Abstract
Daily rainwater samples collected at Lijiang in 2009 were analyzed for pH, electrical conductivity, major ion (SO4 2−, Cl−, NO3 −, Na+, Ca2+, Mg2+, and NH4 +) concentrations, and δ18O. The rainwater was alkaline with the volume-weighted mean pH of 6.34 (range: 5.71 to 7.11). Ion concentrations and δ18O during the pre-monsoon period were higher than in the monsoon. Air mass trajectories indicated that water vapor from South Asia was polluted with biomass burning emissions during the pre-monsoon. Precipitation during the monsoon was mainly transported by flow from the Bay of Bengal, and it showed high sea salt ion concentrations. Some precipitation brought by southwest monsoon originated from Burma; it was characterized by low δ18O and low sea salt, indicating that the water vapor from the region was mainly recycled monsoon precipitation. Water vapor from South China contained large quantities of SO4 2−, NO3 −, and NH4 +. Throughout the study, Ca2+ was the main neutralizing agent. Positive matrix factorization analysis indicated that crustal dust sources contributed the following percentages of the ions Ca2+ 85 %, Mg2+ 75 %, K+ 61 %, NO3 − 32 % and SO4 2− 21 %. Anthropogenic sources accounted for 79 %, 68 %, and 76 % of the SO4 2−, NO3 − and NH4 +, respectively; and approximately 93 %, 99 %, and 37 % of the Cl−, Na+, and K+ were from a sea salt source.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Precipitation throughout the world is contaminated with various types of pollutants, and these contaminants in turn can affect the scavenging of soluble components from the atmosphere (Shrestha et al. 2002; Tang et al. 2005; Zhang et al. 2007; Cao et al. 2009). Precipitation chemistry remains a major environmental issue in several parts of the world (e.g., eastern North America, south-east Asia, and Europe) owing to concerns over acid deposition, eutrophication, trace metal deposition, ecosystem health, biogeochemical cycling, and global climate change (WMO).
The Tibetan plateau is far from industrialized areas, and it is sparsely populated; as a result the plateau is ideal for monitoring global environment changes and for evaluating the impacts of human activities on the atmosphere. Previous studies have shown that because of the buffering effects of natural dust and the relatively small impacts from human activities, precipitation from the Tibetan Plateau is slightly alkaline (Zhang et al. 2003a, b, 2012b; Li et al. 2007). However, precipitation from the northern part of the Tibetan plateau has become increasingly acidic as a result of the rapid industrial development (Zhang et al. 2003a). It was indicated that the chemical composition of precipitation on Tibetan Plateau have been influenced by anthropogenic pollutants. Thereby, the investigation of precipitation chemistry of the Tibetan Plateau and its surrounding area becomes very important.
Changes in precipitation chemistry have led to the inference that the chemical composition Tibetan precipitation is affected by pollutants transported from areas upwind. Indeed, the rapid economic development and increasing energy consumption have resulted in the increased emissions of SO2 and NOx (Lee et al. 2000; Cao et al. 2009), with the unfortunate result that south/southwest China has become the third region of the world most seriously affected by acid rain in the world after North America and Central Europe (Galloway et al. 1976; Cowling 1982; Khwaja and Husain 1990; Heuer et al. 2000; Ito et al. 2002; Smirnioudi and Siskos 1992; Al-Momani et al. 1995; Sanusi et al. 1996; Marquardt et al. 2001; Topcu et al. 2002).
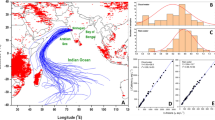
Lijiang is located on the southeastern flank of the Tibetan Plateau (Fig. 1), and it has been used for investigations of precipitation at an inland background site. Studies of precipitation chemistry were first conducted from 1987 to 1989 (Liu et al. 1993), and the daily variations in δ18O in rain during monsoon period have been discussed (Pang et al. 2006). Airflow from the Bay of Bengal, the Tibetan Plateau, and the western Pacific can affect the part of southwestern China where Lijiang is located. Araguás-Araguás et al. (1998) described the weather patterns that controlled air mass movements and rainfall; they concluded that the meteorology and rainfall in southwest China are mainly controlled by three air masses (1) equatorial-maritime air originating in the Indian Ocean, (2) equatorial-maritime air from the western Pacific, and (3) tropical-maritime air originating over the northern Pacific.
Location of Lijiang and classification of backward trajectories. Sector 1 represents south Asia and includes one sample from the Tibetan Plateau; Sector 2 represents northern China including the Sichuan Basin; Sector 3 represents south China which is a region impacted by acid rain region; Sector 4 represents flow from Burma; Sector 5 represents air from the Bay of Bengal
Therefore, the chemical composition of precipitation from southwest China is not only influenced by human activities but also by the various sources of water vapor. Investigations into the precipitation chemistry of the Tibetan plateau and surrounding areas are important for understanding the current impacts of air pollution and for developing scenarios for potential changes in the future. To investigate the influences of the developing tourism industry, a long-term study into chemical composition of precipitation at Lijiang has been conducted (Zhang et al. 2012b). In this work, the chemical composition of rainwater collected at Lijiang in 2009 is presented with the following objectives: (1) to understand the pH and chemical composition of the individual rain events, (2) to identify transport pathways of air mass arrived at Lijiang, and (3) to find the influence on rainwater chemistry by various air masses.
2 Methodology
2.1 Sampling site
Lijiang is an intermontane basin situated at a mean elevation of 2,393 m in the southeastern part Tibetan plateau in Southwest China (Fig. 1); it is a world-famous tourist destination. To promote tourism, industries that emit low concentrations of pollutants have been encouraged. Around Lijiang, there are several cities popular with tourists; the exception is Panzhihua, which is a heavily industrialized city ~200 km to the east of Lijiang. The climate in Lijiang is dominated by the southwest summer monsoon from May to October and by the southern branch of the westerly jet from November to April of the next year. The annual mean temperature and precipitation are 12.6 °C and 900 mm, respectively. Most of the precipitation falls during the summer months when the monsoon flow is strongest, that is, from June to September. The primary source water vapor for the rain during the monsoon is the Indian Ocean (Pang et al. 2006).
