Abstract
Vertical distribution and population structure of Eucalanus bungii were investigated at site H in the Oyashio region (western subarctic Pacific) from September 1996 through October 1997 to evaluate the species’ lifecycle pattern and associated ontogenetic vertical migration. Additional temporary samplings were also made at several stations covering the entire subarctic Pacific, Okhotsk Sea and Japan Sea, as a basis for regional comparison of lifecycle features of this species. At site H, a marked phytoplankton bloom occurred from mid-March to June, and E. bungii spawned in April/May in the surface layer. Resulting nauplii and copepodite stage 1 (C1) formed a prominent abundance peak in early June. The C1 developed and reached C5 by August. The development of nauplii through C4 occurred in the surface layer. From August onwards, C5 and a small fraction of C3–C4 sank gradually deeper, and entered diapause to overwinter at >500 m depth. The C5 molted to C6 males and females in February and March, respectively. The C6 males and females mated at 250–500 m depth, and only mated C6 females ascended to the surface layer in April for spawning. Judging from the size of lipid droplets in the body, the C3–C5 specimens deposited lipids in the body through the phytoplankton bloom period, and the lipids were consumed gradually during overwintering. Taking account of sampling season, temporal changes in population structure, and vertical distribution, the data collected from the western subarctic Pacific and Okhotsk Sea are consistent with a 1-year lifecycle for the site H population, while the data from the central and eastern subarctic Pacific were consistent with a 2-year lifecycle. The populations from the southern and southeastern Japan Sea did not fit the features of either lifecycle scenario, and because of their very small population size it is suggested that they originated from the northern Japan Sea. Regional comparison of the prosome length of C6 females, including those in the Bering Sea, indicated significantly larger specimens from the Japan Sea and Okhotsk Sea, and smaller specimens in the eastern subarctic Pacific, as compared with those in the western subarctic Pacific (including site H) and Bering Sea. A possible overwintering mechanism of E. bungii is discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
While the copepod genus Eucalanus has 20 species and is distributed throughout the world oceans (Mauchline 1998), only Eucalanus bungii occurs in the entire subarctic Pacific Ocean and adjacent Bering, Okhotsk and Japan Seas (Fleminger 1973; Fleminger and Hulsemann 1973). E. bungii is a large copepod (adult body length 5–7 mm), and together with other large copepods such as Neocalanus cristatus, N. plumchrus and N. flemingeri, it accounts for 80–95% of mesozooplankton biomass in the surface zone of these regions during summer (Vinogradov 1970; Tsuda and Sugisaki 1994). For the mesozooplankton biomass in the 0–4,000 m water column in the Kurile-Kamchatka region, the most important species is reported to be N. cristatus, followed by E. bungii (Vinogradov and Arashkevich 1969). From the viewpoint of trophic functions in the pelagic ecosystem, E. bungii prey on phytoplankton, microzooplankton, and other particles (Ohtsuka et al. 1993); in turn, they are preyed upon by pelagic/demersal fishes (Nemoto 1970; Takeuchi 1972; Kamba 1977) and seabirds (Russell et al. 1999), thus playing an intermediate role between primary production and the production of animals at higher trophic levels. In order to estimate that role quantitatively, we need information about the vertical distribution, biomass, and lifecycle patterns of this copepod.
To date, available information about the lifecycle and ontogenetic vertical migration of E. bungii includes those in British Columbia inlets (Krause and Lewis 1979), station P (Miller et al. 1984), Funka Bay, southeastern Hokkaido (western subarctic Pacific; Hirakawa 1984) and the southeastern Bering Sea (Vidal and Smith 1986). According to the most comprehensive study by Miller et al. (1984), the lifecycle of E. bungii in the eastern subarctic Pacific is typically 2 years, and they undergo ontogenetic vertical migration ranging from the surface layer to a depth of 250–500 m. Compared with lifecycle patterns of sympatric Neocalanus spp., E. bungii is similar to Neocalanus spp. in that it develops in the upper layer and enters diapause in the mesopelagic layer for overwintering. However, E. bungii is different from Neocalanus spp. in that adult females of the former continue to feed and spawn in the upper layer, while females of the latter cease feeding and spawn in deep layers. Considering the widespread distribution of E. bungii across the entire subarctic Pacific and its marginal seas, where environmental conditions vary greatly from one region to the next, divergent lifecycles are anticipated. In recent years, there has been growing evidence of regional variations in lifecycles of marine pelagic copepods, including Calanoides acutus (Atkinson et al. 1997) and Rhincalanus gigas (Ward et al. 1997) in the Southern Ocean, Calanus finmarchicus (Planque et al. 1997) and C. hyperboreus (Hirche 1997) in the North Atlantic Ocean, and M. pacifica (Padmavati et al. 2004) in the subarctic North Pacific Ocean.
As part of a research program to evaluate plankton dynamics in the Oyashio region, the present study aims to document the lifecycle pattern of E. bungii and its ontogenetic vertical migration in the Oyashio region by analyzing time-series samples collected from five discrete depths between 0 and 2,000 m. In addition to these time series in the Oyashio region, series from several temporal stations covering the entire subarctic Pacific Ocean, Japan Sea, and Okhotsk Sea were analyzed to examine patterns possibly divergent from those in the Oyashio region. The regional variations in the prosome length and the size of lipid droplets in the body were determined as indices of the confounding effects of temperature and food abundance in the habitats of E. bungii. Similarities/dissimilarities of overwintering mechanism of E. bungii to that of Neocalanus spp. are discussed.
Materials and methods
Field sampling
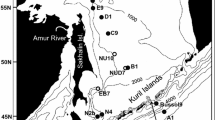
Monthly depth-stratified sampling was done in the Oyashio region (41°30′–42°30′N and 145°00′–146°00′E) off southeastern Hokkaido (hereafter referred to as site H; Fig. 1). We also obtained occasional samples from three additional stations in the western, one station in the central, and two stations in the eastern subarctic Pacific, four stations in the Japan Sea, two stations in the Okhotsk Sea, and one station in the western Bering Sea (Fig. 1; Table 1). Except for the isolated sampling in the Bering Sea in February 1993, all other samples were collected from September 1996 to August 1998.
