%), at the criteria of 0.55. The combination of all parameters had superior accuracy measures compared to clinical data, CSF analysis data or PB immunophenotyping data alone (Fig. 3). The logistic regression equations with all input variables are available as the Supplemental File.
Receiver operating curve analysis for attaining of moderate and higher disability in people with multiple sclerosis. The combination of clinical data, CSF analysis and PB immunophenotyping has the greatest accuracy for EDSS ≥ 3 compared to clinical data, CSF data or PB immunophenotyping data alone. The logistic regression equations with all input variables are available as the Supplemental File. Abbreviations: AUC, area under curve; 95%CI, 95% confidence intervals
Discussion
Considering the heterogeneous clinical presentation of progressive and relapsing-remitting MS forms, as well as unpredictable disease activity in RRMS we have analyzed the peripheral blood immunological status of pwMS and compared it to the CSF analysis biomarkers of intrathecal IgG synthesis and blood-brain barrier function.
Different proportions of CD4 + T-cells were not found in pwMS compared to control subjects in our study even though the key role of CD4 + T-cells in the immunopathogenesis of MS has been demonstrated in multiple studies [10, 11]. Lower frequency of regulatory Th-cells found in MS [12] contributes to the proliferation of autoreactive CD4 + T-cells [13] and is associated with the abnormal CSF IgG synthesis [14]. Autoreactive CD4 + T-cells play the central role in both initiating the disease in the peripheral lymphoid organs, as well as in infiltrating the CNS through the BBB and participating in the demyelination process by producing proinflammatory cytokines like IFN gamma, IL-17, TNF alpha and GM-CSF [15]. Interestingly, higher proportion of CD4 + T-cells was associated with the PPMS, but not the RRMS, phenotype in our study. Previously, a higher frequency of CD4 + T-reg cells [16] and a lower frequency of total CD4 + memory T-cells [17] were associated with the PPMS disease phenotype. There are mixed reports for differences in CD4 + T-cells in newly diagnosed RRMS, with some reporting no differences to controls [18], while others reported that CD4 + T-cells and CD4/CD8 ratio were higher compared to controls [19]. In our study, pwPPMS had a longer disease duration and were on average a decade older compared to the pwRRMS. There are conflicting reports on whether there is an influence of age on the proportion of CD4 + T-cells across the adult lifespan in healthy individuals, as some authors report no differences [20,21,22,23] while others do [24,25,26]. However, the age-related changes in the composition and function of the adaptive and innate immune systems in healthy individuals are well recognized, and eventually lead to a less effective immune response. With aging, MS patients harbour abnormally increased frequencies of CD4 + T-cells with activated and cytotoxic effector profiles [27]. The lasting underlying immunological mechanisms in the respective MS phenotypes could therefore influence pwPPMS more and may be the reason for the observed differences in the CD + T-cell proportion in these individuals.
Unlike previous findings of lower CD8 + T cells count in pwMS [18], especially in patients with PPMS [28], we did not confirm any changes in pwMS related to the CD8 + T-cells. Although PPMS did not differ in the proportion of peripheral CD8 + T-cells in our cohort, we showed that the peripheral CD8 + T-cell compartment was inversely associated with the percentage of IgG intrathecal synthesis and the number of OCBs in PPMS, and more so in the case of the functional BBB. Also in these individuals, the peripheral proportion of CD4 + T-cells positively correlated with the OCB count. Previous research has shown that CNS lesions exhibit a higher presence of CD8 + T cells compared to CD4 + T cells [29] which could imply higher recruitment of CD8 + T cells to the CNS. Moreover, others have demonstrated a correlation between CD8 + T cell presence in CNS lesions and axonal damage [30], a factor strongly linked to MS progression [31]. Given the indication in this study of a heightened recruitment of CD8 + T cells into the CNS, it is plausible that this phenomenon contributes to the progression observed in MS patients.