The sampling site (100.21 °E, 26.90 °N) located at Lijiang was 2,425 m above sea level. During our study which ran from March to October 2009, the total rainfall at the site was 819.6 mm, and a total of 55 rainwater samples were collected. Of these, 45 daily samples with sufficient volumes and without any contamination (such as leaves and insects) from the air were selected for analysis.
2.2 Sampling and analysis
Samples were manually collected with the use of a polyethylene film with a diameter of 25 cm that was placed approximately 1.5 m above the ground. To avoid dry deposition, the film was deployed at the beginning of a rainfall event, and then the collected rainwater was poured immediately into 120 ml polyethylene containers. These containers were pre-cleaned by repeated rinses with purified water (R ≈ 18.2 MΩ). A new polyethylene film was used for each collection. The rainwater samples were frozen for storage, and then they were transported to the State Key Laboratory of Cryospheric Sciences, Chinese Academy of Sciences (Lanzhou) for analysis.
Precautions were taken to minimize contamination both in the field and in the laboratory. To evaluate possible contamination caused by the polyethylene film, five blank samples using Milli-Q water (R ≈ 18.2 MΩ) were prepared and analyzed to simulate the rainwater collection process. The mean results showed the following concentrations (in μg · L−1): Na+ (<MDL (method detection limit)), NH4 + (10.2), K+ (2.1), Mg2+ < (MDL), Ca2+ (<MDL), SO4 2− (8.8), NO3 − (<MDL), and Cl− (3.1). These concentrations are 1 to 3 orders of magnitude lower than the average concentrations measured in the rainwater samples themselves. These mean field-blank values were subtracted from the sample values.
Each sample was divided into three parts. One aliquot was for pH and conductivity analyses, another was for ion determinations, and the last for stable oxygen-18 isotope measurements. The rainwater pH was measured in the laboratory at a temperature of approximately 25 °C using an acidometer (PSH-3B, INESA Scientific Instrument Corp, Shanghai, China). Electric conductivity was measured using a conductivity meter (DDSJ-308A, INESA Scientific Instrument Corp, Shanghai, China). Cation (NH4 +, Mg2+, Na+, and Ca2+) concentrations were determined using a ICS1500 ion chromatograph (Dionex Corp, Sunnyvale, CA) while anions (Cl-, SO4 2−, and NO3 −) were measured with a Dionex DX320 ion chromatograph. The MDLs in μg · L−1 were as follows: Na+ (4.6), NH4 + (4.0), K+ (10.0), Mg2+ (10.0), Ca2+ (10.0), SO4 2− (20.0), NO3 − (15.0), and Cl− (0.5). Standard reference materials produced by the National Research Center for Certified Reference Materials in China were analyzed for quality assurance purposes.
δ18O was measured at the Key Laboratory of Cryosphere and Environment at the Chinese Academy of Sciences with the use of a Finnegan™ MAT-252 isotope-ratio mass spectrometer (Thermo Electron Corporation, Waltham, MA USA). The instrument has an analytical precision of ±0.05‰ (Pang et al. 2012). 18O/16O ratios were expressed in the conventional δ notation in parts per thousand relative to the Vienna standard mean ocean water.
2.3 Sources apportionment
Principal component analysis (PCA) and positive matrix factorization (PMF) were used to identify possible sources for the rainwater ions. PCA is a mathematical procedure that uses orthogonal transformations to convert a set of observations for variables that may be correlated into a set uncorrelated variable called principal components, and it was calculated with the use of SPSS (Version 13.0, SPSS, Inc. Chicago) in this study. PMF is a multi-variate factor analysis tool that utilizes error estimates and produces non-negative results (Paatero and Tapper 1994). PMF also provides factor contributions and factor profiles of the sample data, and it has been applied to data from precipitation studies (Juntto and Paatero 1994; Kitayama et al. 2010; Huston et al. 2012). Here, the PMF 3.0 model of the US EPA (Norris et al. 2008) was used to apportion the source contributions for the ions in the precipitation samples from Lijiang. The design of input files, extraction of principle components and overall usage of the model were based on the US EPA procedure.
3 Results and discussion
3.1 Data quality
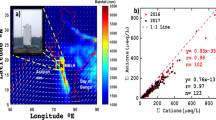
Ion charge balance plots showing the relationship between the measured cations (Ca2++ NH4 ++ Mg2++ Na++ K+) and anions (Cl− + SO4 2− + NO3 −) (Fig. 2) revealed a systematic anion deficit in the majority of the samples (33 samples, 73 %). Only 12 samples (27 %) had an apparent excess of anions. The cation and anion concentrations were positively correlated, but the slopes of both the cation dominated (CD) and anion dominated (AD) samples deviated from unity. The same was true for the relationship between measured and calculated conductivities (Fig. 2). These results suggest that some ions not included in the analyses affected the charge balance in the samples; these presumably include HCO3 −/CO3 2− which also were unmeasured in several other related studies (Li et al. 2006; Zhao et al. 2008). Furthermore, organic compounds such as acetate, formate, and oxalate, are commonly observed in tropical and subtropical rainwater (Honório et al. 2010), and these too, likely contributed to the apparent charge imbalance. Indeed, formic and acetic acids were found to contribute 44 % of the acidity of the precipitation at a rural site in Lijiang (Liu et al. 1993).
