Abstract
Background
BAFF (B-cell activating factor of the tumor necrosis factor family) and APRIL (a proliferation-inducing ligand) are two of the major survival factors for B cells. Many studies have shown that BAFF levels were elevated in MS patients. However, whether the levels of CSF BAFF/APRIL increased in NMO patients was still unclear.
Objective
To measure the CSF BAFF and APRIL concentration of in NMO patients, and explore their relationship with disease activity in NMO.
Methods
CSF BAFF and APRIL was measured by an enzyme-linked immunosorbent assay (ELISA) in NMO (n = 22), MS (n = 18) patients and controls (n = 14).
Results
Concentration of BAFF and APRIL in NMO patients were significantly higher than MS and controls. CSF BAFF and APRIL levels in controls were also lower than MS. Both NMO and MS revealed an increased disease disability with increased CSF BAFF. CSF APRIL was associated with EDSS scores in NMO, but not found in MS.
Conclusions
BAFF/APRIL system considered important for aggressive B cells and T-cell responses, and may stimulates B cells and T cell activation in acute relapse of NMO and MS. In NMO patients, CSF BAFF and APRIL may be key factors of B cell immune response and reflect disease severity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromyelitis optica (NMO) and multiple sclerosis (MS) are autoimmune inflammatory demyelinating disease in the central nervous system (CNS). Most NMO and MS patients are characterized as relapsing-remitting course [1–3]. NMO was once considered to be an optic-spinal form of MS. Until 2004, serum anti-aquaporin 4 antibodies (AQP4-Ab) were suggested to be a specific biomarker of NMO, distinguishing from MS [2].
Oligoclonal immunoglobulin bands (OCBs) in cerebral spinal fluid (CSF) suggested that B cells play an important role in the MS pathogenesis [4–6]. Similarly, AQP4-Ab was believed to have a central pathogenetic role in neuromyelitis optica (NMO) which indicate NMO may be adisease mediated by humoral immunity [7, 8]. BAFF (B-cell activating factor of the tumor necrosis factor family) and APRIL (a proliferation-inducing ligand) are two of the major survival factors for B cells. BAFF/APRIL regulates B cell survival, differentiation and class switching. BAFF/APRIL system determines the size of the peripheral B cell pool [9, 10]. High serum BAFF levels have been reported in several autoimmune diseases [7, 11]. In human brain, BAFF is mainly produced by astrocytes in CNS [12]. BAFF/APRIL mRNA expression is increased in MS patients' peripheral blood [13]. Many studies have shown that BAFF levels in untreated MS patients were no significant higher than controls, and elevated in patients treated with interferon-beta (IFN-β) [14, 15]. However, in acute relapse of NMO and MS, whether CSF BAFF/APRIL was increased in NMO patients or MS patients who did not receive IFN-β treatment were still unclear.
In this study, we evaluate the CSF levels of BAFF and APRIL in NMO and MS patients and explore their relationship with disease activity in those diseases.
Subjects and Methods
Patients and Controls
Twenty-two NMO patients based on the 2006 Wingerchuk diagnostic criteria 2006 [16] and 18 relapsing–remitting MS (RRMS) patients fulfilling 2010 McDonald’s diagnostic criteria [3, 17] from Demyelinating Disease Database of Neurology Department of the Third Affiliated Hospital of Sun yat-sen University were enrolled. Patients enrolled from the database were diagnosed by two specialized neurologists. All samples were taken during relapse phase before treatment. A clinical relapse was defined as a sudden appearance of new symptoms, lasting for at least 24 h, with an increase of EDSS over 1.0 before sampling. Patients were not receiving IFN-β or any other immunomodulatory therapies during remission. 14 controls with noninflammatory neurological diseases (amyotrophic lateral sclerosis n = 2, multiple system atrophy n = 3, sciatica n = 4, and cervical spondylosis n = 5) were recruited. Demographic and clinical features of the patients are shown in Table I. There was no significant difference in age and gender between the groups. Indirect immunofluorescence test systems for human AQP4-Ab detection from EUROIMMUN (EUROIMMUN Medizinische Labordiagnostika, Lübeck, Germany) were used. AQP4-Ab was assessed following the manufacturer’s instructions.
Enzyme-linked Immunosorbentassay (ELISA)
CSF samples were obtained during diagnostic lumbar punctures. All samples were processed within 30 min of withdrawal and stored at −80°C until assay. Enzyme-linked immunosorbent assay was used to quantify BAFF (R&D Systems, Minneapolis, USA) and APRIL (Bender MedSystems, Vienna, Austria) in CSF, according to manufacturer's instructions. The minimal detectable doses were 3.4 pg/ml for BAFF and 400 pg/ml for APRIL. CSF samples were analyzed at a dilution of 1:2, and added in duplicate.
Statistical Analysis
The data were presented as mean ± 1 standard deviation (CSF BAFF, APRIL and EDSS score) or median with range (age, onset age,disease duration, EDSS score). Differences in levels of CSF BAFF and APRIL between different subgroups were analyzed using Mann-Whitney U test. Correlations between CSF BAFF, APRIL and EDSS score were analyzed using Spearman’s rank test. P value <0.05 was considered statistically significant. All statistical analysis was performed using SPSS 16.0 (SPSS Inc, Chicago, IL, USA) for windows.