Although the PB percentage of CD4 + T-cells was associated with OCB count in PPMS with preserved BBB function, these cells inversely correlated with the percentage of intrathecal IgG synthesis in RRMS with BBB dysfunction. Therefore, the heterogeneous underlying mechanism of the CD4 + T-cells involvement in the MS immunopathogenesis is associated with MS phenotype [32, 33] and BBB permeability [34]. We confirm previous findings [35] that CD19 + B-cells in PB are unrelated to disease phenotype, although some reported a deviation in B-cell subpopulations in the progressive MS [16], and not RRMS. The inclusion of people with SPMS could have possibly contributed to the observed differences in B-cells subpopulations compared to our PPMS cohort. In RRMS with BBB dysfunction, the percentage of CD19 + B-cells significantly correlated with the IgG index. The obtained correlation indicates that a higher proportion of activated B-cells on the periphery more easily migrate to the CNS through the damaged BBB [36] and differentiate into antibody-producing plasma cells resulting in intrathecal IgG synthesis [37]. In PPMS, CNS-compartmentalized inflammation supports ongoing neurodegeneration and disability progression [38, 39] explaining the lack of association between any of the biomarkers of intrathecal IgG synthesis and CD19 + B-cells in our study.
The clinical, immunophenotyping, and CSF analysis parameters combined enabled the best discrimination of pwMS with moderate and higher disability, thereby indicating the involvement of PB immunophenotype in neurodegenerative processes in MS. PB lymphocytes immunophenotyping was previously shown to predict disease activity in CIS [40]. The BBB dysfunction was associated with neurodegeneration [34] and disease progression [41].
Due to the retrospective design of our study, we were not able to include pwMS with a lower risk of disease progression who have first started the first-line DMT. Therefore, our conclusions on the immunological status of RRMS are limited to pwMS who were more likely to have a more active disease based on the poor prognostic criteria (≥ 9 T2 or FLAIR lesions on the initial brain and spinal cord magnetic resonance imaging (MRI) or ≥ 3 T1 lesions with post-contrast enhancement on the initial brain and spinal cord MRI or EDSS after treatment of the initial relapse ≥ 3) [6]. Furthermore, the limited antibody panel for PB immunophenotyping in this retrospective analysis limits our conclusions. However, the used panel is commonly available for evaluation and monitoring of patients’ immunological status in clinical centres.
In conclusion, the proportions of CD4 + peripheral blood T-cells are altered in PPMS compared to controls unlike CD8 + T-cells and CD19 + B-cells. The IgG index was associated with the percentage of CD19 + B-cells in RRMS with the dysfunctional BBB who were more likely to have a more active disease course. The CD4 + T-cells and B-cells are seemingly involved in the initiation of the aberrant immune response and demyelination in BBB dysfunction in RRMS while CD8 + T-cells are involved in the autoimmune processes in the CNS with functional BBB leading to neurodegeneration.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Thompson AJ, Banwell BL, Barkhof F et al (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 17:162–173. https://doi.org/10.1016/S1474-4422(17)30470-2
Dastagir A, Healy BC, Chua AS et al (2018) Brain and spinal cord MRI lesions in primary progressive vs. relapsing-remitting multiple sclerosis. eNeurologicalSci 12:42–46. https://doi.org/10.1016/j.ensci.2018.07.002
Manouchehri N, Salinas VH, Rabi Yeganeh N et al (2022) Efficacy of Disease Modifying therapies in Progressive MS and how Immune Senescence may explain their failure. Front Neurol 13:854390. https://doi.org/10.3389/fneur.2022.854390
van Langelaar J, Rijvers L, Smolders J, van Luijn MM (2020) B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers. Front Immunol 11:760. https://doi.org/10.3389/fimmu.2020.00760
Lassmann H (2019) Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol 9:3116. https://doi.org/10.3389/fimmu.2018.03116
Habek M, Adamec I, Barun B et al (2022) Treatment of relapsing multiple sclerosis - recommendations of the Croatian neurological society. Croat Med J 63:379–388. https://doi.org/10.3325/cmj.2022.63.379