3.2 pH
The pH of natural rainwater at equilibrium with atmospheric CO2 is 5.6: in comparison, the pH of the rainwater from Lijiang during our study ranged from 5.71 to 7.11, with a volume-weighted mean (VWM) of 6.34 (Table 1). Thus, the rain can be considered alkalized or alkaline (Ozsoy and Saydam 2000). Figure 3 shows that the pH values of the majority of the samples (60 %) from Lijiang were in the range of 6 to 6.5. Only two samples had pH values greater than 7 while six samples had pHs lower than 6, again indicating that rainwater in the Lijiang region is alkaline relative to equilibrium with the CO2-HCO3 −-CO3 2− system. Figure 3 also shows that the concentrations of some ions increased in proportion as a function of increasing pH while others showed the opposite trend. That is, the combined percentages of Ca2+ and Mg2+ ions increased from 37 %, 39 %, 54 %, and 58 % of the total measured microequivalents at pHs of 5.5 to 6, 6 to 6.5, 6.5 to 7, and 7 to 7.5, respectively. In contrast, the combined percentages of SO4 2− and of NO3 − ions were 41 %, 35 %, 25 %, and 24 % at pHs of 5.5 to 6, 6 to 6.5, 6.5 to 7, and 7 to 7.5.
The increase in Ca2+ and Mg2+ concentrations at higher pHs is consistent with greater abundances of alkaline material in the rain. In fact, the suspended particulate matter in Lijiang precipitation is known to be rich in calcium carbonates and bicarbonates (Zhang et al. 2011). Zhang et al. (2003a) reported that alkaline precipitation on the Tibetan plateau was not caused by low atmospheric pCO2 but rather by the neutralization of acidity by airborne dust particles derived from surface soils. Alkaline rainwater resulting from the neutralizing effects of soil dust also has been found in remote sites and semi-arid regions in India (Khemani et al. 1985). Moreover, significant correlations between SO4 2− and Ca2+ (r = 0.51, P < 0.01) and between NO3 − and Ca2+ (r = 0.80, P < 0.01) suggest that acidic pollutants can be sorbed to particulate matter and be neutralized Ca2+ in the particles.
Alkaline rainwater also has been found at other sites on the Tibetan Plateau. For example, at Waliguan, located on the northern part of the plateau, the average pH of rain collected in 1997 was 6.38 (Tang et al. 2000), while rain from Nam Co, located in the central section of the plateau, had a pH of 6.59 from 2005 to 2006 (Li et al. 2007). On the other hand, various sites in southwestern China that are close to the southeastern Tibetan plateau are classified as acid rain regions; these include Panzhihua, which had an average pH of 4.86 from 1996 to 2000 (Tu et al. 2004), Puding with an average pH of 5.4 in 2008 (Wu et al. 2012), and Chengdu with an average pH value of 5.2 in 2008 (Wang and Han 2011).
Data from several decades ago show that the mean pH in the Lijiang region was 5.00 from 1987 to 1989 (Liu et al. 1993); this can be considered a near background pH value, and it shows that precipitation from Lijiang is naturally slightly acidic. However, with the implementation of industrial policies designed to promote tourism by reducing pollution, the pH precipitation increased to 6.08 from 1989 to 2006 (Zhang et al. 2012b). The acidification of the rain on the Tibetan plateau in Lijiang can therefore be prevented or maintained at low levels by restricting pollution emissions. This situation is in marked contrast to the acidification of rainwater on the North Tibetan plateau, which has been caused by industrial development (Zhang et al. 2003a).
3.3 Chemical composition
VWM pH values, concentrations of major ions, total ionic concentrations, and δ18O values (arithmetic means) are listed in Table 1. The mean total ion concentration in Lijiang was 130.95 μeq · L−1; this is considerably higher than the 26.42 μeq · L−1 reported for the same location by Liu et al. (1993) and slightly higher than the 119.65 μeq · L−1 reported for Lijiang from 1989 to 2006 (Zhang et al. 2012b). The total ion concentration in this study is significantly lower than that observed at regional background stations in East China such as Linan (311.62 μeq · L−1), Shangdianzi (864.02 μeq · L−1), or Longfengshan (273.79 μeq · L−1) (Li et al. 2010).
The sequence of the VWM concentrations of the ionic species in Lijiang rainwater was as follows: Ca2+ > SO4 2− > NH4 + > Mg2+ > NO3 − > Cl− > Na+ > K+. Thus, SO4 2− and Ca2+ were the dominant cations and anions, respectively. The high concentrations of NH4 + and Mg2+ are consistent with the alkaline character of the precipitation; indeed, the sum of the concentrations of Ca2+, NH4 +, and Mg2+ was higher than that of the combined SO4 2− and NO3 −. Sea salt ions were at low concentrations in our rain samples, this may be related to the long-distance transport because the shortest straight-line distance from our site to the nearest coast exceeds 1,000 Km.
It is obvious that the concentration of each ion and the total measured ion concentrations were much greater in the rain samples collected during the pre-monsoon period compared with those from the monsoon (Fig. 4); this was particularly evident in the concentrations of Ca2+ and Mg2+. The differences in precipitation chemistry may have been caused by increased scavenging during the monsoon period due to the greater precipitation. The increased precipitation during the monsoon presumably also suppressed the production or resuspension of windblown dust.
The Cl−/Na+ molar ratio in seawater is 1.16. Previous studies at several sites, including Mt. Lulin (Wai et al. 2008 ) and South Himalayas (Shrestha et al. 2002) have shown that the Cl−/Na+ ratio in monsoonal precipitation is typically higher than the seawater value, that is, the samples showed an excess of Cl. Previous authors (Wai et al. 2008, and references therein) attributed this phenomenon to the following: (1) reactions between the sea-salt particles and H2SO4, which causes a decrease in aerosol Cl− and concomitant increase in Cl− precipitation and (2) the reaction of O3 with Cl− on the surfaces of sea-salt aerosols and the subsequent photochemical conversion of Cl2 to HCl. The HCl emitted by the latter process is eventually scavenged by cloud and by rain droplets. Additional, excess Cl− can be also contributed by anthropogenic sources and biomass burning emission ().
