Abstract
We investigated seasonal changes in carbon demand and flux by mesozooplankton communities at subtropical (S1) and subarctic sites (K2) in the western North Pacific Ocean to compare the impact of mesozooplankton communities on the carbon budget in surface and mesopelagic layers. Fecal pellet fluxes were one order higher at K2 than at S1, and seemed to be enhanced by copepod and euphausiid egestion under high chlorophyll a concentrations. The decrease in pellet volume and the lack of any substantial change in shape composition during sink suggest a decline in fecal pellet flux due to coprorhexy and coprophagy. While respiratory and excretory carbon by diel migrants at depth (i.e., active carbon flux) was similar between the two sites, the actively transported carbon exceeded sinking fecal pellets at S1. Mesozooplankton carbon demand in surface and mesopelagic layers was higher at K2 than S1, and an excess of demand to primary production and sinking POC flux was found during some seasons at K2. We propose that this demand was met by supplementary carbon sources such as feeding on protozoans and fecal pellets at the surface and carnivory of migrants at mesopelagic depths.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The ocean's biological pump, which is recognized as a key component of global biogeochemical cycles (Chisholm 2000), consists of passive and active carbon fluxes (Steinberg et al. 2008b). Sinking particles, including phytoplankton aggregates, zooplankton fecal pellets, feeding mucus, carcasses, and crustacean exoskeletons, represent a major component of passive carbon flux (Flower and Knauer 1986; Turner 2002), and are characterized by an exponential decline with depth (Suess 1980; Martin et al. 1987; Pace et al. 1987) and large variation in attenuation rates among seasons and locations (Buesseler et al. 2007; Buesseler and Boyd 2009). One reason for this may be the significant changes in volume and shape of fecal pellets during sink (e.g., Turner 2002; Wilson et al. 2008) as a result of ingestion by protozoan and metazoan coprophagy and/or fragmentation associated with sloppy feeding and swimming activity (i.e., coprorhexy) and breakdown of their binding membranes (i.e., coprochaly) (e.g., Paffenhöfer and Strickland 1970; Lampitt et al. 1990; Noji et al. 1991). Sinking particles have long been believed to be a major pathway of the biological pump (Fowler and Knauer 1986; Zhang and Dam 1997) and to support mesopelagic carbon demand (Arístegui et al. 2002; Giering et al. 2014).
Active carbon flux, on the other hand, is represented by respiration (dissolved inorganic carbon; DIC), excretion (dissolved organic carbon; DOC), egestion, crustacean exoskeletons and mortality (particulate organic carbon; POC) of diurnal and seasonal migrants at depth (Longhurst and Williams 1992; Steinberg et al. 2000, 2008b, 2012). Steinberg et al. (2008b) noted that sinking particle flux was not sufficient to support the mesopelagic carbon demands of heterotrophic microbes and metazoans. Carbon actively transported by diurnally and seasonally migrating mesozooplankton has been considered to be a supplementary source supporting a portion of the mesopelagic carbon demand (e.g., Kobari et al. 2008a, b, 2013; Steinberg et al. 2008b). Comparisons of carbon actively transported by the migrants to that of sinking POC have shown that respiratory flux by diel migrants was equivalent to 3–72 % of sinking POC flux (Al-Mutairi and Landry 2001; Kobari et al. 2008b, 2013). Thus, actively transported carbon by migrants would not be negligible with respect to mesopelagic carbon budgets.
Large seasonal fluctuations in mesozooplankton biomass are known to occur in the subarctic North Pacific Ocean (e.g., Parsons and Lalli 1988; Mackas and Tsuda 1998; Ikeda et al. 2008). Although the dynamics of mesozooplankton biomass and community structure in the subtropical North Pacific Ocean have traditionally been believed to be spatially and temporally steady-state (McGowan and Walker 1979, 1985), seasonal fluctuations were recently found for some copepod species contributing to subtropical mesozooplankton biomass (e.g., Shimode et al. 2009, 2012a, b). As such, carbon budgets through the mesozooplankton community would show seasonal variability and would have some impact on surface and mesopelagic food webs. While the impact of mesozooplankton communities on carbon budgets and fluxes in surface and mesopelagic layers has long been debated (e.g., Steinberg et al. 2008b; Kobari et al. 2008b, 2013; Giering et al. 2014), the use of a “snapshot” approach has yielded limited information on seasonal variability. As such, repeated seasonal oceanographic observation and zooplankton sampling is necessary to gain a better understanding of the biological pump in pelagic ecosystems.
Here, we investigated seasonal changes in carbon demand and flux (i.e., sinking fecal pellets, and respiration and excretion by diel migrants at depth) by mesozooplankton communities at subarctic and subtropical sites in the western North Pacific Ocean. From these results, we compared the impact of the mesozooplankton community on carbon budgets in surface and mesopelagic layers between the two ecosystems across seasons. This study was also conducted as a follow-up survey to the earlier VERTIGO [Vertical Transport in the Global Ocean] project in order to clarify the impact of zooplankton communities on the biological pump in summer (Buesseler et al. 2007; Kobari et al. 2008b; Steinberg et al. 2008a, b; Wilson et al. 2008) and winter (Kobari et al. 2013).
2 Materials and methods
2.1 Oceanographic observations
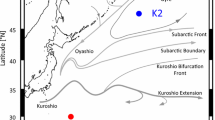
Oceanographic observations, sediment trap experiments and zooplankton collection were conducted at stations K2 (47°N, 160°E) and S1 (30°N, 145°E) in the western North Pacific Ocean (Fig. 1) during cruises aboard the R/V Mirai from October to November 2010 (MR10-06), January to February 2011 (MR11-02), April to May 2011 (MR11-03), and June to August 2011 (MR11-05). Temperature, salinity and chlorophyll fluorescence were recorded from the sea surface to a depth of 1000 m using a CTD system (Sea-Bird SBE 9plus). Water samples for chlorophyll a measurements were collected with a CTD-CMS system and a plastic bucket. The samples were filtered through Whatman GF/F filters, and chlorophyll a pigments on the filters were immediately extracted by direct immersion in N,N-dimethylformamide (DMF) in darkness for more than 24 h (Suzuki and Ishimaru 1990). Chlorophyll a concentration was measured with a Turner Designs fluorometer (Turner Designs, AU-10) using a non-acidification fluorometric method (Welschmeyer 1994). In the present study, a boundary depth between surface and mesopelagic layers of 150 m at K2 and 200 m at S1 was determined at which mixed layer depth (the density exceeded the surface density by 0.125 kg/m3) reached a maximum during winter.
2.2 Passive carbon flux
To measure the sinking POC, including fecal pellets, Knauer-type drifting sediment traps were deployed at 60, 100, 150 and 200 m at both sites for 48–120 h during each cruise. The sediment traps were constructed from eight polycarbonate cylinders with baffles, which were modified from Knauer et al. (1979). Approximately 250 mL polycarbonate sample cups were attached to the bottom of each cylinder (via screw threads) and filled with filtered (0.2 µm) surface seawater with an adjusted salinity of ~39 PSU (addition of 100 g NaCl to 20 L filtered seawater). Each sample was filtered on a pre-combusted Whatman GF/F filter, and swimmers were removed with tweezers. After the inorganic carbon was removed by HCl fume treatment, the carbon content was determined with a CHN analyzer. Details of measurements for sinking POC flux are described by Honda et al. (2015).
