Abstract
Severe combined immunodeficiency is a life-threatening primary immune deficiency characterized by low numbers of naïve T cells. Early diagnosis and treatment of this disease decreases mortality. In 2008, Wisconsin began newborn screening of infants for severe combined immunodeficiency and other forms of T-cell lymphopenia by the T-cell receptor excision circle assay. In total, 207,696 infants were screened. Seventy-two infants had an abnormal assay. T-cell numbers were normal in 38 infants, abnormal in 33 infants, and not performed in one infant, giving a positive predictive value for T-cell lymphopenia of any cause of 45.83% and a specificity of 99.98%. Five infants with severe combined immunodeficiency/severe T-cell lymphopenia requiring hematopoietic stem cell transplantation or other therapy were detected. In summary, the T-cell receptor excision circle assay is a sensitive and specific test to identify infants with severe combined immunodeficiency and severe T-cell lymphopenia that leads to life-saving therapies such as hematopoietic stem cell transplantation prior to the acquisition of severe infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infants born with severe combined immunodeficiency (SCID) typically appear normal at birth but are at a high risk of developing life-threatening infections, a risk that is markedly increased once the level of maternally transferred antibody has declined. Although there are numerous genetic etiologies of SCID, severe deficiency of naïve T cells is a universal finding [1]. Detecting and treating these children within the first 3.5 months of life and prior to the development of severe infections improves outcomes, although the diagnosis may be difficult in an otherwise healthy appearing infant [2, 3].
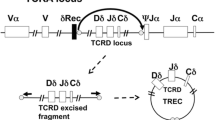
Population-based newborn screening (NBS) for SCID was initiated in Wisconsin in 2008 and has since begun in several states [4]. Currently, the NBS test used to detect SCID is the T-cell receptor excision circle (TREC) assay, which enumerates the number of TRECs by real-time quantitative polymerase chain reaction (RT-qPCR) on DNA extracted from routine NBS cards [5–7]. TRECs are small, circular pieces of DNA that are formed during the differentiation of T cells in the thymus as a result of the rearrangement of T-cell receptor genes and do not replicate with cellular division. One particular TREC, the δRec-ψJα TREC, is produced by approximately 70% of all T cells that express the α/β T-cell receptor. Therefore, quantification of TRECs by RT-qPCR using DNA isolated from NBS blood spots is considered a surrogate marker for the number of naïve T-cells [8]. Prior studies demonstrated that the TREC assay could identify babies with SCID regardless of genetic cause [5, 7]. In addition, we have previously shown that the TREC assay can detect a variety of forms of T-cell lymphopenia (TCL) as well as other serious immune defects in infants [4]. In this report, we summarize the results of 3 years of NBS in Wisconsin for SCID using the TREC assay.
Methods
Study Population
All infants born in the State of Wisconsin from January 1, 2008 through December 31, 2010 were included in the study. The Secretary of the Wisconsin Department of Health and Family Services approved by statute the NBS program to start routine prospective screening for SCID using the TREC assay effective on January 1, 2008. The State of Wisconsin has an “opt out” or informed dissent for all NBS tests that are approved by statutory law. Because NBS for SCID was part of routine NBS in Wisconsin beginning 2008, the screening performed was not considered research and no institutional review board approval was needed. Subsequent analysis of infants detected by NBS was approved by the institutional review board of Children’s Hospital of Wisconsin.
TREC Analyses
TREC analysis was performed as previously described [4, 7]. Briefly, newborn screening cards were obtained, a 3.2-mm disk was punched from the dried blood spot, DNA extracted, and RT-qPCR of TRECs and β-actin performed. TREC values were normalized to microliters of blood based on the estimation that each punch contained 3 μl of blood.