Zooplankton were collected with a closing net (60 cm mouth diameter, 100 µm mesh size; Kawamura 1968, 1989) equipped with a Rigosha flowmeter in its mouth ring and a TSK depth distance recorder or RMD depth meter on its suspension cable at site H and most of the other stations. In addition to the closing net, a WP-2 net (57 cm mouth diameter, 200 µm mesh size) and a Vertical Multiple Plankton Sampler (VMPS) (100×100 cm mouth opening, 90 µm mesh size; Terazaki and Tomatsu 1997) were used for the collection at the station in the Bering Sea and KNOT, respectively. All the nets were towed vertically at a speed of 1 m s−1, usually through five discrete depths: 0 (surface) to the bottom of the thermocline (TC), TC–250 m, 250–500 m, 500–1,000 m, and 1,000–<2,000 m for the closing net and VMPS, with the exception only of 0–500 m for the WP-2 net (Table 1). During the winter season, when the thermocline was not detected, the TC was assumed arbitrarily as 100 m depth. On three dates, one or more depth strata were not sampled. Data for missing samples were interpolated from the nearest samplings of that depth interval before and after the sampling dates (see Table 1). To investigate the diel vertical migration pattern, day-night samplings were conducted on three occasions: 8 December 1996, and 11 April and 5 October 1997 (Table 1).
After collection, zooplankton samples were immediately preserved in 5% formalin-seawater solution buffered with borax. Temperature and salinity profiles were determined with a CTD system (Neil Brown, General Oceanics Inc., or Sea Bird, Electronics Inc., Washington, USA) at each zooplankton sampling. Chlorophyll a profiles at site H have been reported elsewhere (Kasai et al. 2001). Details of the sampling program at KNOT have been reported elsewhere (Yamaguchi et al. 2002).
Identification and enumeration
In the land laboratory, E. bungii was sorted from the entire or 1/2 aliquot of preserved zooplankton samples and counted under a dissecting microscope. The morphological features of each developmental stage of E. bungii are given by Johnson (1937). Naupliar stages 1–6 were pooled and counted as nauplii in this study. Separation of males from females was possible from copepodite stage 4 (C4). The prosome length of 30 arbitrarily selected specimens for each developmental stage was measured under the dissecting microscope to the nearest 0.1 mm. As an index of the nutritional condition of specimens, the diameter of spherical lipid droplets in the posterior prosome of copepodite stages was also measured under the dissecting microscope, and specimens were classified into a ‘rich’ category with oil droplet diameter >1/2 of body width, ‘medium’ category with droplet diameter <1/2 of body width, or ‘poor’ category with no lipid droplet (Fig. 2). As an index of the spawning activity of C6 females, seven maturity categories based on ovary conditions were established for E. bungii by Miller et al. (1984). In this study, we adopted the following simplified system by observing the female’s prosome laterally.
Stage I—the ovary is present. Some granular oocytes or ova are visible in the posterior part of the oviduct [Miller et al.’s (1984) categories I–III].
Stage II—one fully developed row of ova is observed in the ventral edge of the oviduct [Miller et al.’s (1984) category IV].
Stage III—more than two full rows of ova are seen in the oviduct [Miller et al.’s (1984) category V].
Stage IV—lower portion of oviduct does not contain ova, but is filled with lipid-like substances [Miller et al.’s (1984) category VI].
Stage V—ovary and lipid droplets are not seen and the body is transparent (=spent).
Results
Hydrography
Site H
The western boundary current of the subarctic circulation in the northern Pacific is called the ‘Oyashio current’. It flows southwestward along the Kuril Islands and reaches the east coast of northern Honshu, Japan, then turns east at about 40°N (Reid 1973). During its journey, the properties of Oyashio water are modified as a result of exchange with Okhotsk Sea water and Kuroshio water (Kono 1997). Because of its meandering flow pattern, isolated loops of the Kuroshio extension are often entrapped between the downstream and return flows of the Oyashio and are called “warm core rings”.
Site H of this study is near the southern end of the southwestward alongshore flow of the Oyashio current. Over the study period, surface temperatures ranged from 2°C (March–April 1997) to 18°C (September–October 1996 and 1997)(Fig. 3). Oyashio current water, characterized by salinities from 33.0‰ to 33.3‰ and a temperature below 3°C (Ohtani 1971) occurred in the upper 150 m from February to April 1997. After April, less saline, seasonally warmed water (possibly originating from the Okhotsk Sea; T. Kono, personal communication) occupied the upper 50 m of the water column. Surface temperatures above 10°C were observed from September to November 1996 and from June to October 1997, when the thermocline was well established at 20–50 m in the water column. Effects of warm core rings originating from the Kuroshio extension were seen in September of both 1996 and 1997, and from December 1996 to January 1997, as judged by temperature at 200 m (>4°C) and salinity in the 0–200 m layer (>33.5‰)(Fig. 2). The temperature and salinity in the 200–1,500 m layer were stable and nearly constant at 2–3°C and 33.5–34.5‰, respectively, throughout the year.
Phytoplankton biomass, estimated in terms of chlorophyll a concentrations, showed a marked seasonality (Fig. 3). Chlorophyll a at the surface was around 0.4 mg m−3 from August 1996 to the end of February 1997, and then increased rapidly to >9 mg m−3 in May 1997. During this period, concentrations above 2 mg m−3 extended down to a depth of 50 m. The surface chlorophyll a concentrations had decreased to 2 mg m−3 by the end of June and to 0.4 mg m−3 toward the end of 1997. Chlorophyll a concentrations were <0.4 mg m−3 throughout the yearbelow 100 m depth.