Result
CSF BAFF/APRIL in NMO, MS, Controls
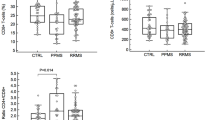
As shown Fig. 1, the concentration of BAFF and APRIL was determined in cell-free CSF from NMO (n = 22), MS (n = 18) patients and controls (n = 14) using ELISA. Mean BAFF was 500.06 ± 387.97 pg/ml for NMO, and 221.60 ± 126.23 pg/ml for MS, compared to 151.88 ± 92.62 pg/ml for controls. BAFF levels were higher in the inflammatory demyelinating diseases cohort compared with the controls (NMO versus controls, p < 0.001; MS versus controls, p = 0.018). When comparing BAFF within subgroups of inflammatory demyelinating diseases, the levels of BAFF were much higher in NMO compared with MS (NMO versus MS, p = 0.001).
The levels of CSF BAFF and APRIL were shown. BAFF levels were higher in the inflammatory demyelinating diseases cohort compared with the controls (NMO versus controls, p < 0.001; MS versus controls, p = 0.018). When comparing BAFF within subgroups of inflammatory demyelinating diseases, the levels of BAFF were much higher in NMO compared with MS (NMO versus MS, p = 0.001). CSF APRIL levels in NMO patients were significantly higher than those in MS (NMO versus MS, p = 0.011) and controls (NMO versus controls, p < 0.001). CSF APRIL levels in controls were lower than MS (MS versus controls, p = 0.027)
Mean concentration of APRIL was 2955.40 ± 1488.51 pg/ml for NMO, 1747.49 ± 1314.29 pg/ml for MS, and 995.90 ± 486.30 pg/ml for controls. CSF APRIL levels in NMO patients were significantly higher than those in MS (NMO versus MS, p = 0.011) and controls (NMO versus controls, p < 0.001). CSF APRIL levels in controls were lower than MS (MS versus controls, p = 0.027).
CSF BAFF/APRIL and EDSS Score
As shown Fig. 2, EDSS scores of all 22 NMO patients and 18 MS patients were reviewed. The median EDSS score was 3.5(1.0–8.5) in NMO and 2.75 (1.0–9.5) in MS subgroup. Both subgroups revealed a trend to an increased disease disability with increased CSF BAFF (NMO: p = 0.008; MS: p = 0.013). CSF APRIL was associated with EDSS scores in NMO (p = 0.003), but not found in MS (p = 0.650).
CSF BAFF/APRIL and CSF Routine
Mean CSF WBC (white blood cells) (106/L) of NMO patients was 6.32 ± 9.81 and 5.00 ± 8.51 in MS subgroup. Mean CSF TP (total protein) (g/L) was 0.25 ± 0.16 for NMO and 0.19 ± 0.11 for MS. In NMO subgroup, BAFF concentrations were associated with CSF WBC (p = 0.004), and APRIL concentrations had a significantly positive correlation with CSF WBC counting (p = 0.012) (Table II).
Discussion
In this case-control study, we confirmed that CSF BAFF and APRIL were increased in NMO and MS, and CSF BAFF had a borderline significant correlation with EDSS scores. CSF APRIL was positive associated with EDSS scores in NMO, but not in MS.
The BAFF/APRIL system was identified almost a decade ago [18]. This system comprises three ligands (BAFF, APRIL, TWE-PRIL), and three signalling receptors (BAFF-R, TACI, BCMA). Studies of BAFF, APRIL and their receptors have highlighted the importance of this ligand/receptor system in regulating B cell homeostasis and tolerance. BAFF/APRIL system is also expressed in activated T cells [19, 20]. In many systemic autoimmune diseases, including rheumatoid arthritis [11], systemic lupus erythematosus [21] and Sjogren’s [22] are associated with higher than normal serum BAFF levels. Previous study shows an increase in BAFF levels in MS patients treated with IFN-β or GA [7, 23]. However, whether the levels of CSF BAFF/APRIL were increased in NMO patients was still unclear. Our results demonstrate that both BAFF and APRIL protein levels are significantly higher in NMO and MS in acute relapse. In acute relapse, T and B cells were activated. BAFF/APRIL system may participate in this process. Higher BAFF and APRIL expression, possibly facilitate B and T cell responses [18]. In the some research, the investigators reported elevated CSF levels of BAFF in MS patients [7]. However, In NMO, there were notable higher BAFF and APRIL in CSF and positive associated with EDSS score. AQP4 is expressed in astrocytes and BAFF is mainly produced by astrocytes in CNS. This result may show astrocyte activation and damage in NMO [7]. On the other hand, It is plausible that more severe inflammation is involved in pathogenetic mechanisms of NMO, especially the humoral immunity mediated by B cell immune response. Activated BAFF/APRIL system may accelerate the AQP4-Ab production.