Samples for quantification of fecal pellet flux were fixed with 4 % borax-buffered formaldehyde solution. The fecal pellets were manually categorized into four shapes: cylindrical, oval, spherical, and amorphous. Pellets in each shape category were counted under a dissecting microscope. The length and width of arbitrarily selected fecal pellets in each category were measured using image analysis software (NIS-Elements Documentation, Nikon). Fecal pellet volumes were estimated by assuming simple geometrical shapes (i.e., cylindrical, oval, spherical, and these combinations for amorphous pellets). The equivalent spherical diameter (ESD: mm) was estimated from the volume. Fecal pellet volume at K2 was converted to carbon using a conversion factor of 0.08 mgC mm−3 (Wilson et al. 2008). Since low conversion factors were likely for fecal pellets at the temperate and subtropical sites in the North Pacific Ocean (0.03 mgC mm−3: Taguchi and Saino 1998; 0.04 mgC mm−3: Urrére and Knauer 1981), the mid-range was used as a conversion factor at S1 (0.035 mgC mm−3).
To identify the producers for each shape of fecal pellet in the sediment trap samples, live copepods were collected above 50 m at K2 and S1 using a NORPAC net (mesh size 0.1 mm) towed vertically at 0.5 m s−1. The net was equipped with a filtering cod-end (2 L) to avoid damaging live animals. Copepods occurring abundantly and swimming actively were placed in 15-mL tubes or 500-mL bottles (in groups of 1–10 animals per tube or bottle) filled with 0.2 µm filtered seawater. Calanoid, cyclopoid, and poecilostomatoid copepods were separately incubated for 2 h, and both the copepods and their fecal pellets were fixed with 4 % borax-buffered formalin. Copepod body length (BL: mm) and fecal pellet volume (PV: mm3) were measured using digital images and NIS-Elements imaging software (see above), and were expressed as the following equation:
where a and b are constants and log PV and log BL are the logarithms of PV and BL, respectively.
2.3 Active carbon flux
Mesozooplankton samples were collected at eight discrete depth intervals (0–50, 50–100, 100–150, 150–200, 200–300, 300–500, 500–750 and 750–1000 m) using the Intelligent Operative Net Sampling System (IONESS: mesh size 0.335 mm, mouth opening 1.5 m2). Note that some metazoans such as small poecilostomatoids and cyclopoids were not collected due to the coarse mesh size. Zooplankton samples were split aboard the vessel, and aliquots (taxonomic samples and others) were fixed with 5 % borax-buffered formaldehyde (final concentration) after removing larger gelatinous zooplankton and micronekton.
In the land laboratory, we identified and classified animals into 13 major taxonomic groups using a dissecting microscope (see Kitamura et al. 2016). We computed the abundance of each taxonomic group in the layer i (GNO i : individuals m−2). Identified animals were transferred to nylon mesh (mesh size 0.1 mm) and briefly rinsed with distilled water. The dry weight of each taxonomic group in the layer i (GDW i : mg m−2) was determined with a microbalance (Sartorius CP224S, accuracy ±0.1 mg) after drying at 60 °C for 24 h, and integrated all identified taxonomic groups (DW i : mg m−2). To minimize the unpredictable effects of patchiness of some metazoans, we adjusted the abundance ( AD GNO i : individuals m−2) and dry weight ( AD GDW i : mg m−2) of each taxonomic group in the layer i using the following equation:
where Y is the adjusted abundance ( AD GNO i ) or dry weight ( AD GDW i ) of each taxonomic group in the layer i, X i is the abundance (GNO i ) or dry weight (GDW i ) of each taxonomic group in the layer i during day or night, and M is the abundance or dry weight of each group, summing the eight layers, and averaged during day and night. The abundance ( AD NO i ) or dry weight ( AD DW i ) of the mesozooplankton community in layer i was computed from the integration of AD GNO i or AD GDW i .
For estimation of carbon demand and respiratory flux, oxygen consumption rates can be calculated from the global model (Eq. 3; Ikeda 1985) or the global-bathymetric model (Eq. 4; Ikeda et al. 2007):
where RO i or RO i′ is the oxygen consumption rate (µLO2 individual−1 h−1), ADW i is the animal dry weight (mgDW individual−1), which is the AD DW i (mgDW m−2) divided by the AD NO i in the layer i (individuals m−2), T i is the ambient temperature (°C), which is the mean temperature in the layer i, OS i is the mean oxygen saturation (1.00 for full saturation) in the layer i, and D i is the mean depth in the layer i. The global-bathymetric model (Eq. 4) calibrated the overestimation of oxygen consumption rates with depth and oxygen saturation, but it was available only for copepods. Thus, we determined a calibration factor (F i ) by comparison of the oxygen consumption rates estimated with each model, applying the copepod community:
where CRO i and CRO i ′ are the oxygen consumption rates of the copepod community in the layer i estimated with the global (Eq. 3) and global-bathymetric models (Eq. 4), respectively. In the mesopelagic layers, we excluded dormant copepods such as C4 for Neocalanus flemingeri, C5 for Calanus, Metridia and Neocalanus spp., C3 to C5 and adult female for Eucalanus bungii, C5 and adult female for Eucalanus californicus and Rhincalanus rostrifrons, and C4 to C5 and adult female for Rhincalanus nasutus (Conover 1988; Kobari and Ikeda 1999, 2001a, b; Padmavati et al. 2004, Shoden et al. 2005; Shimode et al. 2009, 2012a, b) from the DW i and the N i . We also excluded non-feeding C6 males and females of Neocalanus spp. and C6 males of Metridia spp. which were residing at depth throughout the day (Miller et al. 1984; Miller and Clemons 1988; Padmavati et al. 2004). Finally, RO i was summed in each taxonomic group and converted to carbon units (RC i: µgC m−2 h−1) as follows:
A respiratory quotient (RQ) of 0.97 was assumed (protein metabolism; Gnaiger 1983). Due to the atomic weight of carbon (12 g) per 1 mol of CO2 (22.4 L), a conversion factor from oxygen unit (O2) to carbon (CO2) should be 12/22.4. Carbon demand (CD) of the mesozooplankton community integrated in surface and mesopelagic layers was estimated as:
We used 0.6 for assimilation efficiency (AE) and 0.5 for fraction of assimilated carbon respired (R) (Steinberg et al. 2008b; Giering et al. 2014). We performed a sensitivity analysis for the calculation of copepod carbon demand using an upper (AE: 0.5, R: 0.5) and lower (AE: 0.7, R: 0.5) estimate of combined parameters (Steinberg et al. 2008b).
Respiratory carbon flux (DIC) by diel migrants was calculated as the difference between respired carbon (RC) by diel migrants plus residents (RC a) and those of residents (RC b) in the mesopelagic layers. RC a and RC b were calculated as daytime RC (by migrants and residents) plus nighttime RC (only by residents) and nighttime RC in terms of 24 h, respectively. Excretory carbon flux by diel migrants (DOC) was calculated as 31 % of respired carbon by migrants at depth (Steinberg et al. 2000).
3 Results
3.1 Environments
At K2, the mixed layer depth in February and April reached 120 m, where the permanent halocline was evident (Fig. 2). Due to the development of a seasonal thermocline, the mixed layer depth was 30 m in July and 40 m in October. Seasonal variations in mean water temperature and salinity in the mixed layer depth were from 1.6 to 8.5 °C and 32.6 to 33.0 °C, respectively. Below the permanent halocline, salinity increased toward 1000 m, and water temperature showed a maximum around 200 m throughout the seasons. At S1, the mixed layer depth reached below 200 m in February and around 50 m in May. A high-salinity water mass was evident around 50 m in July, when the mixed layer was shallowest. Below the mixed layer depth, water temperature decreased toward 1000 m, and salinity showed a minimum between 600 and 700 m throughout the seasons.