Lymphocyte Subset Analysis by Flow Cytometry
Whole blood collected in sodium heparin tubes was stained with antibodies to CD3-FITC (SK7), CD4-PE (SK3), CD8-PE (SK1), CD19-PE (SJ25C1), and CD45-PE (2D1), all from BD-Immunocytometry Systems; CD56-APC (N901; Beckmann Coulter); and CD45-PerCP (2D1) and CD45RO (UCHL1) from Invitrogen. Samples were analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
Algorithm of Positive Results of TREC Analysis for Newborn Screening for SCID/TCL
An algorithm was developed that established a standard operating protocol for the TREC assay [4]. Briefly, full-term infants with a TREC level below the cut-off were first tested for DNA integrity by analyzing β-actin by qRT-PCR. If the β-actin level was normal with abnormally low TRECs, an abnormal report was issued and the primary care provider was contacted for confirmatory testing by flow cytometry, although a few physicians requested repeat TREC analysis. If the β-actin result was low, an inconclusive report was issued and the screening test was repeated with a new newborn screening card. For pre-term infants [adjusted gestational age (AGA) <37 weeks] with an abnormal or inconclusive TREC assay, the screening test was repeated until either normal or until the infant reached 37 weeks AGA at which time the infant was reclassified as an abnormal. Lymphocyte subset analysis by flow cytometry was recommended on all full-term infants and all pre-term infants with an AGA of 37 weeks with an abnormal TREC assay. Infants with an abnormal flow cytometry were then referred for evaluation by a clinical immunologist.
During the first year of screening, the TREC assay cut-off value was set at 25 TRECS/μl of blood, which represented the lowest 1.1% of TREC values from randomized blood spots during assay development. Due to the low numbers of full-term infants with abnormal TREC assays in the first 19 months of NBS, the cut-off value for TRECs was increased to 40 TRECs/μl in August, 2009 and remained at this level for the next 17 months of screening.
Results
During the first 3 years of screening for SCID/TCL by TREC analysis, 207,696 infants were screened, consisting of 188,741 (90.87%) full-term infants and 18,955 (9.13%) pre-term infants (Table I). Of the full-term infants, 63 (0.03%) were classified as abnormal on initial testing, based on a low TREC assay with normal β-actin analysis, and 51 (0.025%) were inconclusive (i.e. low TREC, low b-actin). Of pre-term infants, 94 infants were classified as abnormal (0.045%) and 241 (0.116%) were classified as inconclusive, giving a repeat testing rate for pre-term infants of 0.16%. Thus, the repeat testing rate of pre-term infants (inconclusive and abnormal results) and full-term infants (inconclusive results) was 0.19%.
In total, 72 infants were ultimately classified as abnormal, including nine full-term or pre-term infants reclassified as abnormal after repeat testing. Of these, 38 infants were ultimately found to be normal, resulting in a false positive rate of 0.018% and specificity of 99.98%. Thirty-three infants were found to have TCL of varying degrees. Thus, the positive predictive value of the test predicting TCL was 45.83%.
Figure 1 and Table II summarize the clinical diagnoses, TREC values, and total T-cell counts of infants with abnormal TRECs. Of infants with TCL, 58% (n = 19) had secondary causes for their TCL such as anatomic abnormalities of the lymphatics, chromosomal abnormalities, multiple congenital anomalies, or a presumed metabolic disorder. The remaining 14 infants had primary TCL, which included five infants with reversible TCL, four infants with 22q11.2 deletion syndrome, and five infants with SCID/severe TCL. Interestingly, a fifth infant with 22q11.2 deletion syndrome was detected on the initial screen, but on follow-up testing the lymphocyte counts were within normal limits, and thus the infant was classified as normal. Interestingly, this infant had a congenital heart defect that was not detected until after the newborn screening result.