The average Cl−/Na+ ratio in our samples was 3.43 and varied widely-from 0.67 to 30.76. If the three highest Cl−/Na+ ratios (23.73, 29.51, and 30.76) are excluded from the calculations, the average Cl−/Na+ ratio in our samples would become 1.59, and the range would decrease to 0.67 ~ 5.74. Thus, the preponderance of the Cl−/Na+ ratios was greater than 1.16, indicating a Cl− excess. Further inspection of the data shows that the average Cl−/Na+ ratios during the pre-monsoon period was 1.71, which is slightly higher than the average during the monsoon (1.56). These results suggest that additional sources for Cl− were present during the pre-monsoon; one possibility is emissions from biomass burning (Zhang et al. 2012a).
The isotopic composition of rain from the monsoon region can be influenced by temperature as well as precipitation amount. The precipitation “amount effect” observed in monsoon-climate regions means that the 18O and deuterium (D) contents decrease with increasing monthly or annual mean precipitation (Lee and Fung 2008). By analyzing summertime (monsoon period) precipitation from Lijiang, Pang et al. (2006) concluded that daily δ18O variations had an obvious “amount effect” and the “temperature effect” could be neglected. The “amount effect” of precipitation also has been observed on the South Tibetan plateau (Tian et al. 2001 and the references therein), which is influenced by the southwest monsoon.
The δ18O values in the samples collected during the monsoon period in our study were clearly lower than those obtained during the pre-monsoon (Table 1). The correlation coefficients between δ18O and precipitation and between δ18O and temperature were −0.45 (N = 45, P < 0.01) and −0.01 (N = 45, P > 0.05), respectively. This indicates that the “amount effect” was the main factor affecting δ18O in our samples.
3.4 Air mass transport and sources
The Hybrid Single-Particle Lagrangian Integrated Trajectory Model (HYSPLIT Version 4.9, http://www.arl.noaa.gov/ready/hysplit4.html) developed by the NOAA Air Resources Laboratory was used to evaluate the transport of water vapor to Lijiang. The terminus for the model was set at the sampling site (100.21 °E, 26.90 °N; 1,000 m above ground level), with an ending time around 4:00 UTC time for each rainfall event. The trajectories were calculated backwards in time for 10 days. Similarities in the trajectories suggest that the sources for air masses transported to Lijiang can be divided into two sectors during the pre-monsoon period (Sectors 1 and 2) and three sectors during the monsoon (Sectors 3, 4, and 5) (Fig. 1, Table 2).
As shown in Fig. 1, for the pre-monsoon samples, Sector 1 (4 samples) represents the water vapor sources in South Asia; this group includes one trajectory from the Tibetan Plateau. Sector 2 (3 samples) represents the sources in northern China, including the Sichuan Basin. For the samples collected during the monsoon, Sector 3 (10 samples) represents the sources in South China, which is an area where the rain is typically acidic. Sector 4 (6 samples) of the monsoon group mainly represents the water vapor sources in Burma. Lastly, Sector 5 (22 samples) represents the sources from the Bay of Bengal. The summary statistics for the chemical data stratified by sector are presented in Table 1 and the results discussed in the next section.
3.4.1 Pre-monsoon period
For the Sector 1 samples from the pre-monsoon, it appeared the concentrations of NO3 − and NH4 + were higher than those from Sector 2. Sector 1 also had the highest concentrations of K+ of all five sectors. The high concentrations of these ions may be caused by anthropogenic emissions, especially biomass burning in south Asia and northern Burma. The Sector 2 samples had a higher concentration of SO4 2− than those from Sector 1, indicating that water vapor from North China contained more SO4 2− than that from South Asia. It is noteworthy that Sector 2 includes the Sichuan basin, which is heavily polluted by sulfate and its precursors and regarded as region seriously affected by acid rain (Wang and Han 2010).
3.4.2 Monsoon period
For the rain samples from Sector 3, a significant relationship (R = 0.89, P < 0.01) between Cl− and Na+ was also found (Fig. 5). However, the relationship between K+ and Cl− and K+ and Na+ was not significant, suggesting that K+ from Sector 3 did not originate from the sea salt source. No significant relationship was found between K+ and other detected species, implying that K+ had complex sources for the Sector 3 samples.
For Sector 4, the relationship between Cl− and K+ (R = 0.92, P = 0.01) had a higher level of statistical significance than that between Cl− and Na+ (R = 0.75, P > 0.05) (Fig. 5). This finding supports the idea that Cl− and K+ are from the same source in air that has passed over Burma. Meanwhile, significant relationships between Cl− and NH4 + (R = 0.94, P < 0.01) and between K+ and NH4 + (R = 0.94, P < 0.01) were found, suggesting that Cl− and K+ from Sector 4 were mainly from anthropogenic emissions, such as those from biomass burning for agriculture and household activities. Na+ in the rainwater samples from Sector 4 can be associated with crustal sources because of its significant relationship with Ca2+ (R = 0.98, P < 0.01).
Most of the rainwater samples were transported from Sector 5, and therefore, the bulk of the precipitation during the study was associated with flow from the Bay of Bengal region. Precipitation affected by marine sources generally contains more sea salt ions, such as Cl− and Na+, compared with rain from freshwater sources; indeed, the Sector 5 samples had the highest concentrations of Cl− and Na+ of the samples collected during the monsoon (Fig. 4). Moreover, the relationship between Cl− and Na+ was significant (R = 0.88, P < 0.01), as was that between Cl− and K+ (R = 0.87, P < 0.01, Fig. 5). A significant relationship between K+ and Na+ (R = 0.67, P < 0.01) also was observed for the Sector 5 group, indicating that at least some of the K+ was contributed by the sea-salt source.