Montalban X, Gold R, Thompson AJ et al (2018) ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Multiple Scler J 24:96–120. https://doi.org/10.1177/1352458517751049
Reiber H (1994) Flow rate of cerebrospinal fluid (CSF) — a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci 122:189–203. https://doi.org/10.1016/0022-510X(94)90298-4
Andersson M, Alvarez-Cermeño J, Bernardi G, Cogato I, Fredman P, Frederiksen J, Fredrikson S, Gallo P, Grimaldi LM, Grønning M et al (1994) Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry 57:897–902. https://doi.org/10.1136/jnnp.57.8.897
Severson C, Hafler DA (2010) T-Cells in multiple sclerosis. Results Probl Cell Differ 51:75–98. https://doi.org/10.1007/400_2009_9012
Liu R, Du S, Zhao L et al (2022) Autoreactive lymphocytes in multiple sclerosis: Pathogenesis and treatment target. Front Immunol 13:1–23. https://doi.org/10.3389/fimmu.2022.996469
Li YF, Zhang SX, Ma XW et al (2019) The proportion of peripheral regulatory T cells in patients with multiple sclerosis: a meta-analysis. Mult Scler Relat Disord 28:75–80. https://doi.org/10.1016/j.msard.2018.12.019
Dhaeze T, Peelen E, Hombrouck A et al (2015) Circulating Follicular Regulatory T Cells Are Defective in multiple sclerosis. J Immunol 195:832–840. https://doi.org/10.4049/jimmunol.1500759
Puthenparampil M, Zito A, Pantano G et al (2018) Peripheral imbalanced TFH/TFR ratio correlates with intrathecal IgG synthesis in multiple sclerosis at clinical onset. Multiple Scler J 25:918–926. https://doi.org/10.1177/1352458518779951
Murúa SR, Farez MF, Quintana FJ (2021) The Immune response in multiple sclerosis. Annual Review of Pathology: mechanisms of Disease. Annu Rev Pathol Mech Dis 17:121–139. https://doi.org/10.1146/annurev-pathol-052920
Cellerino M, Ivaldi F, Pardini M et al (2020) Impact of treatment on cellular immunophenotype in MS: a cross-sectional study. Neurol Neuroimmunol Neuroinflamm 7:e693. https://doi.org/10.1212/NXI.0000000000000693
Canto-Gomes J, Da Silva-Ferreira S, Silva CS et al (2023) People with Primary Progressive multiple sclerosis have a Lower Number of Central Memory T Cells and HLA-DR + Tregs. Cells 12:439. https://doi.org/10.3390/cells12030439
Canto-Gomes J, Silva CS, Rb-Silva R et al (2022) Low Memory T Cells Blood Counts and High Naïve Regulatory T Cells Percentage at relapsing remitting multiple sclerosis diagnosis. Front Immunol 13:901165. https://doi.org/10.3389/fimmu.2022.901165
Uppal SS, Verma S, Dhot PS (2003) Normal values of CD4 and CD8 lymphocyte subsets in healthy Indian adults and the effects of sex, age, ethnicity, and smoking. Cytometry B Clin Cytom 52B:32–36. https://doi.org/10.1002/cyto.b.10011
Bisset LR, Lung TL, Kaelin M et al (2004) Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. Eur J Haematol 72:203–212. https://doi.org/10.1046/j.0902-4441.2003.00199.x
Jentsch-Ullrich K, Koenigsmann M, Mohren M, Franke A (2005) Lymphocyte subsets’ reference ranges in an age- and gender-balanced population of 100 healthy adults—A monocentric German study. Clin Immunol 116:192–197. https://doi.org/10.1016/j.clim.2005.03.020
Valiathan R, Deeb K, Diamante M et al (2014) Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology 219:487–496. https://doi.org/10.1016/j.imbio.2014.02.010
Niu H-Q, Zhao X-C, Li W et al (2020) Characteristics and reference ranges of CD4 + T cell subpopulations among healthy adult Han Chinese in Shanxi Province, North China. BMC Immunol 21:44. https://doi.org/10.1186/s12865-020-00374-9
Xia Y, Liu A, Li W et al (2022) Reference range of naïve T and T memory lymphocyte subsets in peripheral blood of healthy adult. Clin Exp Immunol 207:208–217. https://doi.org/10.1093/cei/uxab038
Yi JS, Rosa-Bray M, Staats J et al (2019) Establishment of normative ranges of the healthy human immune system with comprehensive polychromatic flow cytometry profiling. PLoS ONE 14(12):e0225512. https://doi.org/10.1371/journal.pone.0225512
Zuroff L, Rezk A, Shinoda K et al (2022) Immune aging in multiple sclerosis is characterized by abnormal CD4 T cell activation and increased frequencies of cytotoxic CD4 T cells with advancing age. EBioMedicine 82:104179. https://doi.org/10.1016/j.ebiom.2022.104179