Other areas
Among three stations in the western subarctic Pacific, temperatures of the top 500 m increased in the order of stations KNOT to 19 to HO96098 (Fig. 4). Compared to site H, the upper 500 m of stations KNOT and 19 were colder and HO96098 was similar (except for higher temperatures near the surface layer). The cold temperature in the subsurface layers at stations 19 and KNOT reflects the influence of cold Okhotsk Sea water for the former (cf. Kono 1997) and cold intermediate water formed during winter for the latter (Tsurushima et al. 2002). Taking into account the sampling season, the temperature profiles at station OS97997 in the central subarctic Pacific, and OS97120 (= station P) and OS97130 in the eastern subarctic Pacific were closely comparable to that at site H, except that the top 500 m was slightly warmer at the latter two stations. Comparison of annual temperature variations of the top 500 m at site H (Fig. 3) and station P (Miller et al. 1984) showed that site H was characterized by a wider temperature range in the surface layer (2–18°C versus 6–14°C) and lower temperatures in the 200–500 m stratum (2–3°C versus 4–5°C).
The thermal regimes in the marginal seas (Japan Sea and Okhotsk Sea) differed from those in the subarctic Pacific by the occurrence of near-zero temperatures (Zenkevitch 1963). This very cold water is termed ‘deep water’ in the Japan Sea, and ‘cold intermediate water’ in the Okhotsk Sea. At all four stations in the Japan Sea, the deep water was below 500 m. At stations I09 and J07 in the northern Japan Sea, water temperature in the top 300 m was higher than at stations C08 and HO097104 in the northern Japan Sea, perhaps due to the effect of the Tsushima warm current [a branch of Kuroshio carrying warm water from the south (Nishimura 1969)].
Nishimura (1969) classified Japan Sea regions as ‘subtropical’, ‘subarctic’, and ‘arctic’ based on biological features. According to his classification, stations C08 and HO97103 in the northern Japan Sea are in the subarctic region, and stations I09 and J07 in the southern Japan Sea are in the subtropical region. At two stations in the Okhotsk Sea, the cold intermediate water was at 30–200 m depth, shallower than in the Japan Sea. This was especially evident at the southern station HO97150.
Population structure
Site H
Nauplii and young copepodites (C1–C2) did not occur in September 1996 or October through April of the next year (Fig. 5). They occurred first in early May (midphytoplankton bloom), formed a prominent abundance peak in early June then decreased rapidly. While the C3 occurred throughout the year with a marked abundance peak in early June, they were very few during October and April of the next year. The abundance of C4 and C5 was very low in April–May, followed by moderate annual peaks in June–early July (end of high chlorophyll period) and August (1 month after the high chlorophyll period) (Fig. 5). Seasonal differences in the abundance of the sexes were not evident in C4 or C5, but were pronounced in C6. The C6 males were less abundant (annual mean 11% of the total C6) than the C6 females, and the males showed a sharp abundance peak in March, while the females exhibited irregular peaks in April–July. Maturity stage composition of C6 females indicated predominance of stage I (94–100% of the total C6) in most seasons, except during the high chlorophyll period when stages II–V increased rapidly. The proportion of stage II–V in C6 females became high again in August/September, but their contribution to the overall reproduction of the population must have been modest, because of the low abundance of C6 females at that time.
Other areas
The total abundance of nauplii (N) or C1–C6 individuals in the 0–2,000 m water columns in the western (HO96098, 19, and KNOT) and central subarctic Pacific (OS97097) fell within the annual range recorded at site H, but those in the eastern subarctic Pacific (OS970120 and OS97130), Okhotsk Sea (Ok30 and HO97150), and Japan Sea (HO97103, C08, I09, and J07) were less or significantly less than the lower end of the annual range recorded at site H (Table 2).
In terms of developmental stage composition, the populations at HO96098, 19, and KNOT in the western subarctic Pacific, and OK30 and HO97150 in the Okhotsk Sea were characterized by the predominance of C3–C5, and those at OS97097 in the central, and OS97120 and OS97130 in the eastern subarctic Pacific by nauplii or C6 males (Table 2). For the stage composition of the Japan Sea populations, predominant stages varied greatly (C3–C6 males) between stations (C08, I09, J07, and HO97103).
Vertical distribution
Site H
Day/night differences in vertical distribution patterns of the N–C3, C4, C5, and C6 males and females of E. bungii observed in December 1996 and April and October 1997 were all insignificant (Fig. 6; Kolmogorov-Smirnov test, P>0.1). From these results, vertical distribution patterns of E. bungii observed at different times of the day can be compared directly to reveal their seasonal vertical movements, as follows (Fig. 7). Nauplii through C2 stages occurred almost exclusively in the top 250 m. In particular, they were found above the thermocline (<80 m) when their abundance reached its annual maximum in early June. Similarly, the majority of C3, C4, C5, and C6 females were found above the thermocline during the peak season of their abundance. As an exception, most C6 males at their peak season were seen at 250–500 m depth. From July, C3–C5 sank to a deeper layer, residing mostly at 500–2,000 m (C3 and C5) or 250–500 m (C4) by February 1997, then ascending to the surface layer in March 1997. Seasonal migration patterns of C6 males and females were more or less similar to those of C3–C5, but they started upward migration in February 1997, thus about 1 month earlier than C3–C5.
Other areas
The C3–C5 stages, which predominated at stations in the western subarctic Pacific (HO96098, 19, and KNOT) and Okhotsk Sea (OK30 and HO97150), were distributed in the vertical ranges 250–1,000 m and 500–2,000 m, respectively (Fig. 8). More than 50% of the populations of predominant C4–C5 in the central (OS97097) and N and C6 males in the eastern subarctic Pacific (OS97120 and OS97130) were found in the 0–250, 0–40 (bottom of thermocline), and 250–500 m layers, respectively. The vertical distribution patterns of predominant stages at stations in the Japan Sea were characterized by narrower vertical ranges than those seen at site H: C3 predominated at C08 and occurred from 500 to 1,000 m only; while C5 predominating at I09, and C6 males predominating at J07, occurred from 250 to 500 m only (Fig. 8).