Rainwater samples from Sectors 4 and 5 are both linked to flow driven by the southwest monsoon, but they represent different types of precipitation. First, the rainfall events for Sector 5 mainly occurred during the early monsoon period (June and July), whereas the rainfall for Sector 4 mainly occurred later, in late August and September (Table 2). Second, synoptic-scale variations in δ18O in the summer monsoon rain from Lijiang led Pang et al. (2006) to conclude that low values of δ18O were due to the recycling of precipitation during the late monsoon period. Indeed, the δ18O values for Sector 4 were the lowest among the five sectors, implying that rainwater in the Sector 4 samples was from precipitation recycling. As noted above, the source of the water vapor for those samples apparently was the evaporation of monsoon precipitation in Burma.
Given the different water vapor sources for the Sector 4 and 5 samples, the observed differences the ionic composition of rainwater from the two sectors was not unexpected. As described previously, Sectors 4 and 5 had different sources for Cl−, Na+, and K+. Moreover, the percentages of Cl− and Na+ were highest from Sector 5, whereas those from Sector 4 were the lowest of the monsoon period (Fig. 6). These comparisons suggest that precipitation from the southwest monsoon can be divided into two types, namely, one that directly comes from marine water vapor and another that is indirectly contributed by recycled monsoon precipitation. Moreover, the results imply that the amount of water vapor over Burma during the late monsoon is linked to the precipitation at Lijiang.
Throughout the entire sampling period, a strong correlation between SO4 2− and NO3 − was observed (R = 0.75, P < 0.001, N = 45). For Sectors 3, 4, and 5, the correlation coefficients between SO4 2− and NO3 − were 0.91 (P < 0.001, N = 10), 0.99 (P < 0.001, N = 6), and 0.83 (P < 0.001, N = 22), respectively. These strong correlations suggest that the ions have the same source or at least share a common transport pathway during the monsoon. Evaluations of SO4 2−/NO3 − ratios can provide insight into the anthropogenic sources that affect precipitation in industrial areas (Migliavacca et al. 2004). The average SO4 2−/NO3 − ratio in Lijiang for the entire study was 3.40 (Table 3); this is higher than the ratios found at Kothi (Tiwari et al. 2012) and Mt. Lulin (Wai et al. 2008), two rural sites, whose ratios are comparable to the value in remote Arctic regions (1.16) (Talbot et al. 1992). However, the SO4 2−/NO3 − ratios in Lijian were close to the values reported for Shenzhen (Huang et al. 2010) and Xi’an (Lu et al. 2011). These results indicate that rainwater acidity in Lijiang as well as at some urban regions in China is more strongly affected by SO4 2− than NO3 −.
Meanwhile, the SO4 2−/NO3 − ratio calculated using the background precipitation chemical data of Liu et al. (1993) was 4.24, which is considerably higher than the ratio calculated in the present study. It also has been shown that the SO4 2−/NO3 − ratios in Lijiang followed clear a decreasing trend from 2000 to 2009 (Wang et al. 2012). Therefore, NO3 − apparently has played an increasingly important role in rainwater acidity. This interpretation can be explained by the increased use of motor vehicles as tourism has grown in Lijiang. Along these lines, the Lijiang Statistics Yearbook (in Chinese) indicated that the number of vehicles in the city was 1.33 × 104 in 2000 and increased to 1.07 × 105 in 2009.
As shown in Table 3, the SO4 2−/NO3 − ratio for the Sector 3 samples was the highest of the five sectors, suggesting that rainwater from eastern and southern China is the most heavily impacted by SO4 2−. During the last three decades, the rapid development of heavy industries and the increased usage of fossil fuels in China has resulted in growing emissions of SO2 and NOx (Lee et al. 2000), and these are, of course, precursors for the strong acids H2SO4 and HNO3. Indeed, southern China has become the third region of the world to be severely affected by acid rain (Wang 1994; Ding et al. 1997; Safai et al. 2004; Wang and Han 2010; Wu et al. 2012).
This explains why the concentrations of SO4 2− from Sector 3 were the highest of the three transport sectors for the monsoon (Table 1) and why NO3 − and NH4 + also displayed high concentrations in the monsoon Sector 3 samples. Furthermore, the percentage of SO4 2− from Sector 3 was higher than that of Ca2+ (Fig. 6). Half of the AD samples evidently passed through Sector 3; these accounted to 60 % of the rainwater samples in Sector 3. These results thus indicate that pollutants from East and South China are transported to the Tibetan plateau, and that the chemical contaminants alter precipitation chemistry.
3.4.3 Source apportionments for ions
In our study, three principal factors were retained for discussion from PCA, and these factors accounted for 90.68 % of the total variance in the data (Table 4). Thus, three-factor solution from the PMF (Fig. 7) was used for the interpretation of sources; this decision was also based on knowledge concerning the composition of the potential sources. Factor 1 is interpreted to be crustal dust because it has high loadings of Ca2+ (85 %), Mg2+ (75 %), and K+ (61 %). Nitrate (32 %) and SO4 2− (21 %) were also loaded in Factor 1, indicating that these pollutants react with the dust particles, most likely with species containing carbonate. Indeed, the correlation coefficients between Ca2+ and SO4 2−; Ca2+ and NO3 −; and Ca2+ and NH4 + were 0.51 (P < 0.01), 0.80 (P < 0.01), and 0.66 (P < 0.01), respectively.
Factor 2 represents anthropogenic sources because it has high loadings of SO4 2− (79 %), NO3 − (68 %), and NH4 + (76 %). Factor 2 most likely represents the acids neutralized by ammonium ion because those reactions result in the formation of species such as NH4NO3, NH4HSO4, and (NH4)2SO4. A small fraction of Cl− also was loaded on Factor 2, indicating possible reactions between acid gases and sea salts. Factor 3 was associated with a sea-salt source because it was heavily loaded with Cl− (93 %), Na+ (99 %) and with ~37 % K+ and 17 % Mg2+. PMF results indicated that only 0.1 % SO4 2− was associated with Factor 3, and this is consistent with a previous report that the mean sea-salt SO4 2− was only 0.9 % of the total SO4 2− in Lijiang rain collected from 1989 to 2006 (Zhang et al. 2012b). In other words, the overwhelming majority of the SO4 2− ions in Lijiang are from non-sea salt sources.