At K2, chlorophyll a concentrations in the mixed layer were uniform, at about 0.6 mg m−3 in October, decreasing to 0.4 mg m−3 in February. Chlorophyll a increased to 0.9 mg m−3 at the near-surface in April. In July, a subsurface maximum developed just above the pycnocline, and the concentration reached 1.3 mg m−3. At S1, chlorophyll a concentrations were high near the surface (20 m) in February. A subsurface chlorophyll maximum was evident in November, May and July, when a thermocline was also apparent. Maximum chlorophyll a concentration in the mixed layer was high (0.9 mg m−3) in February and low (0.3 mg m−3) in November.
3.2 Sinking fecal pellets
While fecal pellet flux varied among depths and seasons, the flux at a given depth and season was higher at K2 than at S1 (Fig. 3). The seasonal ranges of fecal pellet flux were 1.6–28.5 mgC m−2 day−1 at K2 and 0.2–4.1 mgC m−2 day−1 at S1. The fecal pellet fluxes at a given depth were highest during July at K2 and during February at S1, when the chlorophyll a concentrations were highest. While the fecal pellet fluxes in the upper 100 m were higher than those below the layers from February to July at both sites, such difference was not apparent in October at K2 or in November at S1. At the bottom of the surface layers (150 m at K2 and 200 m at S1), fecal pellets accounted for 8–21 % of sinking POC flux at K2 and 1–4 % at S1 (Table 1). A positive correlation was found between the sinking fecal pellet and POC fluxes at the same depth at both K2 (Pearson product-moment correlation coefficient: r = 0.547, p < 0.05) and S1 (Pearson product-moment correlation coefficient: r = 0.776, p < 0.01), indicating similar seasonal changes in the sinking fecal pellet flux and sinking POC flux.
Seasonal changes in the depth distribution of fecal pellet flux (FP: mgC m−2 day−1) and the shape composition (%) observed in drifting sediment traps at four different depths in the subarctic (K2: upper level) and subtropical sites (S1: lower level) in the western North Pacific Ocean. CY, OV, SP and AM are cylindrical, oval, spherical and amorphous fecal pellets, respectively. Note that the horizontal scale for fecal pellet fluxes of K2 is one order higher than those of S1
At both stations, cylindrical pellets accounted for 63–91 % of all fecal pellets throughout the study period. Oval or amorphous pellets were of secondary importance. Spherical pellets made only a minor contribution, less than 3 %. There was no significant variation in shape composition among layers at either station. Pearson product-moment correlation coefficients were computed by comparison of fecal pellet fluxes to water temperature and chlorophyll a concentrations at the same depth. Significant positive correlations were found between fecal pellet flux and chlorophyll a concentration at the same depth for both K2 (r = 0.745, p < 0.01) and S1 (r = 0.755, p < 0.01).
3.3 Fecal pellet size
The average ESD of cylindrical pellets was larger in July at K2 and in February at S1 than in the other seasons (ANOVA and Scheffé’s comparison, p < 0.01: Fig. 4), as the large cylindrical pellets were abundant throughout the water column under high chlorophyll a concentrations. At both sites, the ESDs at 200 m were significantly smaller than those at 60 m from February to July (ANOVA and Scheffé’s comparison, p < 0.01), but this difference was not apparent in October at K2 or November at S1. The size of oval pellets followed a seasonal trend: the average ESD was larger during July at K2 and during February at S1 than during the other seasons (ANOVA and Scheffé’s comparison, p < 0.01). In October/November and July, the ESDs of cylindrical and oval pellets were larger at K2 than at S1 (Welch’s t test, p < 0.01). Since spherical and amorphous pellets were found in much lower numbers in samples (i.e., less than 16 pellets) than other pellets throughout the study period at both sites, seasonal changes in the depth distribution of the ESDs were not evaluated.
Seasonal changes in the size distribution of equivalent spherical diameter (ESD: mm) for cylindrical (CYL) and oval (OVA) fecal pellets collected with drifting sediment traps at four different depths at the subarctic (K2) and subtropical sites (S1) in the western North Pacific Ocean. Box shows 25th and 75th percentiles, and vertical bars in boxes indicate medians. Error bars represent 5th and 95th percentiles. The number of pellets observed is shown in parentheses
In the defecation experiments, cylindrical fecal pellets were produced by calanoids and oval pellets by poecilostomatoids and cyclopoids. The log PVs were positively correlated with the log BLs of all copepods (Pearson product-moment correlation coefficient: r = 0.763, p < 0.01), indicating that smaller copepods egested smaller fecal pellets (Fig. 5). The regression between logPV and logBL was statistically significant:
Scatter diagram of fecal pellet volume (PV: mm3) versus body length (BL: mm) of copepods collected from the subarctic (K2) and subtropical sites (S1) in the western North Pacific Ocean. Log-transformed pellet volume (logPV) and body length (logBL) are shown. Regression lines derived from this study (broken line) and the previous studies in the Inland Sea of Japan (solid line, ISJ: Uye and Kaname 1994) and Kagoshima Bay (dotted line, KB: Kobari et al. 2010) are superimposed
The slope of the present regression line was gentler than those previously recorded, indicating small fecal pellets even at the large body size.
3.4 Mesozooplankton biomass
Mesozooplankton biomass in surface and mesopelagic layers was one order higher at K2 than at S1 (Fig. 6). At K2, the surface biomass averaged during day and night showed large seasonal variability, with a minimum in February (1.9 gDW m−2) and a maximum in April (11.6 gDW m−2). The mesopelagic biomass averaged during day and night revealed a seasonal trend, with the highest recorded in October (12.9 gDW m−2) and lowest in April (6.4 gDW m−2). The mesopelagic biomass exceeded the surface biomass across seasons excepted for the month of April. Dormant copepods represented 28–68 % of the mesopelagic biomass and contributed to seasonal variability, while mesopelagic biomass other than dormant copepods showed little variability.
Dry mass-based mesozooplankton biomass in surface and mesopelagic layers (gDW m−2) during daytime (left side panel) and nighttime (right side panel) at the subarctic (K2: upper row) and subtropical sites (S1: lower row) in the western North Pacific Ocean. Open bar: dormant copepods. Solid bar: other mesozooplankton. The bottom of the surface layers was defined as 150 m at K2 and 200 m at S1, based on the seasonal changes in mixed layer depth (see Sect. 2). Note that horizontal scale for K2 is about one order higher than that for S1
At S1, a seasonal trend was found for both surface and mesopelagic mesozooplankton biomass. The surface biomass averaged during day and night reached a maximum in February (1.0 gDW m−2), followed by the mesopelagic biomass at a maximum in May (1.5 gDW m−2). In November, minima were found for both surface (0.3 gDW m−2) and mesopelagic biomass values averaged during day and night (0.4 gDW m−2). Throughout the seasons, surface biomass was greater during night than during the day, indicating a diel vertical migration. Dormant copepods represented 6–24 % of the mesozooplankton biomass below 200 m.
3.5 Carbon budgets
The seasonal variation in mesozooplankton carbon demand in the surface layers differed between K2 and S1 (Fig. 7). At K2, the carbon demand fluctuated from 72 to 313 mgC m−2 day−1. Demand was lower than primary production in July (15 %) and February (57 %), but exceeded primary production in October (126 %) and April (142 %). At S1, the carbon demand showed a seasonal trend, reaching a maximum in February (169 mgC m−2 day−1) and a minimum in November (52 mgC m−2 day−1), and corresponding to less than 54 % of primary production throughout the seasons. Using the lower combination (AE: 0.7, R: 0.6) of a sensitivity analysis, the carbon demand was nearly equal to primary production in October and April at K2.