Prosome length
Site H
Prosome lengths of C1–C6 individuals fluctuated to some extent throughout the year at station H (Fig. 9), but we detected no significant trend in the seasonal pattern (Runs test, P>0.05). On this basis, mean prosome lengths were calculated as (mean±SD) 1.19±0.06 mm for C1, 1.73±0.25 mm for C2, 2.70±0.07 mm for C3, 3.67±0.10 mm for C4 males, 3.72±0.12 mm for C4 females, 4.89±0.15 mm for C5 males, 5.19±0.19 mm for C5 females, 4.90±0.18 mm for C6 males, and 6.79±0.20 mm for C6 females. Between-stage differences were significant, except for C5 males and C6 males for which the difference was not significant (U-test, P>0.7). For C4–C6, the prosome lengths of females were greater than those of males (U-test, P<0.001).
Other areas
Prosome length data of C6 females and C5 males from the subarctic Pacific, Bering Sea, Okhotsk Sea, and Japan Sea are summarized in Table 3. Data for C5 males were used because the number of C6 males collected was too small to make meaningful regional comparisons. As noted above, the differences in the prosome length between C6 and C5 males were very small at site H (Fig. 9). Statistical tests (one-way ANOVA and Scheffé’s F) revealed that the prosome lengths of site H specimens are similar to those at HO96098, 19, and KNOT in the same western subarctic Pacific and to those at W302 in the Bering Sea, but are larger than those at OS97097, OS97120, and OS97130 in the central and western subarctic Pacific, and smaller than those at OK30, HO97150, and HO97103 in the Okhotsk Sea and Japan Sea (Table 3).
Lipid droplets
Site H
Since preliminary analysis indicated that the sexual differences in the lipid deposition pattern were not appreciable, the male and female data of C4–C6 were pooled in each stage (Fig. 10). For C3–C5, the proportion of specimens in the rich category decreased gradually from September 1996 and was nearly zero by the middle of the phytoplankton bloom period. At the same time, the proportion of medium category and/or poor category specimens increased gradually. After the phytoplankton bloom, the poor category almost disappeared in C3–C5, and the proportions of medium and rich category (C4 and C5) increased rapidly. During the phytoplankton bloom period, C6 exhibited a similar lipid deposition pattern to that of C3–C5, but the pattern was rather irregular either prior to or after the bloom period. On average (annual mean), medium was the most common type for C3 (60%), C4 (57%), and C6 (49%), while the rich category was most common for C5 (59%).
Other areas
The proportion of the three lipid deposition types was calculated for the C5 and C6 female data (Fig. 11). Across 12 stations, the fraction of rich category exceeded 50% of the total at all four stations in the Japan Sea and one station (HO96998) in the western subarctic Pacific. Conversely, the poor category occupied >30% of the total at one station (KNOT) in the western, one station (OS87997) in the central, and one station (OS97130) in the western subarctic Pacific, and one station (HO97150) in the Okhotsk Sea. In three other stations (19, OS97120, and Ok30), the medium category contained more specimens than the other two.
Discussion
Lifecycle/ontogenetic vertical migration
Combining seasonal sequences of abundance of each developmental stage (Fig. 5) with those of their vertical distribution (Fig. 7), the lifecycle and associated ontogenetic vertical migration patterns of E. bungii at site H can be deduced. Judging from a rapid increase in the number of C6 females of advanced maturity stages in April, which was then followed by prominent abundance peak of nauplii in early June, major spawning can be estimated to have occurred in April/May at site H. By tracing the abundance peaks, it is clear that nauplii developed and reached C5 by August. The entire sequence, including spawning, egg hatching and subsequent postembryonic development up to C5, occurred in the surface layer (see Fig. 7). From August onward, some C3 and C4 individuals might have halted development, migrated down to >500 m (see Fig. 7) and overwintered together with C5, then developed to C6. The C6 males increased in February/March, 1 month earlier than the increase in C6 females. Mating occurred in the 250–500 m stratum, just before the upward migration of C6 females to the surface layer for spawning. From this E. bungii developmental scenario, their generation time is estimated as 1 year at site H (Fig. 12). It is noted, however, that C3, C4, C5, and C6 females never disappeared completely from the 0–2,000 m water column by the time of the onset of new cohorts at site H, so longer a generation time than 1 year cannot be ruled out for a small part of the population.
Miller et al. (1984) studied the lifecycle of E. bungii at station P (50°N, 145°W) in the eastern subarctic Pacific. Some of their results are consistent with ours, e.g. near equal sex ratios of C4 and C5, extremely female-biased sex ratios of C6, and incidence of possible mating of C6 males and females at 250–500 m depth. However, there are some differences in lifecycle features of the populations between these two regions. At station P, a cohort produced in June/July develops to C3–C4 by the end of summer of the same year. In the second year, overwintered C3–C4 individuals develop to C5 through summer, overwinter again, and then develop to C6 in the third year. Thus, one lifecycle of the population at station P requires typically 2 years, in contrast to 1 year at site H. In terms of ontogenetic vertical migration, the C3, C5, and C6 female populations at station P overwinter in a shallower depth stratum (250–500 m) than those at site H (>500 m depth, see Fig. 7). The population size of C3–C6 over 0–2,000 m depth at station P ranged from 2,800 (July) to 10,200 (March) individuals m−2, many fewer than the 5,500 (May) to 61,400 (July) individuals m−2 at site H.
These differences in lifecycle features of E. bungii at site H and station P may be due to dissimilar environmental conditions between these two regions. At station P, phytoplankton biomass shows no clear seasonality throughout the year (0.1–0.6 mg chlorophyll a m−3), although primary production reaches its maximum in May to July (Welschmeyer et al. 1993; Harrison et al. 1999). In contrast, the occurrence of a phytoplankton bloom (2–9 mg chlorophyll a m−3) between March and June is a regular annual event at site H in the Oyashio region (Kasai et al. 2001). A later spawning season at station P (June/July) as compared to site H (April/May) may reflect the dissimilar peak seasons of high phytoplankton stock (or productivity). The close-coupling of spawning and high phytoplankton has been observed in the other copepods of the subarctic Pacific, such as M. pacifica and M. okhotensis (Padmavati et al. 2004). With regard to thermal conditions of the habitats, the annual range in surface temperatures at station P is narrower [6–14°C; Fig. 6 in Miller et al. (1984)] than that at site H (2–18°C; see Fig. 2). Station P is warmer than site H in general; temperatures as low as 3°C throughout the year are only seen below 1,000 m at the former but below 200 m at the latter. Among the large grazing copepods predominant in the subarctic Pacific, including Neocalanus cristatus, N. plumchrus, and N. flemingeri, E. bungii is considered to be the most cold-adapted species (Vinogradov and Arashkevich 1969). This, combined with higher phytoplankton concentrations at site H, may be why they develop faster there than at station P. In addition to faster development, larger population size (mentioned above) and longer prosome (Table 3) at site H further support the view that this is a more favorable habitat than station P.