3.5 Acidification and neutralization potentials
The main acidifying components in precipitation samples are typically H2SO4, HNO3 and organic acids (Kaya and Tuncel 1997). In the rainwater samples from our study, SO4 2− and NO3 − were the main acidic components contributed by human activities. By calculating the ratio of [NO3 −]/([SO4 2−] + [ NO3 −]), one finds that ~23 % of the rainwater acidity can be ascribed to NO3 − and 77 % to SO4 2−. This result has implications for sources, control measures, and future scenarios because the two ions are from different sources, that is, SO4 2− and NO3 − is of the relative importance of stationary vs mobile sources of sulfur and nitrogen in the atmosphere (Arimoto et al. 1996).
Crustal materials, especially the non-sea-salt fraction of Ca2+ and Mg2+, and NH4 + were the main acid-neutralizing species in rainwater; here the non-sea-salt fraction was calculated based on the results of PMF analyses. Neutralization factors (NF) were calculated using the following equation (Possanzini et al. 1988):
where X = NH4 +, nssCa2+, or nssMg2+. From Eq. 1, the average NF for NH4 +, nssCa2+, and nssMg2+ were found to 0.43, 1.39, and 0.16, respectively. This result shows that the main neutralizing component in Lijian is Ca2+, a finding consistent with previous studies. For example, Ca2+ was identified as the main neutralizing component in studies at Lijiang from 1989 to 2006 (Zhang et al. 2012b). In comparison, the average NF of Ca2+ was more than 1.21 in Pune (Safai et al. 2004), 0.7 in Xian (Lu et al. 2011), and 0.56 in Chengdu (Wang and Han 2010).
To assess the neutralization capacity and the balance between alkalinity and acidity, the ratios of the neutralizing potential (NP) to the acidifying potential (AP) were calculated from the following formula:
The average value for the NP/AP ratio was 1.82, indicating that the acidity was completely neutralized by the alkaline constituents in the samples from our study.
4 Conclusions
The rainwater from Lijiang was alkaline relative to equilibrium with the CO2-HCO3 −-CO3 2− system; the mean pH was 6.34, and 60 % of the pH values ranged from 6.0 to 6.5. Most of the rainwater acidity (77 %) was contributed by SO4 2− and 23 % by NO3 −; the main neutralizing component in this study was Ca2+, followed by NH4 + and Mg2+. The dominant cations and anions were Ca2+ and SO4 2−, respectively. The combined concentration of Ca2+ and NH4 + was higher than that of SO4 2− and NO3 −, and this is further evidence that the rainwater ions are dominated by alkaline components. Ion concentrations and δ18O during the pre-monsoon period were both higher than those obtained during the monsoon period. By calculating backward trajectories, the main water vapor sources were divided into five sectors, demonstrating diversity in the pathways that bring water vapor to Southwest China. Rainwater samples from Sector 1 evidently were affected by pollutants from South Asia while samples from Sectors 2 and 3 were associated with high concentrations of SO4 2− that more than likely resulted from anthropogenic emissions in South China. Comparisons of the rainfall dates, δ18O, and ion composition, show that precipitation from Sectors 4 and 5 differs even though both are transported by southwest monsoon.
PMF analysis indicated that 85, 75 and 61 % of the Ca2+, Mg2+, and K+, respectively, were associated with Asian dust sources. Moreover 32 % of the NO3 − and 21 % of the SO4 2− were also loaded on the dust factor, presumably as a result of reactions occurring between the acidic components and the dust particles. Anthropogenic sources accounted for 79 % of the SO4 2−, 68 % of the NO3 −, and 76 % of the NH4 +, and comparisons of neutralizing potentials with acidifying potentials show that ammonium contributed to neutralization of the acids and that the acidity in the entire sample set was fully neutralized by the alkaline constituents. Sea salt accounted for 93 % of the Cl− and 99 % of the Na+; this is a smaller but still important (37 %) source for K+.