Seasonal changes in primary production (PP: mgC m−2 day−1, solid) and carbon demand of mesozooplankton communities in the surface layers (CD: mgC m−2 day−1, open) at subarctic (K2) and subtropical sites (S1) in the western North Pacific Ocean. PP is quoted from K. Matsumoto (unpublished data). Bars show upper and lower limits of the sensitive analyses. Open circles indicate carbon demand estimated with 0.7 for AE and 0.6 for R
The seasonal variation in carbon demand of mesopelagic mesozooplankton differed between the two sites (Fig. 8). At K2, seasonal variability was small (coefficient of variation of 9 % against 54 % at S1), ranging from 89 mgC m−2 day−1 in February to 107 mgC m−2 day−1 in April. Demand exceeded the sinking POC flux at 150 m by a factor of 1.2 (July) to 4.0 (April). At S1, the carbon demand of the mesopelagic metazoans was lowest in November (17 mgC m−2 day−1) and highest in May (69 mgC m−2 day−1). The values corresponded to less than 67 % of the sinking POC flux at 200 m in February and July, but exceeded the flux in November (178 %) and May (389 %). Even in the lower combination (AE: 0.7, R: 0.5) of a sensitivity analysis, the carbon demand did not meet the sinking POC flux from October to April at K2 or in November and May at S1.
Seasonal changes in sinking flux of particulate organic carbon (POC) at the bottom of the surface layers (mgC m−2 day−1, solid bars) and carbon demand (CD) of mesozooplankton communities in the mesopelagic layers (mgC m−2 day−1, open bars) at the subarctic (K2) and subtropical sites (S1) in the western North Pacific Ocean. Bars show upper and lower limits of sensitive analyses. Open circles indicate carbon demand estimated with 0.7 for AE and 0.5 for R
The seasonal variation in passive carbon flux facilitated by the surface mesozooplankton community (i.e., fecal pellet flux) differed between the two sites (Fig. 9). At K2, the fecal pellet flux at 150 m was lowest in April (2 mgC m−2 day−1) and highest in July (12 mgC m−2 day−1). At S1, no clear pattern was observed for fecal pellet flux at 200 m, ranging from 0.3 (May) to 1.0 mgC m−2 day−1 (February). The active carbon flux by diel migrants (i.e., respiratory DIC and excretory DOC) fluctuated from 1 to 7 mgC m−2 day−1 at K2 and from 2 to 7 mgC m−2 day−1 at S1. The active carbon flux at S1 reached a maximum in May and a minimum in November, but there was no clear seasonal trend at K2.
Seasonal changes in fecal pellet (FP) flux (mgC m−2 day−1, dotted bars) and active carbon flux (mgC m−2 day−1) at the bottom of surface layers of the subarctic (K2) and subtropical sites (S1) in the western North Pacific Ocean. Active carbon flux is integrated with respiratory dissolved inorganic carbon (DIC: open bars) and excretory dissolved organic carbon (DOC: diagonal-lined bars) by diel migrants at depth
4 Discussion
4.1 Production and change of fecal pellets during sink
Calanoid copepods and euphausiids are known to be major producers of cylindrical pellets (Wilson et al. 2008; Yoon et al. 2001), while cyclopoids, poecilostomatoids, harpacticoids and copepod nauplii egest oval and spherical pellets (Martens 1978; Paffenhöfer and Knowles 1979; Lampitt et al. 1990). In our sediment trap samples, cylindrical pellets were the most common among the four shapes at both sites (63–91 %; Fig. 3), suggesting that calanoids and euphausiids contribute to the fecal pellet flux. Indeed, copepods and euphausiids dominated zooplankton biomass in the surface layers at both K2 and S1 (Kitamura et al. 2016), and calanoids were the predominant group among copepods. Oval and spherical pellets were minor components, even though their producers (i.e., small copepods) constituted more than half of the surface mesozooplankton abundance collected with a fine mesh net at both sites (T. Kobari unpublished data). Small pellets are known to be susceptible to metazoan and protozoan coprophagy and bacterial decomposition during sink (Iversen et al. 2010). The average ESD of oval pellets was smaller than that of cylindrical pellets across seasons and depths (Fig. 4). These findings suggest that oval and spherical pellets egested from small copepods disappear during sink and do not contribute to the passive carbon flux facilitated by the mesozooplankton community.
It has long been understood that sinking POC flux is positively correlated with primary productivity (Pace et al. 1987) and that it declines with increasing depth (Martin et al. 1987; Suess 1980). Indeed, our observations demonstrate a significant positive correlation between fecal pellet flux and chlorophyll a concentration (see Sect. 3.2). The slope of the regression equation between pellet volume and copepod body length derived from our defecation experiments was lower than the slopes obtained in previous studies at coastal sites where chlorophyll a concentrations were high (Fig. 5). Fecal pellet production, therefore, is likely enhanced by mesozooplankton egestion under conditions of high food availability. However, the decline in fecal pellets during sink appears complex. In the present study, the vertical attenuation of fecal pellet flux was highly variable among seasons and locations (Fig. 3). Since the contribution of fecal pellets to POC flux is highly variable among locations (Table 1), the rate of vertical attenuation could differ between fecal pellets and other POC. Sediment trap samples revealed no substantial change in shape composition (Fig. 3) and showed a significant decline in pellet volume for both cylindrical and oval pellets (Fig. 4), suggesting that cylindrical and oval pellets are broken down into smaller particles of the same shape during sink.
Over the last two decades, arguments have been advanced by many researchers regarding the mechanisms underlying the decline in fecal pellet flux, including copepod coprorhexy and coprophagy (e.g., González and Smetacek 1994; Svensen and Nejstgaard 2003; Poulsen and Kiørboe 2006) or copepod coprorhexy and protozoan coprophagy (e.g., Iversen and Poulsen 2007; Paulsen and Iversen 2008; Svensen et al. 2012). Taking into account the probable sinking velocity (20–150 m day−1; McDonnell and Buesseler 2010) and assuming no significant decomposition within 3 days (Svensen et al. 2012), bacterial decomposition would be negligible for sinking fecal pellets observed in the present study. Given the lack of substantial change in shape composition but the significant reduction in flux and pellet volume, as described above, the fecal pellet fluxes at both sites may have been reduced by coprorhexy and coprophagy during sink.
4.2 Impacts of mesozooplankton on surface carbon budgets
The ocean environments were considerably different between K2 and S1, with cold and mesotrophic conditions at K2, and warm and oligotrophic waters at S1 (Table 2). However, annual mean chlorophyll a concentrations and primary production were comparable between the two sites K. Matsumoto (unpublished data). Based on previous results in the Western Subarctic Gyre (Tsuda et al. 2003; Fujiki et al. 2014; Matsumoto et al. 2014), phytoplankton growth at K2 may be affected by iron and light limitations. On the other hand, as the N:P ratio at S1 (ca., 11:1) was lower than the Redfield ratio (Table 2), N-limitation on primary production at S1 was suggested by K. Matsumoto (unpublished data). Under these limitations on phytoplankton communities, chlorophyll a concentrations and primary production showed seasonal fluctuations: maxima in July at K2 and in February at S1 (Figs. 2, 7). The seasonal changes in the surface mesozooplankton biomass were synchronized with those of chlorophyll a and primary production at S1, but such synchronization was not clear for the surface mesozooplankton biomass at K2. Moreover, the carbon demand of the surface mesozooplankton community exceeded primary production in October and April at K2 (Fig. 7).