D’Amico E, Zanghì A, Parrinello NL et al (2022) Immunological subsets characterization in newly diagnosed relapsing–remitting multiple sclerosis. Front Immunol 13:819136. https://doi.org/10.3389/fimmu.2022.819136
Pender MP, Csurhes PA, Pfluger CMM, Burrows SR (2014) Deficiency of CD8 + effector memory T cells is an early and persistent feature of multiple sclerosis. Multiple Scler J 20:1825–1832. https://doi.org/10.1177/1352458514536252
Booss J, Esiri MM, Tourtellotte WW, Mason DY (1983) Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis. J Neurol Sci 62:219–232. https://doi.org/10.1016/0022-510X(83)90201-0
Bitsch A, Schuchardt J, Bunkowski S et al (2000) Acute axonal injury in multiple sclerosis: correlation with demyelination and inflammation. Brain 123:1174–1183. https://doi.org/10.1093/brain/123.6.1174
Tallantyre EC, Bø L, Al-Rawashdeh O et al (2010) Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Multiple Scler J 16:406–411. https://doi.org/10.1177/1352458510364992
Peeters LM, Vanheusden M, Somers V et al (2017) Cytotoxic CD4 + T cells drive multiple sclerosis progression. Front Immunol 8:1160. https://doi.org/10.3389/fimmu.2017.01160
Christensen JR, Börnsen L, Ratzer R et al (2013) Systemic inflammation in progressive multiple sclerosis involves follicular T-Helper, Th17- and activated B-Cells and correlates with progression. PLoS ONE 8:e57820. https://doi.org/10.1371/journal.pone.0057820
Puthenparampil M, Tomas-Ojer P, Hornemann T et al (2021) Altered CSF albumin quotient links peripheral inflammation and brain damage in MS. Neurol Neuroimmunol Neuroinflamm 8:e951. https://doi.org/10.1212/NXI.0000000000000951
Yılmaz V, Tura DA, Ulusoy C et al (2017) Multipl Skleroz hastalarında Periferik Kan B lenfosit alt gruplarının akım sitometrisi ile değerlendirilmesi. Turk Noroloji Dergisi 23:219–224. https://doi.org/10.4274/tnd.87523
Kutzelnigg A, Lucchinetti CF, Stadelmann C et al (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128:2705–2712. https://doi.org/10.1093/brain/awh641
Villar LM, Masjuan J, González-Porqué P et al (2002) Intrathecal IgM synthesis predicts the onset of new relapses and a worse disease course in MS. Neurology 59:555. https://doi.org/10.1212/WNL.59.4.555
Greenfield AL, Dandekar R, Ramesh A et al (2019) Longitudinally persistent cerebrospinal fluid B-cells resist treatment in multiple sclerosis. JCI Insight 4:e126599. https://doi.org/10.1101/490938
Lassmann H (2017) Targets of therapy in progressive MS. Mult Scler 23:1593–1599. https://doi.org/10.1177/1352458517729455
Posová H, Horáková D, Čapek V et al (2017) Peripheral blood lymphocytes immunophenotyping predicts disease activity in clinically isolated syndrome patients. BMC Neurol 17:145. https://doi.org/10.1186/s12883-017-0915-1
Kroth J, Ciolac D, Fleischer V et al (2019) Increased cerebrospinal fluid albumin and immunoglobulin A fractions forecast cortical atrophy and longitudinal functional deterioration in relapsing-remitting multiple sclerosis. Multiple Scler J 25:338–343. https://doi.org/10.1177/1352458517748474
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft; JK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft; LJZ: Data curation, Formal analysis, Investigation; MH: Data curation, Methodology, Writing review & editing; MKS: Data curation, Methodology, Writing review & editing; AB: Formal analysis, Methodology, Writing review & editing; ZV: Methodology, Project administration, Writing review & editing.
Corresponding author
Ethics declarations
Ethical approval
The study was approved by the institution’s Ethical Committee (approval No. 8.1–23/69 − 2).
Competing interests
M.H. participated as a clinical investigator and/or received consultation and/or speaker fees from: Biogen, Sanofi Genzyme, Merck, Bayer, Novartis, Pliva/Teva, Roche, Alvogen, Actelion, Alexion Pharmaceuticals, TG Pharmaceuticals. MKS received speaker fees from Sanofi Genzyme and Roche. The other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Turčić, A., Knežević, J., Zaninović, L. et al. Association between peripheral blood immunological status and intrathecal inflammatory markers differentiate multiple sclerosis clinical phenotypes. Acta Neurol Belg (2024). https://doi.org/10.1007/s13760-024-02597-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13760-024-02597-8