Taken together, these data could suggest that BAFF/APRIL system considered important for aggressive B cells and T-cell responses, and may stimulates B cells and T cell activation in acute relapse of NMO and MS. In NMO patients, CSF BAFF and APRIL may be key factors of B cell immune response and reflect disease severity.
References
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K, Nakashima I, Weinshenker BG. A serum autoantibody marker of neuromyelitis optica: distinction frommultiple sclerosis. Lancet. 2004;364:2106–12.
McKeon A, Pittock SJ. Neuromyelitis optica and the evolving spectrum of water channel autoimmunity: a new direction. Eur J Neurol. 2009;16(4):433–5.
Sellner J, Boggild M, Clanet M, Hintzen RQ, Illes Z, Montalban X, Du Pasquier RA, Polman CH, Sorensen PS, Hemmer B. EFNS guidelines on diagnosis and management of neuromyelitis optica. Eur J Neurol. 2010;17(8):1019–32.
Wu GF, Alvarez E. The immunopathophysiology of multiple sclerosis. Neurol Clin. 2011;29(2):257–78.
Archelos JJ, Storch MK, Hartung HP. The role of B cells and autoantibodies in multiple sclerosis. Ann Neurol. 2000;47:694–706.
Meinl E, Derfuss T, Krumbholz M, Pröbstel AK, Hohlfeld R. Humoral autoimmunity in multiple sclerosis. J Neurol Sci. 2011;306:180–2.
Vaknin-Dembinsky A, Brill L, Orpaz N, Abramsky O, Karussis D. Preferential increase of B-cell activating factor in the cerebrospinal fluid of neuromyelitis optica in a white population. Mult Scler. 2010;16(12):1453–7.
Chan KH, Ramsden DB, Yu YL, Kwok KH, Chu AC, Ho PW, Kwan JS, Lee R, Lim E, Kung MH, Ho SL. Neuromyelitis optica-IgG in idiopathic inflammatory demyelinating disorders amongst Hong Kong Chinese. Eur J Neurol. 2009;16(3):310–6.
Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast forward on autoimmunity and signaling. Curr Opin Immunol. 2007;19:327–36.
Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–9.
Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–9.
Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–45.
Thangarajh M, Gomes A, Masterman T, Hillert J, Hjelmstrom P. Expression of B-cell-activating factor of the TNF family (BAFF) and its receptors in multiple sclerosis. J Neuroimmunol. 2004;152:183–90.
Krumbholz M, Faber H, Steinmeyer F, Hoffmann LA, Kümpfel T, Pellkofer H, Derfuss T, Ionescu C, Starck M, Hafner C, Hohlfeld R, Meinl E. Interferon-beta increases BAFF levels in multiple sclerosis: implica-tions for B cell autoimmunity. Brain. 2008;131:1455–63.
Piazza F, DiFrancesco JC, Fusco ML, Corti D, Pirovano L, Frigeni B, Mattavelli L, Andreoni S, Frigo M, Ferrarese C, Tredici G, Cavaletti G. Cerebrospinal fluid levels of BAFF and APRIL in untreated multiple sclerosis. J Neuroimmunol. 2010;220:104–7.
Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–9.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O'Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Ng LG, Mackay CR, Mackay F. The BAFF/APRIL system: life beyond B lymphocytes. Mol Immunol. 2005;42:763–72.
Mukhopadhyay A, Ni J, Zhai Y, Yu GL, Aggarwal BB. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-kappaB, and c-Jun NH2-terminal kinase. J Biol Chem. 1999;274:15978–81.
Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999;65:680–3.
Eilertsen GO, Van Ghelue M, Strand H, Nossent JC. Increased levels of BAFF in patients with systemic lupus erythematosus are associated with acute-phase reactants, independent of BAFF genetics: a case-control study. Rheumatology (Oxford). 2011;Oct 8. PubMed PMID: 21984763.
Vadacca M, Margiotta D, Sambataro D, Buzzulini F, Lo Vullo M, Rigon A, Afeltra A. BAFF/APRIL pathway in Sjögren syndrome and systemic lupus erythematosus: relationship with chronic inflammation and disease activity. Reumatismo. 2011;62:259–65.
Ragheb S, Li Y, Simon K, VanHaerents S, Galimberti D, De Riz M, Fenoglio C, Scarpini E, Lisak R. Multiple sclerosis: BAFF and CXCL13 in cerebrospinal fluid. Mult Scler. 2011;17:819–29.
Acknowledgments
This study was supported by the Natural Science Foundation of China (grant no.81171126).
Author information
Authors and Affiliations
Corresponding author
Additional information
Honghao Wang, Kai Wang, Xiaonan Zhong and Wei Qiu contributed equally to the manuscript.
Rights and permissions
About this article
Cite this article
Wang, H., Wang, K., Zhong, X. et al. Cerebrospinal Fluid BAFF and APRIL Levels in Neuromyelitis Optica and Multiple Sclerosis Patients During Relapse. J Clin Immunol 32, 1007–1011 (2012). https://doi.org/10.1007/s10875-012-9709-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-012-9709-9