References
Al-Momani, I.F., Tuncel, S., Eler, U., Ortel, E., Sirin, G., Tuncel, G.: Major ion composition of wet and dry deposition in the eastern Mediterranean basin. Sci. Total Environ. 164, 75–85 (1995)
Araguás-Araguás, L., Froehlich, K., Rozanski, K.: Stable isotope composition of precipitation over southeast Asia. J. Geophys. Res. 103(D22) (1998). doi:10.1029/98JD02582
Arimoto, R., Duce, R.A., Savoie, D.L., Prospero, J.M., Talbot, R., Cullen, J.D., Tomza, U., Lewis, N.F., Ray, B.J.: Relationships among aerosol constituents from Asia and the North Pacific during Pem-West A. J. Geophys. Res. 101, 2011–2023 (1996)
Cao, Y., Wang, S., Zhang, G., Luo, J., Lu, S.: Chemical characteristics of wet precipitation at an urban site of Guangzhou, South China. Atmos. Res. 94, 462–469 (2009)
Cowling, E.B.: Acid precipitation in historical perspective. Environ. Sci. Technol. 16(2), 110–123 (1982)
Ding, G., Xu, X., Fang, X., Jin, S., Xu, X., Tang, J., Liu, Q., Wang, S., Wang, W.: The actuality and developing trend of acid rain in China. Chin. Sci. Bull. 42(2), 169–173 (1997)
Galloway, J.N., Likens, G.E., Edgerton, E.S.: Acid precipitation in the northeastern United States: pH and acidity. Science 194, 722–724 (1976)
Heuer, K., Tonnessen, K.A., Ingersill, G.P.: Comparison of precipitation chemistry in the Central Rocky Mountains, Colorado, USA. Atmos. Environ. 34, 1713–1722 (2000)
Honório, B.A.D., Horbe, A.M.C., Seyler, P.: Chemical composition of rainwater in western Amazonia –Brazil. Atmos. Res. 98, 416–425 (2010)
Huang, X.F., Li, X., He, L.Y., Feng, N., Hu, M., Niu, Y.W., Zeng, L.W.: 5-Year study of rainwater chemistry in a coastal mega-city in South China. Atmos. Res. 97, 185–193 (2010)
Huston, R., Chan, Y.C., Chapman, H., Gardner, T., Shaw, G.: Source apportionment of heavy metals and ionic contaminants in rainwater tanks in a subtropical urban area in Australia. Water Res. 46, 1121–1132 (2012)
Ito, M., Mitchell, M., Driscoll, C.T.: Spatial patterns of precipitation quantity and chemistry and air temperature in the Adirondack region of New York. Atmos. Environ. 36, 1051–1062 (2002)
Juntto, S., Paatero, P.: Analysis of daily precipitation data by positive matrix factorization. Environmetrics 5, 127–144 (1994)
Kaya, G., Tuncel, G.: Trace element and major ion composition of wet and dry deposition in Ankara, Turkey. Atmos. Environ. 31, 3985–3998 (1997)
Khemani, L.T., Momin, G.A., Naik, M.S., Prakasarao, P.S., Kumar, R., Ramanamurty, B.H.V.: Impact of alkaline particulates on pH of rain water in India. Water Air Soil Pollut. 25, 365–376 (1985)
Khwaja, H.A., Husain, L.: Chemical characterization of acid precipitation in Albany, New York. Atmos. Environ. 24A, 1869–1882 (1990)
Kitayama, K., Murao, N., Hara, H.: PMF analysis of impacts of SO2 from Miyakejima and Asian Continent on precipitation sulfate in Japan. Atmos. Environ. 44, 95–105 (2010)
Lee, J.E., Fung, I.: “Amount effect” of water isotopes and quantitative analysis of post-condensation processes. Hydrol. Process. 22(1), 1–8 (2008)
Lee, B.K., Hong, S.H., Lee, D.S.: Chemical composition of precipitation and wet deposition of major ions on the Korean peninsula. Atmos. Environ. 34, 563–575 (2000)
Li, Z.Q., Ross, E., Thompson, E.M., Wang, F.T., Dong, Z.B., You, X.N., Li, H.L., Li, C.J., Zhu, Y.M.: Seasonal variability of ionic concentrations in surface snow and elution processes in snow-firn packs at the PGPI site on Glacier No. 1 in eastern Tianshan, China. Ann. Glaciol. 43, 250–256 (2006)
Li, C.L., Kang, S.C., Zhang, Q.G., Kaspari, S.: Major ionic composition of precipitation in the Nam Co region, Central Tibetan Plateau. Atmos. Res. 85, 351–360 (2007)
Li, Y., Yu, X.L., Cheng, H.B., Lin, W.L., Tang, J., Wang, S.F.: Chemical characteristics of precipitation at three Chinese regional background stations from 2006 to 2007. Atmos. Res. 96, 173–183 (2010)
Liu, J.Q., Keene, W.C., Wu, G.P.: Study of precipitation background value in Lijiang, China. China Environ. Sci. 13(4), 246–251 (1993)
Lu, X.W., Li, L.Y., Li, N., Yang, G., Luo, D.C., Chen, J.H.: Chemical characteristics of spring rainwater of Xi’an city, N W China. Atmos. Environ. 45, 5058–5063 (2011)
Marquardt, W., Brüggemann, E., Auel, R., Herrmann, H., Möller, D.: Trends of pollution in rain over East Germany caused by changing emissions. Tellus 53B(5), 529–545 (2001)
Migliavacca, D., Teixeira, E.C., Pires, M., Fachel, J.: Study of chemical elements in atmospheric precipitation in South Brazil. Atmos. Environ. 38(11), 1641–1656 (2004)
Norris, G.A., Vedantham, R., Wade, K., Brown, S., Prouty, J., Foley, C.: EPA Positive Matrix Factorization (PMF) 3.0 Fundamentals & User Guide. U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-08/108 (2008)
Ozsoy, T., Saydam, A.C.: Acidic and alkaline precipitation in the Cilician Basin, north-eastern Mediterranean Sea. Sci. Total Environ. 253, 93–109 (2000)
Paatero, P., Tapper, U.: Positive matrix factorization: a non-negative factor model with optimal utilization of error estimates of data values. Environmetrics 5, 111–126 (1994)
Pang, H.X., He, Y.Q., Lu, A.G., Zhao, J.D., Ning, B.Y., Yuan, L.L., Song, B.: Synoptic-scale variation of δ18O in summer monsoon rainfall at Lijiang, China. Chin. Sci. Bull. 51, 2897–2904 (2006)
Pang, H.X., He, Y.Q., Hou, S.G., Zhang, N.N.: Changes in ionic and oxygen isotopic composition of the snow-firn pack at Baishui Glacier No. 1, southeastern Tibetan Plateau. Environ. Earth Sci. 67, 2345–2358 (2012)