From a methodological point of view, the carbon demand might be overestimated by our choice of R value. While we used a conservative combination of AE (0.6) and R (0.5) in the present study, higher AE and R might be available for the surface mesozooplankton community at K2 (Fig. 7). Indeed, the higher AE (~0.8) was evident for copepods feeding on phytoplankton species with lower ash content (Abe et al. 2013). Le Borgne (1982) demonstrated average net growth efficiency of 0.372 for the mesozooplankton community in the eastern tropical Atlantic Ocean (i.e., 0.628 for R). If we apply 0.7 for AE and 0.6 for R, the carbon demand is estimated to be nearly equal to or less than primary production at K2 throughout the seasons (shown as open circles in Fig. 7).
At K2, the dominant metazoans were N. plumchrus in October and N. cristatus in April (T. Kobari unpublished data). These copepods were known to feed on sinking particles, copepod nauplii and protozoans (Dagg 1993; Greene et al. 1988; Gifford 1993), especially in seasons when phytoplankton biomass was low (Kobari et al. 2003; Doi et al. 2010). Although heterotrophic bacteria were too small for grazing by particle-feeding metazoans (e.g., Frost et al. 1983; Liu et al. 2005) except for pelagic tunicates (e.g., Deibel and Lee 1992; Bedo et al. 1993), bacterial production could be transferred to metazoans through protozoans such as heterotrophic nanoflagellates and ciliates (e.g., Sherr et al. 2002). Mesozooplankton fecal pellets are also known to be food resources for the other metazoans (e.g., Turner 2002). The sinking fecal pellets measured by drifting sediment traps were equivalent to less than 30 % of those egested from the surface mesozooplankton community at K2 (29–125 mgC m−2 day−1), indicating rapid consumption of fecal pellets by metazoans and protozoans. Food resources other than phytoplankton would be supplementary for the carbon demand of the surface mesozooplankton community at K2.
4.3 Impacts of mesozooplankton on mesopelagic carbon budgets
Vertical distribution of the mesozooplankton communities at the two sites was characterized by high biomass in the surface and mesopelagic layers (Kitamura et al. 2016). These findings are consistent with previous reports (Steinberg et al. 2008a; Kobari et al. 2013). At both sites, we found seasonally migrating copepods in the mesopelagic layers (Fig. 6), including Calanus, Eucalanus, Metridia, Neocalanus and Rhincalanus, which resided at mesopelagic depths for dormancy and reproduction without feeding (Conover 1988; Kobari and Ikeda 1999, 2001a, b; Padmavati et al. 2004; Shoden et al. 2005; Shimode et al. 2009, 2012a, b). The expatriated copepods from the subarctic regions (e.g., Kobari et al. 2008a, 2013) were also included among the seasonal migrants residing between 500 and 750 m at S1, with no substantial contribution to the mesopelagic biomass (<1 %). Based on the dry-weight biomass, the other predominant components of the mesopelagic biomass were chaetognaths, cnidarians, and no dormant copepods and euphausiids at either K2 and S1 (T. Kobari unpublished data). Taking into account that euphausiids were major migrators (Kitamura et al. 2016), the mesopelagic carbon demand would result from residents such as chaetognaths, cnidarians and the other copepods, excluding diel and seasonal migrants.
In the present study, most of the mesopelagic carbon demand estimates exceeded the POC flux at both sites (Fig. 8). As demonstrated by sensitivity analyses, the mesopelagic carbon demand can be lowered if higher AE and R are applied for the mesopelagic metazoans. Copepods contributed the most mesopelagic biomass at both sites, as carnivorous copepods showed higher biomass at the mesopelagic depths than those at the surface in neighboring waters (Yamaguchi et al. 2002). Generally, higher AE was found in carnivores than herbivores (Maucline 1998). Le Borgne (1982) demonstrated average net growth efficiency of 0.282 for mesozooplankton communities at stations where carnivores were predominant (i.e., 0.718 for R). These facts suggest that a lower combination of AE and R than ours was likely for the mesopelagic metazoans. However, the mesopelagic carbon demand still did not meet the POC flux during some seasons at either site, even if we applied 0.7 for AE and 0.5 for R on the mesopelagic metazoans (shown as open circles in Fig. 8). Since we could not collect small metazoans such as poecilostomatoids and cyclopoids, which appeared abundantly in mesopelagic layers (Yamaguchi et al. 2002), our estimates of the mesopelagic carbon demand would still be conservative. A similar excess of metazoan carbon demand in mesopelagic layers relative to POC flux has been reported in the North Pacific Ocean (Steinberg et al. 2008b) and North Atlantic Ocean (Giering et al. 2014). While carbon demand has previously been overestimated due to the oxygen consumption rates calculated by the global model (Eq. 3), Steinberg et al. (2008b) reported that the mesopelagic carbon demand could be supported by carnivory of mesopelagic residents on diel and seasonal migrants. Interestingly, the excess of mesopelagic carbon demand to POC flux was larger at K2 (Fig. 8), where the seasonally migrating copepods reproduced and died at depth, than at the other site (Extended Data Fig. 8 in Giering et al. 2014). Therefore, the carnivory of mesopelagic residents on the migrants (including their reproduced eggs and nauplii at depth), as well as the low combination of AE and R, may support the excess mesopelagic carbon demand to POC flux.
4.4 Active and passive carbon fluxes facilitated by mesozooplankton
Sinking particles, including fecal pellets, have been accepted as a major contributor to the biological pump (e.g., Fowler and Knauer 1986; Zhang and Dam 1997). In the last two decades, however, many studies have demonstrated that carbon actively transported by diel and seasonal migrants is significant (e.g., Longhurst et al. 1990; Steinberg et al. 2000; Bradford-Grieve et al. 2001; Kobari et al. 2003, 2008b; Takahashi et al. 2009) and is of greater importance than sinking metazoan fecal pellets (Kobari et al. 2013). Compared with the fecal pellet flux, active DIC and DOC fluxes by diel migrants were greater in all seasons at S1 but only in February at K2 (Fig. 9). Due to the higher ambient temperatures at mesopelagic depths (Fig. 2) and smaller fecal pellet flux at S1 than at K2 throughout the seasons (Fig. 3), active carbon flux by diel migrants was a relatively important component of the biological pump compared with passive carbon flux, even with the low biomass of diel migrants at S1 (Table 2).
Respiratory carbon flux by diurnally migrating mesozooplankton (Table 2) was within the range of previous estimates from 1 to 20 mgC m−2 day−1 (Kobari et al. 2013). Even though the migrant biomass at K2 was much higher than that at S1, the seasonal range and annual mean of actively transported DIC and DOC were comparable between the two sites (Fig. 9; Table 2). However, the coefficient of variation (standard deviation divided by average, %) for the DIC and DOC was larger at K2 (75 %) than at S1 (48 %), which suggests that the active carbon flux at the subarctic site was more seasonally variable than that at the subtropical site (Kobari et al. 2013). While the relative importance of actively transported DIC + DOC versus sinking POC was not great (3–26 % at K2 and 8–40 % at S1), and the DIC was not available for mesopelagic heterotrophs, actively transported DOC by diel migrants may affect the seasonal variability of mesopelagic microbes at K2.