The laboratory characteristics of the five infants with SCID/TCL detected by the TREC assay are summarized in Table III. Importantly, in all cases of SCID/TCL, an initial TREC value of zero was obtained on NBS, suggesting that the absence of detectable TRECs in the presence of normal β-actin is highly predictive of severe T-cell lymphopenia requiring urgent clinical evaluation. The first infant identified by the NBS presented with recurrent abscesses and was found to have a dominant negative mutation in RAC2 resulting in defects in both neutrophils and lymphocytes [4, 9]. This infant underwent an umbilical cord blood transplant with myeloablative conditioning and is currently doing well off all medications (e.g., immunosuppressants or intravenous immunoglobulin) for approximately 3 years. In only one of the remaining four infants with SCID/TCL was the molecular cause of TCL ascertained (i.e., ADA deficiency, infant 2) despite DNA sequencing for genes typically responsible for SCID/TCL. The third infant exhibited deficiencies of T and B cells with normal NK cells numbers, although sequencing for mutations in the RAG1, RAG2, or DCLRE1C were negative (data not shown). Mitogen responses were low (Table III). This infant received an umbilical cord blood transplant, is fully engrafted off all medications, and doing clinically well. A fourth infant was identified with deficiency of T cells but with relatively normal B and NK cell counts. Identification of this infant led to the evaluation of a sibling with a similar phenotype that was suffering from recurrent opportunistic infections and autoimmune enteropathy [4]. The sibling subsequently underwent HSCT and is engrafted and doing well while the proband is awaiting HSCT. Analysis of the fifth infant similarly demonstrated low T-cell numbers with relatively normal NK and B cell counts. Sequence analysis for mutations in CD3, ZAP-70, and IL-7R, or microdeletions at 22q11.2 failed to detect a known defect (data not shown). T-cell proliferative responses to phytohemmaglutinin were reduced, but the infant’s T cells demonstrated virtually no proliferative response to IL-7 (Fig. 2). Because of this abnormal finding in conjunction with the persistent and profound TCL and undetectable TRECs, this infant underwent a matched unrelated HSCT and is currently engrafted but early in the course of immune reconstitution. In total, three infants detected by the TREC assay have undergone HSCT for SCID/TCL, and all are engrafted and alive to date.
Proliferative responses to IL-2 and IL-7 in infants with TCL. PBMCs were stimulated with antibodies to CD3 alone or with IL-2 or IL-7 (10 U/ml) as indicated. Anti-IL-2 antibody (10 μg/ml) was added to some samples as indicated. Three days later, 3H-thymidine was added for 16 h and analyzed. Results are representative of two separate experiments. **p < 0.01, ***p < 0.001 by ANOVA
Correlation of TREC Assay and T-Lymphocyte Numbers as Determined by Flow Cytometry
Finally, we wished to determine the correlation between TREC values obtained from newborn screening cards and CD3 T-cell counts determined by flow cytometry. It was not possible to determine if an abnormal TREC assay resulted from low T-lymphocyte counts at the time of the initial test since the confirmatory flow cytometry was performed up to 10 days after the initial TREC assay. Therefore, we investigated how well TREC values from newborn screening cards correlated with T-lymphocyte counts by spotting newborn screening cards at the time of T-lymphocyte enumeration by flow cytometry. As shown in Fig. 3, there was a strong correlation between TREC value and the absolute CD3 T-cell count (R 2 = 0.423, p ≤ 0.0001). However, there were instances where the T-cell count was either higher or lower than what would be predicted by the TREC assay.
Discussion
Newborn screening for SCID began in Wisconsin in 2008 and was followed by the States of Massachusetts, New York, Louisiana, and California. Our data over 3 years of NBS in Wisconsin demonstrates that the TREC assay is a highly sensitive test that detects known causes of SCID. In addition, this assay detects infants with persistent severe TCL, as well as infants with other defects of the hematopoietic system (i.e., RAC2 deficiency). During this 3-year period, no cases of SCID have been diagnosed in the State of Wisconsin outside of the NBS, as no cases were reported in the two major pediatric referral centers in the state. This assay is also highly specific, as there were only 38 false positive tests out of over 200,000 infants, giving a false positive rate of 0.018%. One important contributing factor for the low false positive rate in Wisconsin is the algorithm whereby premature infants have repeat TREC assays performed until they reach an AGA of 37 weeks. This is standard practice in Wisconsin for other NBS assays since there is a high false positive rate in pre-term infants for other NBS tests. Even with the repeat screening of pre-term infants, the repeat testing rate was only 0.19%, which is well below the repeat testing rate of other NBS assays (data not shown).