From dissimilar seasonal sequences in E. bungii population structure and depth distribution of dominant stages between the 1- and 2-year lifecycle scenarios, we can predict the life history patterns of temporal populations sampled at several stations in the western, central and eastern subarctic Pacific, the Okhotsk Sea, and the Japan Sea (see Fig. 1). Taking into account different sampling seasons, dominant stages, and vertical distribution patterns among stations in the western subarctic Pacific and Okhotsk Sea (Table 2; Fig. 7), we suggest their populations all have a 1-year lifecycle. Incidentally, available information about developmental sequences of E. bungii during limited seasons in Funka Bay, southwestern Hokkaido (Hirakawa 1984), in the southeastern Bering Sea (Vidal and Smith 1986), and the populations in British Columbia inlets [sketchily described by Krause and Lewis (1979)] all fit the 1-year lifecycle scenario. On the other hand, these criteria lead us to conclude that E. bungii at all stations in the central and eastern subarctic Pacific (Table 2; Fig. 8) are populations with a 2-year lifecycle. Although specific environmental conditions resulting in the alternation of lifecycle patterns are not yet fully understood, regional variations in copepod lifecycles have been documented in other copepods, such as N. flemingeri (Miller and Terazaki 1989), Pseudocalanus minutus and P. newmani (Yamaguchi et al. 1998), Calanoides acutus (Atkinson et al. 1997), Rhincalanus gigas (Ward et al. 1997), Calanus finmarchicus (Planque et al. 1997), Calanus hyperboreus (Hirche 1997), and M. pacifica (Padmavati et al. 2004).
The stage composition data for E. bungii at four stations in the Japan Sea (Table 2) do not fit either the 1- or 2-year cycle scenarios. Upper layers of the four stations in the Japan Sea (Fig. 1) are under the influence of the warm Tsushima current (a branch of the Kuroshio), so that subarctic zooplankton like E. bungii and subtropical zooplankton occur together in samples collected vertically (Nishimura 1969). Considering the very small population sizes of E. bungii at these stations, they may have been transported from the northern part of the Japan Sea, as has been considered for anomalous stage composition of N. cristatus collected at the same stations previously (Kobari and Ikeda 1999). In light of close coupling of phytoplankton blooms and spawning events of E. bungii (Fig. 5), a complex lifecycle pattern of E. bungii is expected to be a reflection of high regional variations in seasonal phytoplankton production cycles within the Japan Sea (Kim et al. 2000).
Body size
The effects of water temperature and the quality/quantity of food on the body size of marine planktonic copepods are well documented either in the laboratory (Vidal 1980; Escribano and McLaren 1992; Lee et al. 2003) or field (Digby 1954; Deevey 1960; Kobari et al. 2003). As a general rule, lower temperature and higher food supply yield copepods of larger body size. From this view, the annual ranges of water temperature and phytoplankton abundance which the major population of E. bungii with 1-year lifecycles are 2–18°C and 0.2–>9.0 mg chlorophyll a m−3 at site H (Fig. 3). Nevertheless, the prosome lengths of C3 through C6 female E. bungii throughout the year showed irregular patterns with no clear seasonal trend (Fig. 9). Anticipated effects of temperature and food abundance on seasonal prosome length data were not realized. As a plausible explanation for these results, it may be considered that, relative to the length of one generation (1 year), the changes of these variables were too short to produce the effects.
The regional comparison of the prosome length of C6 female E. bungii revealed larger specimens in the Japan and Okhotsk Seas, and smaller specimens in the central and eastern subarctic Pacific (Table 3). Specimens at site H and in the Bering Sea are of intermediate size. Because there were fewer specimens, comparison of C6 males was not possible in this study, but the results for C5 males were consistent with those of C6 females. Kobari and Ikeda (1999, 2001a, 2001b) analyzed regional variation patterns in body size of sympatric Neocalanus spp. at the same stations shown in Fig. 1. Their results show that the regional variation patterns are not necessarily the same among Neocalanus spp., despite them all being grazers with similar, mostly annual lifecycles and associated large ontogenetic vertical migrations. In comparison, the present results for E. bungii are similar to those for N. cristatus (Kobari and Ikeda 1999) and N. flemingeri (Kobari and Ikeda 2001a), but different from those for N. plumchrus (Kobari and Ikeda 2001b). For E. bungii, N. cristatus, and N. flemingeri, larger specimens in the Japan and Okhotsk Seas may result from lower temperatures (Nishimura 1969; Kitani and Shimazaki 1972) and higher phytoplankton abundance in these habitats (Saitoh et al. 1996; Kim et al. 2000), while smaller specimens in the central and eastern subarctic Pacific result from higher temperatures and lower phytoplankton abundance in these habitats (as discussed for station P above). Among the three Neocalanus spp., the prosome length of N. plumchrus exhibited the smallest regional variations, and the length of the specimens in the Japan and Okhotsk Seas did not differ appreciably from those in the other regions. Less marked regional differences in the prosome length of N. plumchrus may be explained by their shallow distribution during the course of copepodite development (cf. Mackas et al. 1993), thereby receiving the widespread influence of the Tsushima warm current in the Japan Sea, or warm upper layer waters above the cold intermediate water in the Okhotsk Sea (see Fig. 4). Beside regional patterns of size variation differing among species of large grazing copepods, those variations showed different amplitudes. The ratio between the largest and smallest specimens is 1.27 (7.62 mm/5.98 mm) for E. bungii, which falls within the variations of 1.08 (4.08 mm/3.79 mm) for N. plumchrus, 1.22 (8.35 mm/6.84 mm) for N. cristatus, and 1.31 (4.57 mm/3.50 mm) for N. flemingeri.