As annual means, the ratio of mesozooplankton fecal pellet flux to POC flux was 15 % at K2 and 2 % at S1 (Table 2), which were similar to previous estimates at K2 and ALOHA during the VERTIGO project (Wilson et al. 2008). The active carbon flux by mesozooplankton respiration (DIC) and excretion (DOC) at depth was equivalent to 8 % of the POC flux at K2 and 12 % at S1. Since the carbon demand of mesopelagic protozoans and metazoans exceeded the POC flux by a factor of 5–20 (Steinberg et al. 2008b; Giering et al. 2014), actively transported DOC would not be a sufficient supplement to account for the excess. Compared with the fecal pellet flux, however, these active carbon fluxes were smaller at K2 (56 %, 3.9/7.0) and greater at S1 (750 %, 4.5/0.6). In previous studies, these active carbon fluxes reached 532 % of the fecal pellet flux at K2 and 348 % at ALOHA (Steinberg et al. 2008b; Wilson et al. 2008). Thus, the actively transported carbon would not be negligible for pathways of downward carbon flux mediated by the mesozooplankton community.
5 Conclusions
We demonstrated the seasonal variability in passive (i.e., sinking fecal pellets) and active carbon fluxes (respired DIC and excreted DOC by diel migrants at depth) by mesozooplankton communities at both subarctic and subtropical sites, which was similar to the variability observed in the VERTIGO project (Steinberg et al. 2008b; Wilson et al. 2008; Kobari et al. 2008a, b) and others (e.g., Kobari et al. 2013). We also found that the relative importance of active carbon flux versus sinking POC flux was greater at the subtropical site than at the subarctic site. This means that the relative importance of active carbon flux by mesozooplankton communities to the biological pump is higher at locations where attenuation efficiency of sinking POC flux is high, even under conditions of low mesozooplankton biomass. Moreover, we still found mesopelagic carbon demand to be in excess of sinking POC flux, which is consistent with the results of the VERTIGO project (Steinberg et al. 2008b). Such subtle differences may be explained by trophic interaction among mesopelagic metazoans (i.e., the carnivory of mesopelagic residents on the migrants).
References
Abe Y, Natsuike M, Matsuno M, Terui T, Yamaguchi A, Imai I (2013) Variation in assimilation efficiencies of dominant Neocalanus and Eucalanus copepods in the subarctic Pacific: consequences for population structure models. J Exp Mar Biol Ecol 449:321–329
Al-Mutairi H, Landry MR (2001) Active export of carbon and nitrogen at Station ALOHA by diel migrant zooplankton. Deep-Sea Res II 48:2083–2103
Arístegui J, Duarte CM, Agustí S, Doval M, Álvarez-Salgado XA, Hansell DA (2002) Dissolved organic carbon support of respiration in the dark ocean. Science 298:1967
Ayukai T, Hattori H (1992) Production and downward flux of zooplankton fecal pellets in the anticyclonic gyre off Shikoku, Japan. Oceanol Acta 15:163–172
Bedo AW, Acuña JL, Robins D, Harris RP (1993) Grazing in the micron and the sub-micron particle size range: the case of Oikopleura dioica (Appendicularia). Bull Mar Sci 53:2–14
Borgne Le (1982) Zooplankton production in the eastern tropical Atlantic Ocean: net growth efficiency and P: B in terms of carbon, nitrogen, and phosphorous. Limnol Oceanogr 27:681–698
Bradford-Grieve JM, Nodder SD, Jillett KB, Currie K, Lassey KR (2001) Potential contribution that the copepod Neocalanus tonsus makes to downward carbon flux in the Southern Ocean. J Plankton Res 23:963–975
Buesseler KO, Boyd PW (2009) Shedding light on processes that control particle export and flux attenuation in the twilight zone of the open ocean. Limnol Oceanogr 54:1210–1232
Buesseler KO, Lamborg CH, Boyd PW, Lam PJ, Trull TW, Bidigare RR, Bishop JKB, Casciotti KL, DeHairs F, Karl DM, Siegel D, Silver MW, Steinberg DK, Valdez J, Van Mooy B, Wilson SE (2007) Revisiting carbon flux through the ocean’s twilight zone. Science 316:567–570
Chisholm SW (2000) Oceanography: stirring times in the Southern Ocean. Nature 407:685–687
Conover RJ (1988) Comparative life histories in the genera Calanus and Neocalanus in high latitudes of the northern hemisphere. Hydrobiol 167(168):127–142
Dagg MJ (1993) Sinking particles as a possible source of nutrition for the large calanoid copepod Neocalanus cristatus in the subarctic Pacific Ocean. Deep-Sea Res I 40:1431–1445
Deibel D, Lee SH (1992) Retention efficiency of sub-micrometer particles by the pharyngeal filter of the pelagic tunicate Oikopleuira vanhoeffeni. Mar Ecol Prog Ser 81:25–30
Doi H, Kobari T, Fukumori K, Nishibe Y, Nakano S-I (2010) Trophic niche breadth variability differs among three Neocalanus species in the subarctic Pacific Ocean. J Plankton Res 32:1733–1737
Fowler SW, Knauer GA (1986) Role of large particles in the transport of elements and organic compounds through the oceanic water column. Prog Oceanogr 16:147–194
Fowler SW, Small LF, LaRosa J (1991) Seasonal particulate carbon flux in the coastal northwestern Mediterranean Sea, and the role of zooplankton fecal matter. Oceanol Acta 14:77–85
Frost BW, Landry MR, Hassett RP (1983) Feeding behavior of large calanoid copepods Neocalanus cristatus and N. plumchrus from the subarctic Pacific Ocean. Deep-Sea Res 30:1–13
Fujiki T, Matsumoto K, Mino Y, Sasaoka K, Wakita M, Kawakami H, Honda MC, Watanabe S, Saino T (2014) The seasonal cycle of phytoplankton community structure and photo-physiological state in the western subarctic gyre of the North Pacific. Limnol Oceanogr 59:887–900
Giering SLC, Sanders R, Lampitt RS, Anderson TR, Tamburini C, Boutrif M, Zubkov MV, Marsay CM, Henson SA, Saw K, Cook K, Mayor DJ (2014) Reconciliation of the carbon budget in the ocean’s twilight zone. Nature 507:480–483
Gifford DJ (1993) Protozoa in the diets of Neocalanus spp. in the oceanic subarctic Pacific Ocean. Prog Oceanogr 32:223–237
Gleiber MR, Steinberg DK, Ducklow HW (2012) Time series of vertical flux of zooplankton fecal pellets on the continental shelf of the western Antarctic Peninsula. Mar Ecol Prog Ser 471:23–36
Gnaiger E (1983) Calculation of energetic and biochemical equivalents of respiratory oxygen consumption. In: Gnaiger E, Forstner H (eds) Polarogranic oxygen sensors. Springer, Berlin, pp 337–345
González HE, Smetacek V (1994) The possible role of the cyclopoid copepod Oithona in retarding vertical flux of zooplankton fecal material. Mar Ecol Prog Ser 113:233–246
Gowing MM, Garrison DL, Kunze HB, Winchell CJ (2001) Biological components of Ross Sea short-term particle fluxes in the austral summer of 1995–1996. Deep-Sea Res I 48:2645–2671
Greene CH, Landry MR (1988) Carnivorous suspension feeding by the subarctic calanold copepod Neocalanus cnstatus. Can J Fish Aquat Sci 45:1069–1074