Forty-eight percent of infants with an abnormal TREC test had T-cell lymphopenia to varying degrees. Many of these cases were expected causes, such as 22q11.2 deletion syndrome or lymphatic malformations resulting in chylous effusions. Importantly, of the five infants with 22q11.2 deletion syndrome, two infants were not detected at birth, and one of these infants had a significant heart defect. A variety of chromosomal or presumed metabolic defects were also detected. One case of Jacobsen syndrome was found, and this chromosomal abnormality has been associated with the development of immune deficiency with increasing age [10]. Two cases of Down syndrome were detected, and both were associated with duodenal atresia. Three infants expired due to a presumed metabolic defect, and none succumbed to infectious complications. Numerous other congenital anomalies were detected, such as Barth syndrome (dilated cardiomyopathy), VACTERL syndrome (vertebral anomalies, anal atresia, cardiovascular anomalies, tracheoesophageal fistula, esophageal atresia, radial/renal anomalies, limb defects), thrombocytopenia-absent-radius (TAR) syndrome, and a case of ectrodactyly ectodermal dysplasia cleft syndrome. In addition, three infants without 22q11.2 deletion syndrome had associated heart defects, one infant had a heart defect as well as multiple congenital anomalies, and one infant had a degenerative neuromuscular disorder. Whether T-cell lymphopenia is a primary or secondary phenomenon in these critically ill patients is unknown. However, in nearly all of these cases, the lymphopenia was mild to moderate.
Seven percent of infants with abnormal TREC assays were found to exhibit SCID/severe TCL. Surprisingly, only one of these five cases resulted from a known cause of SCID (ADA deficiency), and one infant had a dominant negative mutation in RAC2 that was previously described to be only associated with a neutrophil defect [9]. The three remaining cases highlight a dilemma of this screening program, namely what is the correct medical therapy in a child with isolated but profound TCL, with no known genetic cause, and who does not have a SCID-defining infection due to early identification and prophylactic antibody replacement and antimicrobials. In the case of the infant with T-B-NK + SCID, the T- and B-cell counts and functional studies were low enough to recommend transplantation even though a SCID-associated genetic mutation could not be identified. In addition, the lack of B cells points strongly to defects in genes involved in lymphocyte receptor recombination or DNA repair genes, or to a defect affecting growth or survival of T and B lymphocytes. The two cases of T-B + NK + SCID/TCL described above were even more difficult to recommend transplantation. In one case, there was a family history of a similarly affected sibling that demonstrated failure to thrive, invasive fungal infections, and autoimmune enteropathy [4]. However, one infant had significant lymphopenia, persistent TREC values of 0, but no family history of SCID. This infant did not have 22q11.2 deletion syndrome, and a thymus was present. This example is particularly difficult, as there is no consensus as to what T-cell count is consistent with a classic SCID phenotype. However, recently it has been shown in patients with 22q11.2 deletion syndrome that there is an increase in non-cardiac mortality with T-cell counts less than the 10th percentile for age, with four of 11 children succumbing to infections or Epstein–Barr virus associated lymphoproliferative disease [11]. However, the clinical consequences of T-cell lymphopenia in 22q11.2 deletion syndrome may be fundamentally different compared to infants with profound, idiopathic T-cell lymphopenia. Ultimately, we demonstrated that this infant’s T cells had diminished IL-7-dependent proliferation, thereby explaining her persistent T-cell lymphopenia and providing a strong rationale to proceed with HSCT.
Although there is no clear consensus of what T-cell count is consistent with SCID, a T-cell count of less than 200 cells/μl is highly suggestive. When T-cell counts are greater than 200 cells/μl with relatively normal T-cell proliferative responses and no genetic cause, we initially take a “watch and wait” approach over the first few months of life to determine if the T-cell lymphopenia resolves. During this period of time, every effort is made to ascertain the genetic cause of the TCL. In the absence of a defined genetic defect, we formally present each case to a panel of experts in PID/HSCT to aid in the decision to transplant while at the same time performing additional testing to help guide our ultimate diagnostic decisions. A potential downside to this approach is that additional testing may not yield a definitive diagnosis, and in the intervening time complications due to SCID/TCL may occur, including potentially life-threatening infections.