The body size (prosome length) increased with the progress of developmental stage, with the exception that there was no significant increase from C5 males to C6 males (Fig. 9). In terms of dry mass, C5 males and C6 males weigh (mean±SD) 353±136 µg and 397±112 µg, respectively (unpublished data), not a significant difference (P>0.05). Little or no increase has been reported from C5 to C6 in males of other copepods, such as Paraeuchaeta elongata in the Japan Sea (Ikeda and Hirakawa 1996), and Gaidius variabilis (Yamaguchi and Ikeda 2000), M. pacifica, and M. okhotensis in the Oyashio region (Padmavati and Ikeda, unpublished data), all suggesting little or no feeding at C6. This may explain why C6 males remain at depths of 250–500 m and do not migrate up to the surface layer (Fig. 7).
Lipid droplets
It is clear that the phytoplankton bloom (mostly diatoms) induces spawning and facilitates rapid development of E. bungii in the Oyashio region (Fig. 5). For grazing copepods in high latitude seas where phytoplankton abundance is highly seasonal (see Fig. 3), mechanisms to survive the phytoplankton-reduced winter season have been a long-standing issue. Atkinson (1998), working on the copepods in the Southern Ocean, classified the mechanisms into three types: (1) herbivorous in summer, a short reproductive period, and winter diapause at depth; (2) predominantly omnivorous/detritivorous, an extended period of feeding, growth, and reproduction, and less reliance on diapause at depth; and (3) overwintering and feeding within sea ice as early nauplii or copepodites. While the third type is not applicable for the oceanic copepods in the subarctic North Pacific since there is no ice-cover in winter, the first type has been hypothesized previously for Neocalanus spp. and E. bungii (Miller et al. 1984; Tsuda et al. 1999; Kobari and Ikeda 1999, 2001a, 2001b), and the second type for M. pacifica and M. okhotensis (Padmavati et al. 2004). For the first type, specimens in diapause at depth have been known to cease feeding, reduce metabolism, and have large lipid droplets in the body, of which major components are wax esters for Neocalanus spp. (cf. Hagen and Schanack-Schiel 1996; Saito and Kotani 2000) or triacylglycerols for Eucalanus spp. (Ohman et al. 1998; Saito and Kotani 2000). Energy needed to sustain low metabolism in diapause is supplied from these lipid droplets. Thus, the size of the lipid droplets reduces continuously during overwintering, as was observed for C3–C5 (but not C6) E. bungii in this study (Fig. 10). Since the few specimens were available for C6 in fall and winter (Fig. 5), the results cannot be taken as precise in this study. As the winter season progresses, a gradual reduction in the size of lipid droplets in the body has also been reported for Neocalanus spp. at site H (Kobari and Ikeda 1999, 2001b).
The incidence of rich, medium, and poor categories of E. bungii is a widespread phenomenon across the subarctic Pacific, Okhotsk Sea, and Japan Sea (Fig. 11). While the proportion of each type varied greatly from one station to the next, those at stations in the western subarctic Pacific and Okhotsk Sea are similar to those at site H when the differences in sampling seasons are taken into account. For those in the eastern and central surbarctic Pacific, where the populations with a 2-year lifecycle predominated, specimens of poor and medium category were most common, suggesting greater nutritional constraints. This explanation is consistent with their smaller prosome length mentioned above (see Prosome length). In the Japan Sea, the proportions of the three categories at one out of four stations (HO97103) is identical to that at site H at the same season, but samples from the other three stations, collected in January, correspond to the proportions found at site H in July–September. As mentioned above (see Lifecycle), we consider that these specimens do not complete lifecycles at each station but are transported from the northern part of the Japan Sea.
E. bungii is characterized by a very transparent body, which is reflected in their high water content (90–92% of wet weight; unpublished data) compared with water contents (82–84%) recorded for most other copepods (Båmstedt 1986). In addition to a watery body, the size of lipid droplets (see Fig. 2) relative to the body volume of E. bungii is much smaller than is found in Neocalanus spp. Rich category specimens may contain body carbon that is 50% of body dry weight (DW) (Omori 1969) while those of the poor category contain carbon at 35% of DW (Ikeda, unpublished data). The carbon content of rich category E. bungii is much less than the 60–64% found in Neocalanus spp. (Ikeda et al. 2004). In the 1-year lifecycle scenario of the E. bungii population at site H (Fig. 12), the C5 are in the diapause phase from August to February of the next year (i.e., for 7 months) mostly at >500 m depth (Fig. 7). If one assumes that oxygen consumption rates in diapause of E. bungii are similar to those of N. cristatus [0.075 µl O2 (mg DW)−1 h−1, see Ikeda et al. 2004] and the DW of C5s is 0.33 mg, the amount of lipids [0.33×(0.50−0.35)=0.0495 mg or 49.5 µg] could supply the energy needed for the metabolism of C5 for 222 days [=49.5/(0.075×0.33×0.7×24×12/22.4), where 0.7 is RQ for lipid metabolism (Gnaiger 1983), 24 is hours in a day, and 12/22.4 is C mass in 1 mol CO2] or 7.4 months, thus demonstrating that rich category C5 E. bungii could overwinter at site H through Atkinson’s first overwintering mechanism.