Possanzini, M., Buttini, P., Dipalo, V.: Characterization of a rural area in terms of dry and wet deposition. Sci. Total Environ. 74, 111–120 (1988)
Safai, P.D., Rao, P.S.P., Momin, G.A., Ali, K., Chate, D.M., Praveen, P.S.: Chemical composition of precipitation during 1984–2002 at Pune, India. Atmos. Environ. 38, 1705–1714 (2004)
Sanusi, A., Wortham, H., Millet, M., Mirabel, P.: Chemical composition of rainwater in eastern France. Atmos. Environ. 30, 59–71 (1996)
Shrestha, A.B..., Wake, C.P., Dibb, J.E., Whitlow, S.I.: Aerosol and precipitation chemistry at a remote Himalayan site in Nepal. Aerosol Sci. Technol. 36, 441–456 (2002)
Smirnioudi, V.N., Siskos, P.A.: Chemical composition of wet and dust deposition in Athens, Greece, in relation to meteorological conditions. Atmos. Environ. 26B, 483–490 (1992)
Talbot, R.W., Vijgen, A.S., Harriss, R.C.: Soluble species in the Arctic summer troposphere: acidic gases, aerosols and precipitation. J. Geophys. Res. 97(16), 531–543 (1992)
Tang, J., Xue, H.S., Yu, X.L., Cheng, H.B., Xu, X.B., Zhang, X.C., Ji, J.: The preliminary study on chemical characteristics of precipitation at Mt. Waliguan. Acta Sci. Circumst. 20(4), 420–425 (2000)
Tang, A., Zhuang, G., Wang, Y., Yuan, H., Sun, Y.: The chemistry of precipitation and its relation to aerosol in Beijing. Atmos. Environ. 39, 3397–3406 (2005)
Tian, L.D., Yao, T.D., Numaguti, A., Duan, K.Q.: Relation between stable isotope in monsoon precipitation in southern Tibetan Plateau and moisture transport history. Science in China Series D: Earth Sci. 44(1), 267–274 (2001)
Tiwari, S., Chate, D.M., Bisht, D.S., Srivastava, M.K., Padmanabhamurty, B.: Rainwater chemistry in the North Western Himalayan Region, India. Atmos. Res. 104–105, 124–138 (2012)
Topcu, S., Incecik, S., Atimtay, A.: Chemical composition of rainwater at EMEP station in Ankara, Turkey. Atmos. Res. 65, 77–92 (2002)
Tu, Y., Wang, X.J., Feng, Y.K.: Preliminary discussion on pollution characteristics and formation cause of acid rain in Panzhihua City. Sichuan Environ. 23(5), 36–39 (2004)
Wai, K.M., Lin, N.H., Wang, S.H., Dokiya, Y.: Rainwater chemistry at a high-altitude station, Mt. Lulin, Taiwan: comparison with a background station, Mt. Fuji. J Geophys Res 113, Do6305 (2008). doi:10.1029/2006JD008248
Wang, W.: Study on the origin of acid rain formation in China. China Environ. Sci. 14(5), 323–329 (1994)
Wang, H., Han, G.L.: Chemical composition of rainwater and anthropogenic influences in Chengdu, Southwest China. Atmos. Res. 99, 190–196 (2011)
Wang, C.F., He, Y.Q., Zhang, N.N., Li, Z.X., Du, J.K., Niu, G.: Chemical characteristics of rainwater in different regions of Lijiang- Yulong snow mountain. Res. Environ. Sci. 25(1), 18–23 (2012)
WMO, http://www.wmo.int/pages/prog/arep/gaw/precip_chem.html
Wu, Q.X., Han, G.L., Tao, F.X., Tang, T.: Chemical composition of rainwater in a karstic agricultural area, Southwest China: the impact of urbanization. Atmos. Res. 111, 71–78 (2012)
Zhang, D.D., Jim, C.Y., Peart, M.R., Jim, C.Y., Shi, C.X.: Rapid changes of precipitation pH in Qinghai province, the northeastern Tibetan Plateau. Sci. Total Environ. 305, 241–248 (2003a)
Zhang, D.D., Peart, M.R., Jim, C.Y., He, Y.Q., Li, B.S., Chen, J.A.: Precipitation chemistry of Lhasa and other remote towns, Tibet. Atmos. Environ. 37, 231–240 (2003b)
Zhang, M.Y., Wang, S.J., Wu, F.C., Yuan, X.H., Zhang, Y.: Chemical compositions of wet precipitation and anthropogenic influences at a developing urban site in southeastern China. Atmos. Res. 84, 311–322 (2007)
Zhang, N.N., He, Y.Q., Wang, C.F., He, X.Z., Xin, H.J.: The chemical characteristic of soluble ions in total suspended particles (TSP) at Lijiang winter time. Environ. Sci. 32(2), 26–33 (2011)
Zhang, N.N., Cao, J.J., Ho, K.F., He, Y.Q.: Chemical characterization of aerosol collected at Mt. Yulong in wintertime on the southeastern Tibetan Plateau. Atmos. Res. 107, 76–85 (2012a)
Zhang, N.N., He, Y.Q., Cao, J.J., Ho, K.F., Shen, Z.X.: Long-term trends in chemical composition of precipitation at Lijiang, southeast Tibetan Plateau, southwestern China. Atmos. Res. 106, 50–60 (2012b)
Zhao, Z.P., Tian, L.D., Li, M., Fischer, Z.Q., Jiao, K.Q.: Study of chemical composition of precipitation at an alpine site and a rural site in the Urumqi River Valley, Eastern Tien Shan, China. Atmos. Environ. 42, 8934–8942 (2008)
Acknowledgments
This work was supported by the Natural Science Foundation of China (NSFC40801028, 40925009), the West Light Foundation of Chinese Academy of Sciences, projects from the Chinese Academy of Sciences (No O929011018, KZCX2-YW-BR-10 and KZCX2-YW-148), the Ministry of Science & Technology (2012BAH31B03, 2009IM030100) and Meteorological Innovative Research Project of Baoji Meteorological Bureau (No.T2012-01). We sincerely thank the staffs at the Yulong Snow Mountain glacial and environment observation station who were instrumental in the field work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, N., Cao, J., He, Y. et al. Chemical composition of rainwater at Lijiang on the Southeast Tibetan Plateau: influences from various air mass sources. J Atmos Chem 71, 157–174 (2014). https://doi.org/10.1007/s10874-014-9288-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10874-014-9288-7