Honda MC, Kawakami H, Matsumoto K, Fujiki T, Mino Y, Sukigara C, Kobari T, Uchimiya M, Kaneko R, Saino T (2015) Comparison of sinking particle upper 200 m between subarctic station K2 and subtropical station S1 based on drifting sediment trap experiment. J Oceanogr. doi:10.1007/s2-015-0289-x
Ikeda T (1985) Metabolic rates of epipelagic marine zooplankton as a function of body mass and temperature. Mar Biol 85:1–11
Ikeda T, Sano F, Yamaguchi A (2007) Respiration in marine pelagic copepods: a global-bathymetric model. Mar Ecol Prog Ser 339:215–219
Ikeda T, Shiga N, Yamaguchi A (2008) Structure, biomass distribution and trophodynamics of the Pelagic ecosystem in the Oyashio Region, Western Subarctic Pacific. J Oceanogr 64:339–354
Iversen MH, Poulsen LK (2007) Coprorhexy, coprophagy, and coprochaly in the copepods Calanus helgolandicus, Pseudocalanus elongates, and Oithona similis. Mar Ecol Prog Ser 350:79–89
Iversen MH, Nowald N, Ploug H, Jackson GA, Fischer G (2010) High resolution profiles of vertical particulate organic matter export off Cape Blanc, Mauritania: degradation processes and ballasting effects. Deep-Sea Res I 57:771–784
Kitamura M, Kobari T, Honda MC, Matsumoto K, Sasaoka K, Nakamura R, Tanabe K (2016) Seasonal changes in the mesozooplankton biomass and community structure in subarctic and subtropical time-series stations in the western North Pacific. J Oceanogr. doi:10.1007/s10872-015-0347-8
Knauer GA, Martin JH, Bruland KW (1979) Fluxes of particulate carbon, nitrogen, and phosphorus in the upper water column of the northeast Pacific. Deep-Sea Res A 26:97–108
Kobari T, Ikeda T (1999) Vertical distribution, population structure and life cycle of Neocalanus cristatus (Crustacea: Copepoda) in the Oyashio region, with notes on its regional variations. Mar Biol 134:683–696
Kobari T, Ikeda T (2001a) Life cycle of Neocalanus flemingeri (Crustacea: Copepoda) in the Oyashio region, western subarctic Pacific, with notes on its regional variations. Mar Ecol Prog Ser 209:243–255
Kobari T, Ikeda T (2001b) Ontogenetic vertical migration and life cycle of Neocalanus plumchrus (Crustacea: Copepoda) in the Oyashio region, with notes on regional variations in body size. J Plankton Res 23:287–302
Kobari T, Shinada A, Tsuda A (2003) Functional roles of interzonal migrating mesozooplankton in the western subarctic Pacific. Prog Oceanogr 57:279–298
Kobari T, Moku M, Takahashi K (2008a) Seasonal appearance of expatriated boreal copepods in the Oyashio-Kuroshio mixed region. ICES J Mar Sci 65:469–476
Kobari T, Steinberg DK, Ueda A, Tsuda A, Silver MW, Kitamura M (2008b) Impacts of ontogenetically migrating copepods on downward carbon flux in the western subarctic Pacific Ocean. Deep-Sea Res II 55:1648–1660
Kobari T, Akamatsu H, Minowa M, Ichikawa T, Iseki K, Fukuda R, Higashi M (2010) Effects of the copepod community structure on fecal pellet flux in Kagoshima Bay, a deep, semi-enclosed embayment. J Oceanogr 66:673–684
Kobari T, Kitamura M, Minowa M, Isami H, Akamatsu H, Kawakami H, Matsumoto K, Wakita M, Honda MC (2013) Impacts of the wintertime mesozooplankton community to downward carbon flux in the subarctic and subtropical Pacific Oceans. Deep-Sea Res I 81:78–88
Lampitt RS, Noji TT, von Bodungen B (1990) What happens to zooplankton faecal pellets? Implications for material flux. Mar Biol 104:15–23
Lane PVZ, Smith SL, Urban JL, Biscayn PE (1994) Carbon flux and recycling associated with zooplanktonic fecal pellets on the shelf of the Middle Atlantic Bight. Deep-Sea Res II 41:437–457
Liu H, Dagg MJ, Strom S (2005) Grazing by the calanoid copepod Neocalanus cristatus on the microbial food web in the coastal Gulf of Alaska. J Plankton Res 27:647–662
Longhurst AR, Bedo AW, Harrison WG, Head EJH, Sameoto DD (1990) Vertical flux of respiratory carbon by oceanic diel migrant biota. Deep-Sea Res. 37(4):685–694
Longhurst A, Williams R (1992) Carbon flux by seasonal vertical migrant copepods is small number. J Plankton Res 14:1495–1509
Mackas DL, Tsuda A (1998) Mesozooplankton in the eastern and western subarctic Pacific: community structure, seasonal life histories, and interannual variability. Prog Oceanogr 43:335–363
Maita Y, Odate T, Yanada M (1988) Vertical transport of organic carbon by sinking particles and the role of zoo- and phytogenic matters in neritic waters. Bull Fac Fish Hokkaido Univ 39:265–274
Martens P (1978) Faecal pellets. FichIdent Zooplancton 162:1–4
Martin JH, Knauer GA, Karl DM, Broenkow WW (1987) VERTEX: carbon cycling in the northeast Pacific. Deep-Sea Res 34:267–285
Matsumoto K, Honda CM, Sasaoka K, Wakita M, Kawakami H, Watanabe S (2014) Seasonal variability of primary production and phytoplankton biomass in the western Pacific subarctic gyre: control by light availability within the mixed layer. J Geophys Res 119:6523–6534
Mauchline J (1998) The biology of calanoid copepods. Adv Mar Biol 33:1–710
McDonnel AMP, Buesseler KO (2010) Variability in the average sinking velocity of marine particles. Limnol Oceanogr 55:2085–2096
McGowan JA, Walker PW (1979) Structure in the copepod community of the North Pacific central gyre. Ecol Monogr 49:195–226
McGowan JA, Walker PW (1985) Dominance and diversity maintenance in an oceanic ecosystem. Ecol Monogr 55:103–118
Miller CB, Clemons MJ (1988) Revised life history analysis for large grazing copepods in the subarctic Pacific Ocean. Prog Oceanogr 20:293–313
Miller CB, Frost BW, Batchelder HP, Clemons MJ, Conway RE (1984) Life histories of large, grazing copepods in a subarctic ocean gyre: Neocalanus plumchrus, Neocalanus cristatus, and Eucalanus bungii in the northeast Pacific. Prog Oceanogr 13:201–243
Noji TT, Estep KW, MacIntyre F, Norrbin F (1991) Image analysis of faecal material grazed upon by three species of copepods: evidence for coprohexy, coprophagy, and coprochaly. J Mar Biol Ass United Kingdom 71:465–480
Pace ML, Knauer GA, Karl DM, Martin JH (1987) Primary production, new production and vertical flux in the eastern Pacific Ocean. Nature 325:803–804
Padmavati G, Ikeda T, Yamaguchi A (2004) Life cycle, population structure and vertical distribution of Metridia spp. (Calanoida: Copepoda) in the Oyashio region (NW Pacific Ocean). Mar Ecol Prog Ser 270:181–198
Paffenhöfer G-A, Knowles SA (1979) Ecological implications of fecal pellet size, production and consumption by copepods. J Mar Res 37:35–49
Paffenhöfer G-A, Strickland JDH (1970) A note on the feeding of Calanus helgolandicus on detritus. Mar Biol 5:97–99
Parsons TR, Lalli CM (1988) Comparative oceanic ecology of the plankton communities of the subarctic Atlantic and Pacific Oceans. Oceanogr Mar Biol Ann Rev 26:317–359
Poulsen LK, Iversen MH (2008) Degradation of copepod fecal pellets: key role of protozooplankton. Mar Ecol Prog Ser 367:1–13