References
Atkinson A (1998) Lifecycle strategies of epipelagic copepods in the Southern Ocean. J Mar Syst 15:289–311
Atkinson A, Schnack-Schiel SB, Ward P, Marin V (1997) Regional differences in the lifecycle of Calanoides acutus (Copepoda: Calanoida) within the Atlantic sector of the Southern Ocean. Mar Ecol Prog Ser 150:99–111
Båmstedt U (1986) Chemical composition and energy content. In: Corner EDS, O’Hara SCM (eds) The biological chemistry of marine copepods. Clarendon Press, Oxford, pp 1–58
Deevey GB (1960) Relative effects of temperature and food on seasonal variations in length of marine copepods in some eastern American and western European waters. Bull Bingham Oceanogr Coll 17:54–87
Digby PSB (1954) The biology of the marine planktonic copepods of Scoresby Sound, east Greenland. J Anim Ecol 23:298–338
Escribano R, McLaren IA (1992) Influence of food and temperature on length and weights of two marine copepods. J Exp Mar Biol Ecol 159:77–88
Fleminger A (1973) Pattern, number, variability and taxonomic signification of integumental organs (sensilla and glandular pores) in the genus Eucalanus (Copepoda, Calanoda). Fish Bull 71:965–1010
Fleminger A, Hulsemann (1973) Relationship of Indian Ocean epiplanktonic calanoids to the world oceans. In: Zeitzschel B, Gerlach SA (eds) The biology of the Indian Ocean. Springer, Berlin Heidelberg New York, pp 339–348
Gnaiger E (1983) Calculation on energetic and biochemical equivalents of respiratory oxygen consumption. In: Gnaiger E, Forstner H (eds) Polarographic oxygen sensors. Springer, Berlin Heidelberg New York, pp 337–345
Hagen W, Schanack-Schiel SB (1996) Seasonal lipid dynamics in dominant Antarctic copepods: Energy for overwintering or reproduction? Deep Sea Res 43:139–158
Harrison PJ, Boyd PW, Varela DE, Takeda S, Shiomoto A, Odate T (1999) Comparison of factors controlling phytoplankton productivity in the NE and NW subarctic Pacific gyres. Prog Oceanogr 43:205–234
Hirakawa K (1984) Seasonal distribution of the planktonic copepods, and life histories of Calanus pacificus, Calanus plumchrus and Eucalanus bungii bungii in the waters of Funka Bay, southern Hokkaido, Japan. Marine Biological Research Institute of Japan (in Japanese with English abstract)
Hirche H-J (1997) Lifecycle of the copepod Calanus hyperboreus in the Greenland Sea. Mar Biol 128:607–618
Ikeda T, Hirakawa K (1996) Early development and estimated lifecycle of the mesopelagic copepod Paraeuchaeta elongata in the southern Japan Sea. Mar Biol 126:261–270
Ikeda T, Sano F, Yamaguchi A (2004) Metabolism and body composition of a copepod Neocalanus cristatus (Crustacea) from bathypelagic zone of the Oyashio region, western subarctic Pacific. Mar Biol (in press)
Johnson MW (1937) The developmental stages of the copepod Eucalanus elongatus Dana var. bungii Giesbrecht. Trans Am Microscop Soc 56:79–98
Kamba M (1977) Feeding habits and vertical distribution of walleye Pollock, Theragra chalcogramma (Pallas), in early life stage in Uchiura Bay, Hokkaido. Res Inst N Pac Fish Hokkaido Univ 175–197
Kasai H, Saito H, Kashiwai M, Taneda T, Kusaka A, Kawasaki Y, Kono T, Taguchi S, Tsuda A (2001) Seasonal and interannual variations in nutrients and plankton in the Oyashio region: a summary of a 10-years observation along the A-line. Bull Hokkaido Natl Fish Res Inst 65:55–134
Kawamura A (1968) Performance of Peterson type closing net. Bull Plankton Soc Jpn 15:11–12
Kawamura A (1989) Fast sinking mouth ring for closing NORPAC net. Bull Jpn Soc Sci Fish 55:1121
Kim SW, Saitoh S, Ishizaka J, Isoda Y, Kishino M (2000) Temporal and spatial variability of phytoplankton pigment concentration in the Japan sea derived from CZCS images. J Oceanogr 56:527–538
Kitani K, Shimazaki K (1972) On the hydrography of the northern part of the Okhotsk Sea in summer. Bull Fac Fish Hokkaido Univ 22:231–242
Kobari T, Ikeda T (1999) Vertical distribution, population structure and lifecycle of Neocalanus cristatus (Crustacea: Copepoda) in the Oyashio region, with notes on its regional variations. Mar Biol 134:683–696
Kobari T, Ikeda T (2001a) Lifecycle of Neocalanus flemingeri (Crustacea: Copepoda) in the Oyashio region, western subarctic Pacific, with notes on its regional variations. Mar Ecol Prog Ser 209:243–255
Kobari T, Ikeda T (2001b) Ontogenetic vertical migration and lifecycle of Neocalanus plumchrus (Crustacea: Copepoda) in the Oyashio region, with notes on its regional variations in body size. J Plankton Res 23:287–302
Kobari T, Shinada A, Tsuda A (2003) Functional roles of inerzonal migrating mesozooplankton in the western subarctic Pacific. Prog Oceanogr 57:279–298
Kono T (1997) Modification of the Oyashio Water in the Hokkaido and Tohoku areas. Deep Sea Res 44:669–688
Krause EP, Lewis AG (1979) Ontogentic migration and the distribution of Eucalanus bungii (Copepoda; Calanoida) in British Columbia inlets. Can J Zool 57:2211
Lee HW, Ban S, Ikeda T, Matsuishi T (2003) Effect of temperature on development, growth and reproduction in the marine copepod Pseudocalanus newmani at satiating food condition. J Plankton Res 25:261–271
Mackas DL, Sefton H, Miller CB, Raich A (1993) Vertical habitat partitioning by large calanoid copepods in the oceanic subarctic Pacific during spring. Prog Oceanogr 32:259–294
Mauchline J (1998) The biology of Calanoid copepods. Adv Mar Biol 33:1–710
Miller CB, Terazaki M (1989) The life histories of Neocalanus flemingeri and Neocalanus plumchrus in the Sea of Japan. Bull Plankton Soc Jpn 36:27–41
Miller CB, Frost BW, Batchelder HP, Clemons MJ, Conway RE (1984) Life histories of large, grazing copepods in the subarctic ocean gyre: Neocalanus plumchrus, Neocalanus cristatus and Eucalanus bungii in the Northeast Pacific. Prog Oceanogr 13:201–243