As newborn screening for SCID is increasingly implemented throughout the USA and the world, the phenotypic spectrum of SCID and other forms of profound T-cell lymphopenia will increase with a concomitant increase in disorders without a known genetic cause. The decision regarding immune reconstitution in these cases will be difficult as we must not only consider the risk of HSCT but also the serious long-term consequences of preparative regimens and whether or not these regimens should be used. Ultimately, the decision to perform HSCT in these situations should be based on the constellation of immunological findings and their persistence over time and should not require a molecular diagnosis [12]. Properly controlled clinical trials are urgently needed to determine the role of preparative conditioning regimens in infants with SCID.
In summary, our 3-year experience of newborn screening for SCID/TCL by the TREC assay has shown that this assay is sensitive, specific, and detects known and unknown causes of SCID/TCL. With the recommendation for the institution of nation-wide NBS screening for SCID/TCL by the Secretary of Health and Human Services, these programs will teach us the exact incidence of SCID as well as other defects of T-cell development, and more importantly will guide practitioners as to how to treat these infants to obtain the best possible outcomes.
Abbreviations
- SCID:
-
Severe combined immunodeficiency
- HSCT:
-
Hematopoietic stem cell transplantation
- NBS:
-
Newborn screening
- TREC:
-
T-cell receptor excision circles
References
Puck JM. Population-based newborn screening for severe combined immunodeficiency: steps toward implementation. J Allergy Clin Immunol. 2007;120(4):760–8.
Brown L, Xu-Bayford J, Allwood Z, Slatter M, Cant A, Davies EG, et al. Neonatal diagnosis of severe combined immunodeficiency leads to significantly improved survival outcome: the case for newborn screening. Blood. 2011;117(11):3243–6.
Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: longterm outcomes. Immunol Res. 2011;49(1–3):25–43.
Routes JM, Grossman WJ, Verbsky J, Laessig RH, Hoffman GL, Brokopp CD, et al. Statewide newborn screening for severe T-cell lymphopenia. JAMA. 2009;302(22):2465–70.
Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115(2):391–8.
Baker MW, Laessig RH, Katcher ML, Routes JM, Grossman WJ, Verbsky J, et al. Implementing routine testing for severe combined immunodeficiency within Wisconsin's newborn screening program. Public Health Rep. 2010;125 Suppl 2:88–95.
Baker MW, Grossman WJ, Laessig RH, Hoffman GL, Brokopp CD, Kurtycz DF, et al. Development of a routine newborn screening protocol for severe combined immunodeficiency. J Allergy Clin Immunol. 2009;124(3):522–7.
Douek DC, Vescio RA, Betts MR, Brenchley JM, Hill BJ, Zhang L, et al. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet. 2000;355(9218):1875–81.
Accetta D, Syverson G, Bonacci B, Reddy S, Bengtson C, Surfus J, et al. Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. J Allergy Clin Immunol. 2011;127(2):535–8.
Sirvent N, Monpoux F, Pedeutour F, Fraye M, Philip P, Ticchioni M, et al. Jacobsen's syndrome, thrombocytopenia and humoral immunodeficiency. Arch Pediatr. 1998;5(12):1338–40.
Eberle P, Berger C, Junge S, Dougoud S, Buchel EV, Riegel M, et al. Persistent low thymic activity and non-cardiac mortality in children with chromosome 22q11.2 microdeletion and partial DiGeorge syndrome. Clin Exp Immunol. 2009;155(2):189–98.
Griffith LM, Cowan MJ, Notarangelo LD, Puck JM, Buckley RH, Candotti F, et al. Improving cellular therapy for primary immune deficiency diseases: recognition, diagnosis, and management. J Allergy Clin Immunol. 2009;124(6):1152–60.
This work was supported by funding from the Children's Hospital of Wisconsin, the Jeffrey Modell Foundation, the Wisconsin State Laboratory of Hygiene and by Cooperative Agreement 5U01EH000365 from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verbsky, J.W., Baker, M.W., Grossman, W.J. et al. Newborn Screening for Severe Combined Immunodeficiency; The Wisconsin Experience (2008–2011). J Clin Immunol 32, 82–88 (2012). https://doi.org/10.1007/s10875-011-9609-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9609-4