Poulsen LK, Kiørboe T (2006) Vertical flux and degradation rates of copepod fecal pellets in a zooplankton community dominated by small copepods. Mar Ecol Prog Ser 323:195–204
Roy S, Silverberg N, Romero N, Deibel D, Kleine B, Savenkofff C, Vézinaf A, Tremblaye J-É, Legendree L, Rivkind RB (2000) Importance of mesozooplankton feeding for the downward flux of biogenic carbon in the Gulf of St. Lawrence (Canada). Deep-Sea Res II 47:519–544
Sherr EB, Barry F, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. Ant Leeuwen 81:293–308
Shimode S, Hiroe Y, Hidaka K, Takahashi K, Tsuda A (2009) Life history and ontogenetic vertical migration of Neocalanus gracilis in the western North Pacific Ocean. Aquat Biol 7:295–306
Shimode S, Takahashi K, Shimizu Y, Nonomura T, Tsuda A (2012a) Distribution and life history of the planktonic copepod, Eucalanus californicus, in the northwestern Pacific: mechanisms for population maintenance within a high primary production area. Prog Oceanogr 86:1–13
Shimode S, Takahashi K, Shimizu Y, Nonomura T, Tsuda A (2012b) Distribution and life history of two planktonic copepods, Rhincalanus nasutus and Rhincalanus rostrifrons, in the northwestern Pacific Ocean. Deep-Sea Res I 65:133–145
Shoden S, Ikeda T, Yamaguchi A (2005) Vertical distribution, population structure and lifecycle of Eucalanus bungii (Copepoda: Calanoida) in the Oyashio region, with notes on its regional variations. Mar Biol 146:497–511
Steinberg DK, Carlson CA, Bates NR, Goldthwait SA, Madin LP, Michaels AF (2000) Zooplankton vertical migration and the active transport of dissolved organic and inorganic carbon in the Sargasso Sea. Deep-Sea Res I 47:137–158
Steinberg DK, Cope JS, Wilson SE, Kobari T (2008a) A comparison of mesopelagic mesozooplankton community structure in the subtropical and subarctic North Pacific Ocean. Deep-Sea Res II 55:1615–1635
Steinberg DK, Van Mooy BAS, Buesseler KO, Boyd PW, Kobari T, Karl DM (2008b) Microbial vs. zooplankton control of sinking particle flux in the ocean’s twilight zone. Limnol Oceanogr 53:1327–1338
Steinberg DK, Lomas MW, Cope JS (2012) Long-term increase in mesozooplankton biomass in the Sargasso Sea: linkage to climate and implications for food web dynamics and biogeochemical cycling. Glob Biogeochem Cyc 26:GB1004
Stukel MR, Ohman MD, Benitez-Nelson CR, Landry MR (2013) Contributions of mesozooplankton to vertical carbon export in a coastal upwelling system. Mar Ecol Prog Ser 491:47–65
Suess E (1980) Particulate organic carbon flux in the oceans-surface productivity and oxygen utilization. Nature 288:260–263
Suzuki R, Ishimaru T (1990) An improved method for the determination of phytoplankton chlorophyll using N, N-dimethylformamide. J Oceanogr Soc Japan 46:190–194
Svensen C, Nejstgaard JC (2003) Is sedimentation of copepod faecal pellets determined by cyclopoids? Evidence from enclosed ecosystems. J Plankton Res 25:917–926
Svensen C, Riser CW, Reigstad M, Seuthe L (2012) Degradation of copepod faecal pellets in the upper layer: role of microbial community and Calanus finmarchicus. Mar Ecol Prog Ser 462:39–49
Taguchi S, Saino T (1998) Net zooplankton and the biological pump off Sanriku, Japan. J Oceanogr 54:573–582
Takahashi K, Kuwata A, Sugisaki H, Uchikawa K, Saito H (2009) Downward carbon transport by diel vertical migration of the copepods Metridia pacifica and Metridia okhotensis in the Oyashio region of the western subarctic Pacific Ocean. Deep-Sea Res I 56:1777–1791
Taylor GT (1989) Variability in the vertical flux of microorganisms and biogenic material in the epipelagic zone of a North Pacific central gyre station. Deep-Sea Res 36:1287–1308
Tsuda A, Takeda S, Saito H, Nishioka J, Nojiri Y, Kudo I, Kiyosawa K, Shiomoto A, Imai K, Ono T, Shimamoto A, Tsumune D, Yoshimura T, Aono T, Hinuma A, Kinugasa M, Suzuki S, Sohrin Y, Noiri Y, Tani H, Deguchi Y, Tsurushima N, Ogawa H, Fukami K, Kuma K, Saino T (2003) A mesoscale iron enrichment in the western subarctic Pacific induces a large centric diatom bloom. Science 300:958–961
Turner JT (2002) Zooplankton fecal pellets, marine snow and sinking phytoplankton blooms. Aquat Microb Ecol 27:57–102
Urrére MA, Knauer GA (1981) Zooplankton fecal pellet fluxes and vertical transport of particulate organic material in the pelagic environment. J Plankton Res 3:369–387
Uye S, Kaname K (1994) Relations between fecal pellet volume and body size for major zooplankters of the Inland Sea of Japan. J Oceanogr 50:43–49
Wassmann P, Hansen L, Andreassen IJ, Riser CW, Urban-Rich J (1999) Distribution and sedimentation of faecal pellets on the Nordvestbanken shelf, northern Norway, in 1994. Sarsia 84:239–252
Wassmann P, Ypma JE, Tselepides A (2000) Vertical flux of faecal pellets and microplankton on the shelf of the oligotrophic Cretan Sea (NE Mediterranean Sea). Prog Oceanogr 46:241–258
Welschmeyer NA (1994) Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and phaeopigments. Limnol Oceanogr 39:1985–1992
Wilson S, Steinberg D, Buesseler K (2008) Changes in fecal pellet characteristics with depth as indicators of zooplankton repackaging of particles in the mesopelagic zone of the subtropical and subarctic North Pacific Ocean. Deep-Sea Res II 55:1636–1647
Yamaguchi A, Watanabe I, Ishida H, Harimoto T, Furusawa K, Suzuki S, Ishizaka J, Ikeda T, Takahashi MM (2002) Structure and size distribution of plankton communities down to the greater depths in the western North Pacific Ocean. Deep-Sea Res II 49:5513–5529
Yoon WD, Kim SK, Han KN (2001) Morphology and sinking velocities of fecal pellets of copepod, molluscan, euphausiid, and salp taxa in the northeastern tropical Atlantic. Mar Biol 139:923–928
Zhang X, Dam HG (1997) Downward export of carbon by diel migrant mesozooplankton in the central equatorial Pacific. Deep-Sea Res II 44:2191–2202
Acknowledgments
We are grateful to Drs. C. B. Miller and S. S. Villamor for English editing and the two anonymous reviewers and a handling editor for valuable comments and suggestions. We thank the captain and crew of the R/V Mirai, and the staff of Marine Works Japan Ltd for their help with sampling in the field and with sample analyses. This study was supported in part by grants from the Japan Society for the Promotion of Science (21710012, 23310020, 25340011) and the Ministry of Education, Culture, Sports, Science and Technology of Japan (SKED: The Study of Kuroshio Ecosystem Dynamics for Sustainable Fisheries).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobari, T., Nakamura, R., Unno, K. et al. Seasonal variability in carbon demand and flux by mesozooplankton communities at subarctic and subtropical sites in the western North Pacific Ocean. J Oceanogr 72, 403–418 (2016). https://doi.org/10.1007/s10872-015-0348-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10872-015-0348-7