Nemoto T (1970) Chlorophyll pigments in the stomach and gut of some macrozooplankton species. In: Takenouchi AY et al. (eds) Biological oceanography of the northern North Pacific Ocean. Idemitsu Shoten, Tokyo, pp 411–418
Nishimura S (1969) The zoogeographical aspects of the Japan sea: part V. Publ Seto Mar Biol Lab 17:67–142
Ohman MD, Drits AV, Clarke ME, Plourde S (1998) Differential dormancy of cooccurring copepods. Deep Sea Res 45:1709–1740
Ohtani K (1971) Studies on the change of the hydrographic conditions in the Funka Bay. II. Characteristics of the waters occupying the Funka Bay. Bull Fac Fish Hokkaido Univ 22:58–66
Ohtsuka S, Ohaye S, Tanimura A, Fukuchi M, Hattori H, Sasaki H, Matsuda O (1993) Feeding ecology of copepodid stages of Eucalanus bungii in the Chukchi and northern Bering Seas in October 1988. Proc NIPR Symp Polar Biol 6:27–37
Omori M (1969) Weight and chemical composition of some important oceanic zooplankton in the North Pacific Ocean. Mar Biol 3:4–10
Padmavati G, Ikeda T, Yamaguchi A (2004) Lifecycle, population structure and vertical distribution of Metridia spp. (Copepoda: Calanoida) in the Oyashio region (NW Pacific Ocean). Mar Ecol Prog Ser 270:181–198
Planque B, Hays GC, Ibanez F, Gamble JC (1997) Large-scale spatial variations in the seasonal abundance of Calanus finmarchicus. Deep Sea Res 44:315–326
Reid JL (1973) North Pacific Ocean waters in winter. The Johns Hopkins Oceanographic Studies No. 5. The Johns Hopkins Press, Baltimore, pp 1–9
Russell RW, Harrison NM, Hunt GL Jr (1999) Foraging at a front: hydrography, zooplankton, and avian planktivory in the northern Bering Sea. Mar Ecol Prog Ser 182:77–93
Saito H, Kotani Y (2000) Lipids of four boreal species of calanoid copepods: origin of monene fats of marine animals at higher trophic levels in the grazing food chain in the subarctic ocean ecosystem. Mar Chem 71:69–82
Saitoh S, Kishino M, Kiyohuji H, Taguchi S, Takahashi M (1996) Seasonal variability of phytoplankton pigment concentration in the Okhotsk Sea. J Rem Sen Soc Jpn 16:86–92
Takeuchi I (1972) Food animals collected from the stomachs of three salmonid fishes (Oncorhynchus) and their distribution in the natural environments in the northern North Pacific. Bull Hokkaido Reg Fish Res Lab 38:1–119 (in Japanese with English abstract)
Terazaki M, Tomatsu C (1997) A vertical multiple opening and closing plankton sampler. J Adv Mar Sci Technol Soc 3:127–132
Tsuda A, Sugisaki H (1994) In situ grazing rate of the copepod population in the western subarctic North Pacific during spring. Mar Biol 120:203–210
Tsuda A, Saito H, Kasai H (1999) Life histories of Neocalanus flemingeri and Neocalanus plumchrus (Calanoida: Copepoda) in the western subarctic Pacific. Mar Biol 135:533–544
Tsurushima N, Nojiri Y, Imai K, Watanabe S (2002) Seasonal variations of carbon dioxide system and nutrients in the surface mixed layer at station KNOT (44°N, 155°E) in the subarctic western North Pacific. Deep Sea Res 49:5377–5394
Vidal J (1980) Physioecology of zooplankton. IV. Effects of phytoplankton concentration, temperature, and body size on the net production efficiency of Calanus pacificus. Mar Biol 56:203–211
Vidal J, Smith SL (1986) Biomass, growth, and development of populations of herbivorous zooplankton in the southeastern Bering Sea during spring. Deep Sea Res 33:523–556
Vinogradov ME (1970) Vertical distribution of the oceanic zooplankton. Israel Program for Scientific Translations, Jerusalem
Vinogradov ME, Arashkevich EG (1969) Vertical distribution of interzonal copepod filter feeders and their role in communities at different depths in the northwestern Pacific. Oceanology 9:399–409
Ward P, Atkinson A, Schnack-Schiel SB, Murray AWA (1997) Regional differences in the lifecycle of Rhincalanus gigas (Copepoda: Calanoida) in the Antarctic sector of the Southern Ocean: re-examination of existing data (1928 to 1993). Mar Ecol Prog Ser 157:261–275
Welschmeyer NA, Strom S, Goericke R, Ditullio G, Belvin M, Petersen W (1993) Primary production in the subarctic Pacific Ocean: project SUPER. Prog Oceanogr 32:101–135
Yamaguchi A, Ikeda T (2000) Vertical distribution, lifecycle, and developmental characteristics of the mesopelagic calanoid copepod Gaidius variabilis (Aetideidae) in the Oyashio region, western North Pacific Ocean. Mar Biol 137:99–109
Yamaguchi A, Ikeda T, Shiga N (1998) Population structure and lifecycle of Pseudocalanus minutus and Pseudocalanus newmani (Copepoda: Calanoida) in Toyama Bay, southern Japan Sea. Plankton Biol Ecol 45:183–193
Yamaguchi A, Watanabe Y, Ishida H, Harimoto T, Furusawa K, Suzuki S, Ishizaka J, IkedaT, Takahashi MM (2002) Community and trophic structures of pelagic copepods down to greater depths in the western subarctic Pacific (WESTCOSMIC). Deep Sea Res 49:1007–1025
Zenkevitch L (1963) Biology of the seas of the USSR. Allen and Unwin, London
Acknowledgements
We are grateful to C.B. Miller for reviewing earlier drafts of this paper; his constructive comments significantly improved the manuscript. This study was supported in part by grant JSPS KAKENHI 14209001 to T.I. The authors hereby declare that experiments performed during this study comply with current laws of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Shoden, S., Ikeda, T. & Yamaguchi, A. Vertical distribution, population structure and lifecycle of Eucalanus bungii (Copepoda: Calanoida) in the Oyashio region, with notes on its regional variations. Marine Biology 146, 497–511 (2005). https://doi.org/10.1007/s00227-004-1450-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1450-3